Introduction
Zingiber mioga (known as yangha in Korea,
myoga in Japan and ranghe in China) is a perennial herb with short
vegetative shoots that belongs to the ginger family (Zingiberaceae)
(1). Z. mioga is widely
cultivated in central and southeast China, North Vietnam and South
Korea (1). Z. mioga is used
medicinally to treat coughing and rheumatism in China, and is
widely consumed throughout Japan (2). Furthermore, Z. mioga is
commercially cultivated on Jeju Island in South Korea. The leaves
of Z. mioga are used to wrap and preserve manjyu, a popular
traditional Japanese confection, due to their potent antimicrobial
activity (3).
Numerous compounds have been extracted from Z.
mioga. Mioganal, miogadial and miogatial are found at high
levels in the flower buds. Additionally, high levels of galanal A
and B are present in the leaves and rhizomes (3–6). These
compounds have various biological activities, including anticancer,
antimicrobial, anti-inflammatory and anti-platelet aggregation
effects (3,6–8). Cho
et al previously reported that Z. mioga can improve
memory and neurocognitive performance (9). Although Z. mioga flower buds
have various beneficial effects, including antimicrobial and
anti-inflammatory properties, few studies have evaluated their
anti-obesity effects (10,11). A prior report suggested that Z.
mioga inhibits fat accumulation in 3T3-L1 adipocytes and
decreases body weight gain in mice (12); however, the molecular mechanism
underlying this effect remains unclear.
In the current study, we investigated the effect of
Z. mioga flower bud water extract (ZMW) on obesity and
insulin resistance. To clarify the mechanisms underlying the
regulation of obesity, the expression of lipid
metabolism-associated genes in the liver and adipose tissue were
analyzed. Furthermore, we examined the effect of ZMW on
obesity-induced inflammation.
Materials and methods
Plant material and extract
preparation
Z. mioga Roscoe flower buds were collected
from a traditional market on Jeju Island (South Korea) in September
2013 and authenticated by Professor Seong-gyu Ko of the College of
Oriental Medicine, Kyunghee University (Seoul, South Korea). A
voucher specimen was deposited at the KFRI Herbarium (K00234) on
September 23, 2012. Freshly picked plants were freeze-dried, ground
using a mill and passed through a 50-mesh sieve. The ground powder
was subjected to extraction with water or 70% ethanol twice using
repeat sonication for 30 min at room temperature. The extract was
filtered using a Whatman No. 2 paper filter (Advantec MFS, Inc.,
Dubulin, CA, USA) and concentrated using a vacuum evaporator
(R-200; BÜCHI Labortechnik AG, Flawil, Switzerland). The extracts
were freeze-dried and stored at −80°C.
Chemicals and reagents
Mouse anti-β-actin monoclonal antibody (sc-47778),
rabbit anti-sterol regulatory element-binding protein (SREBP)-1c
polyclonal antibody (sc-366), rabbit anti-CCAAT-enhancer-binding
protein α (C/EBPα) polyclonal antibody (sc-61), mouse
anti-peroxisome proliferator-activated receptor (PPAR)-γ monoclonal
antibody (sc-7273), rabbit anti-phosphorylated (p)-nuclear factor
(NF)-κB polyclonal antibody (sc-33039), rabbit anti-NF-κB
polyclonal antibody (sc-109) and horseradish peroxidase
(HRP)-conjugated anti-rabbit (sc-2004) and anti-mouse (sc-2005)
secondary antibodies, were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Rabbit anti-p-AMP-activated kinase
(AMPK; 2531), anti-AMPK (2532) and anti-FAS (318) polyclonal
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Oil Red O (O0625) and 3-(4,5-dime
thylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's
modified Eagle's medium (DMEM), fetal bovine serum (FBS),
penicillin-streptomycin, bovine calf serum (CS) and
phosphate-buffered saline (PBS) were obtained from Gibco (Thermo
Fisher Scientific, Inc., Grand Island, NY, USA).
Cell culture and adipocyte
differentiation
3T3-L1 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM
containing 10% CS, 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C under 5% CO2. The cells were seeded onto a
6-well plate (4×105 cells/well) and maintained for 2
days. To induce differentiation, 2-day post-confluent 3T3-L1 cells
(day 0) were incubated in DMEM containing 0.5 mM
3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich), 1 µM
dexamethasone (Sigma-Aldrich), 1 µg/ml insulin (Sigma-Aldrich) and
10% FBS for 3 days. Following induction, the medium was replaced
with DMEM containing 10% FBS and 1 µg/ml insulin, and the cells
were incubated for 2 days. Cells were then maintained in DMEM
containing 10% FBS until they reached maturity. Between days 0 and
3, the cells were exposed to Z. mioga extract.
Cytotoxicity assay
3T3-L1 cells were seeded in a 96-well plate
(2×103 cells/well). After 24 h of preconditioning, the
cells were exposed to various concentrations of Z. mioga
extracts for 24 or 48 h. Subsequently, 50 µl cell counting kit-8
solution (#CK04; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) was added to each well, and the plate was incubated for an
additional 3 h at 37°C, to detect cell survival. Cell viability was
calculated by measuring the absorbance on a microplate reader
(Infinite M200; Tecan US, Inc., Morrisville, NC, USA) at 450
nm.
Oil Red O staining
Cells were washed with PBS, fixed with 10% formalin
for 1 h at room temperature, washed with PBS again and stained with
filtered 0.5% Oil Red O solution in 60% isopropanol for 1 h. After
the stained lipid droplets were washed with distilled water, they
were observed under a fluorescence microscope (IX71; Olympus
Corporation, Tokyo, Japan). Oil Red O was extracted from the cells
with 100% isopropanol, and the optical density was determined using
a microplate reader (Infinite M200) at an excitation wavelength of
490 nm.
Treatment of animals
Four-week-old male C57BL/6J mice were purchased from
Orient Bio, Inc. (Seoul, South Korea) and acclimatized for 1 week
prior to random assignment into experimental groups. The animals
were housed in individual cages with free access to a regular diet
and water in a room with a 12-h light cycle, a temperature of 24°C
and a humidity of 55%. The mice were divided into four groups:
Normal group fed the American Institute of Nutrient-76 diet
(AIN-76; N group); a group fed a high-fat diet (HFD; HFD group);
and two groups fed a HFD with 0.25 or 0.5% ZMW (HFD + ZMW 0.25 or
0.5% groups). The experimental diets were based on the AIN-76 diet,
which contained 20% fat and 0.5% cholesterol (w/w). Animal studies
were conducted in accordance with institutional and national
guidelines, and all experimental procedures were approved by the
Korea Food Research Institute Animal Care and Use Committee
(#2014-0120). After consuming the experimental diets for 8 weeks,
the mice were subcutaneously anesthetized with Zoletil 50 (30
mg/kg; Virbac Philippines Inc., Taguig, Philippines) in PBS and
then sacrificed by cervical dislocation. Blood samples were
collected from the abdominal aorta. The liver and epididymal fat
tissues were harvested in liquid nitrogen and stored at −80°C for
protein and RNA extraction.
Glucose tolerance test
The oral glucose tolerance test (OGTT) was performed
after 8 weeks of ZMF administration. OGTT was determined in
response to oral glucose administration (1.2 g/kg body weight)
after fasting 12 h. Blood glucose was collected from the tail vein
0, 14, 30, 60 and 120 min after oral glucose administration. Blood
glucose concentrations were determined by the glucose oxidase
method using a blood glucose monitoring meter
(Accu-Chek® Performa Nano; Roche Diagnostics, Basel,
Switzerland).
Total triglyceride (TG), total
cholesterol (TC) and high-density lipoprotein (HDL)
determination
TG, TC and HDL were measured enzymatically using
commercial kits (cat. nos. 20267, 20186 and 20081, respectively;
Shinyang Chemical Co., Ltd., Busan, South Korea).
Immunoblotting
Cells were harvested in lysis buffer (Sigma-Aldrich)
containing 40 mM HEPES (pH 7.4), 120 mM NaCl, 1 mM EDTA, 50 mM NaF,
1.5 mM Na3VO4, 10 mM β-glycerophosphate and
1% Triton X-100, supplemented with protease and phosphatase
inhibitor cocktails (78440; Pierce; Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). Protein concentration was determined using the
Bradford protein assay. The proteins were separated by 7.5% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene fluoride (PVDF) membranes, followed
by blocking with 5% non-fat dry milk for 1 h. Subsequently, the
membranes were incubated overnight at 4°C with mouse anti-β-actin
monoclonal antibody (1:2,000), rabbit anti-SREBP-1c polyclonal
antibody (1:1,000), rabbit anti-C/EBPα polyclonal antibody
(1:1,000), mouse anti-PPAR-γ monoclonal antibody (1:1,000), rabbit
anti-p-NF-κB polyclonal antibody (1:1,000), rabbit anti-NF-κB
polyclonal antibody (1:1,000), rabbit anti-p-AMPK polyclonal
antibody (1:1,000), rabbit anti-AMPK polyclonal antibody (1:1,000)
and rabbit anti-FAS polyclonal antibody (1:1,000), followed by
washing with tris-buffered saline containing Tween-20 and
incubation with HRP-conjugated anti-rabbit and anti-mouse IgG
(1:1,000; Santa Cruz Biotechnology, Inc.) at room temperature for 1
h. The bands were visualized using a chemiluminescence reagent (GE
Healthcare, Little Chalfont, UK), and the relative intensities of
the bands to β-actin signal was quantified by ImageJ software v1.45
(National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 20–30 mg liver tissue
using a NucleoSpin RNA II kit (Macherey-Nagel GmbH & Co. KG,
Düren, Germany) according to the manufacturer's protocol. Following
genomic DNA elimination with the gDNA elimination buffer in the
RT2 First Strand kit (cat. no. 330401, Qiagen, Inc.
Valencia, CA, USA), cDNA was synthesized using 1 µg RNA and the
ReverTra Ace® qPCR RT kit (Toyobo, Co., Ltd., Osaka,
Japan). qPCR was conducted on an StepOnePlus Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc., Foster City,
CA, USA) using SYBR Green real-time PCR Master Mix (Toyobo, Co.,
Ltd.) as follows: Pre-denaturation at 95°C for 5 min, followed by
40 cycles of 95°C for 15 sec, 60°C for 15 sec and 75°C for 45 sec.
The PCR reaction was performed in a volume of 20 µl, containing 2
µl cDNA template and 10 pmol primers. The specific primer sequences
were as follows: Phosphoenolpyruvate carboxykinase (PEPCK) forward,
AAAAGCCTTTGGTCAACAAC and reverse, AAACTTCATCCAGGCAATGT; G6Pase
forward, GAGTCTTGTCAGGCATTGCT and reverse, GGTACATGCTGGAGTTGAGG;
interleukin (IL)-6 forward, AGTTGCCTTCTTGGGACTGA and reverse,
TCCACGATTTCCCAGAGAAC; and tumor necrosis factor (TNF)-α forward,
ACGGCATGGATCTCAAAGAC and reverse, AGATAGCAAATCGGCTGACG. The
experiment was conducted in duplicate with a negative control (no
cDNA) and RT control (no reverse transcription). RNA levels were
expressed as the ratio of the signal intensity for each gene
relative to β-actin and were calculated using the 2−ΔΔCq
method (13).
Statistical analysis
Differences between groups were evaluated using a
one-way analysis of variance (ANOVA) with Prism5 software (GraphPad
Software, Inc., San Diego, CA, USA). The Bonferroni post-hoc
correction for multiple comparisons was used when significant
differences were identified using ANOVA (P<0.05). Data are
expressed as the mean ± standard deviation.
Results
ZMW inhibits 3T3-L1 preadipocyte cell
differentiation
To establish the optimal extract concentration for
use in vitro, the cells were treated with media containing
0–500 µg/ml ZMW or Z. mioga ethanol extracts (ZME) for 24 or
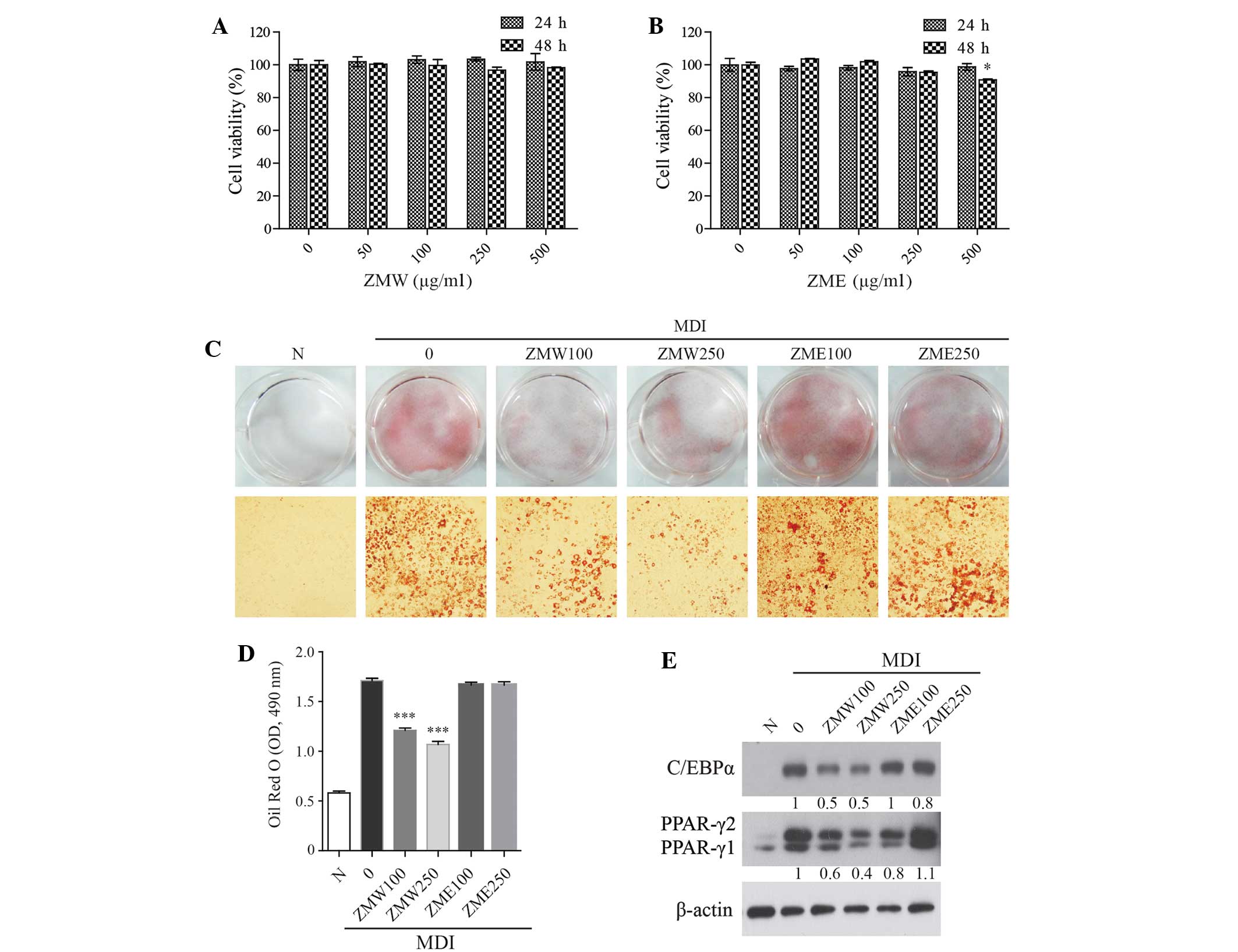
48 h. The viability of 3T3-L1 preadipocytes was not significantly
affected by extract treatment up to 250 µg/ml (Fig. 1A and B). We next compared the
inhibitory effects of the extract (0–250 µg/ml) on lipid
accumulation during 3T3-L1 preadipocyte cell differentiation. Oil
Red O staining was used to determine lipid droplet accumulation in
3T3-L1 adipocytes. ZMW significantly and dose-dependently inhibited
lipid accumulation, whereas ZME had no effect (Fig. 1C and D). The expression of several
proteins involved in adipogenesis, including C/EBPα and PPARγ, were
markedly suppressed by ZMW (Fig.
1E). These results suggest that ZMW, but not ZME, may have
anti-obesity effects.
ZMW reduces body and liver weight in
HFD-fed mice
We next investigated whether ZMW was able to prevent
HFD-induced obesity in mice. Total body and liver weight were
significantly decreased in the HFD + ZMW 0.5% group, as compared to
the HFD group (Fig. 2A and B).
Unexpectedly, ZMW treatment did not affect adipose tissue weight
(Fig. 2C), although ZMW inhibited
adipogenesis in vitro.
ZMW reduced serum and liver
lipids
We next evaluated the effects of ZMW on the serum
and liver lipid levels in mice fed HFD. ZMW significantly reduced
the serum TG concentration (N, 37.5±2.3; HFD, 62.3±5.1; HFD + ZMW
0.5%, 48.3±4.2) and TC (N, 162.0±14.2; HFD, 243.2±14.3; HFD + ZMW
0.5%, 208.4±22.6) (Fig. 2D),
although there was no significant difference in HDL cholesterol.
Furthermore, hepatic TC and TG in the ZMW group were significantly
reduced compared with the HFD group (Fig. 2E), although the total lipid levels in
the ZMW group were not significantly different compared with the
HFD group. These results indicate that ZMW effectively lowers serum
and liver cholesterol.
ZMW improves insulin resistance
Lipid accumulation in the liver can lead to insulin
resistance by interfering with insulin signal transduction
(14). We next examined the effect
of ZMW on insulin resistance in HFD-induced obese mice. The OGTT
was used to evaluate insulin resistance. The ZMW group showed
significantly lower blood glucose levels compared with the HFD
group (Fig. 3A). Consequently, the
area under the curve (AUC) in the ZMW group was significantly
reduced (P<0.05) (Fig. 3B). To
test the effect of ZMW on the expression of gluconeogenic genes,
including PEPCK and G6Pase, the mRNA expression levels of PEPCK and
G6Pase were determined using RT-qPCR. ZMW supplementation
significantly reduced hepatic mRNA expression of PEPCK and G6Pase
compared with the control group (Fig. 3C
and D), indicating that HFD impairs hepatic insulin signaling,
and ZMW supplementation effectively restores hepatic insulin
sensitivity.
ZMW reduces the expression of lipid
metabolism-associated genes in the liver, but not the epididymal
fat
The AMPK signaling pathway serves a crucial function
in liver lipid metabolism; when AMPK is activated, it inhibits the
fatty acid and sterol synthesis pathway, and activates the fatty
acid oxidation pathway (15).
Furthermore, activated AMPK suppresses SREBP-1c, a
lipogenesis-associated gene (16).
Consistent with the reduced liver weight, western blot analysis
indicated that ZMW treatment increased AMPK activation and
decreased the SREBP-1c (Fig. 4A and
B), although it did not suppress adipogenesis (Fig. 4C and D). These results suggest that
ZMW may reduce body weight via the downregulation of hepatic
lipogenesis.
ZMW ameliorates hepatic
inflammation
Diet-induced obesity triggers inflammatory responses
(17). When hepatic proinflammatory
cytokine mRNA levels were examined, HFD-fed mice displayed a
significant increase in IL-6, although TNF-α levels were slightly
decreased, as compared with normal mice (Fig. 5A and B). The increased IL-6 levels
were significantly decreased by ZMW supplementation (Fig. 5A). However, ZMW had no effect on
TNF-α levels (Fig. 5B). We also
examined whether ZMW inhibits NF-κB activation (Fig. 5C and D). ZMW reduced NF-κB
phosphorylation, indicating that ZMW ameliorates HFD-induced
inflammatory enzymes, such as NF-κB. Collectively, the present
results suggest that ZMF improves hepatic steatosis while
decreasing liver inflammation.
Discussion
Z. mioga is also a traditional medicine that
is used to relieve insect bites, eye inflammation, cough and
rheumatism (2,18). Z. mioga is a member of the
ginger family and its biological function is unclear. It was
previously reported that Z. mioga exerts an anti-obesity
effect (12); however, the mechanism
underlying obesity inhibition remains unclear. In the present
study, ZMW inhibited adipogenesis in vitro, whereas ZME had
no effect. Several studies have characterized the chemical
composition and biological activity of Z. mioga (5–7,19). Phenolic compounds are more abundant
in ZMW compared with ZME (9),
indicating that the phenolic compounds are water soluble, which is
unique as the majority of phenolic compounds are hydrophobic.
Miogadial, galanal A and galanal B have been identified in Z.
mioga, and display potent anticancer activity in vitro,
as compared with other ginger-specific constituents (7). However, these compounds were isolated
from the ethyl acetate fraction and are water insoluble (5). Thus, these compounds are likely to not
be involved in the anti-obesity effects. Rather, water-soluble
compounds are responsible for the inhibiting adipogenesis. Flavor
components were also identified, with numerous terpenes, including
β-pinene, β-terpinene, β-phellandrene, α-pinene and 1,4-terpineol
(20).
In animal models, the present results showed that
ZMW lowers body weight and serum and hepatic lipid levels in
HFD-fed mice. ZMW also attenuates the increase in liver weight
observed in mice fed with a HFD. Western blot data indicated that
ZMW effectively suppresses SREBP-1c protein expression, a lipogenic
gene, and restores AMPK activation. These results are consistent
with previous work that showed AMPK activation suppresses SREBP-1c
cleavage and nuclear translocation (16). Unlike the in vitro studies,
ZMW administration did not decrease adipose tissue weight, although
ZMW markedly inhibited adipogenesis during 3T3-L1 cell
differentiation. Furthermore, the HFD + ZMW 0.5% group did not
alter C/EBPα and PPARγ expression in epididymal white adipose
tissue, as compared with the mice that received a HFD. It is
possible that ZMW does not penetrate the fat tissue, or that levels
were too low for an effect. These results also suggest that ZMW is
able to reduce body weight without decreasing adipose tissue fat
accumulation.
A HFD has been shown to affect lipid and glucose
metabolism, impair insulin sensitivity and induce inflammation
(15,21,22). In
the present study, ZMW reversed the HFD-induced insulin resistance.
Furthermore, decreased PEPCK and G6Pase gene expression was
observed in the liver tissue of the HFD + ZMW group, suggesting
that reduced gluconeogenesis may contribute to improved insulin
sensitivity. Inflammation has an important role in the pathogenesis
of obesity-related insulin resistance (15,23). The
proinflammatory cytokines IL-6 and TNFα mediate insulin action and
glucose transport through multiple targets (22,24).
Therefore, we evaluated the effects of ZMW treatment on the
expression of cytokines in the liver. The data presented herein
showed that ZMW reduced the expression of IL-6 in the liver, as
compared with the HFD group. However, hepatic TNF-α expression was
not significantly different between the normal and HFD groups,
which differs from previous studies (25,26). It
is possible that HFD administration for 4 weeks may not markedly
increase inflammation. Additionally, it was found that the HFD
upregulated the phosphorylation of NF-κB genes, which were
significantly reduced following ZMF supplementation.
Collectively, the results of in vivo
experiments support the anti-obesity effect of Z. mioga. ZMW
may function by inhibiting hepatic gluconeogenesis, resulting in
increased insulin sensitivity and reduced inflammation. The present
study supports the further investigation of ZMW as a potential
anti-hepatic steatosis agent.
Acknowledgements
The present study was supported by the Korea Food
Research Institute (grant no. E0080201-08).
References
|
1
|
Cole TCH and Nürnberger S: Zingiber
mioga and its cultivars. Plantsman: New Series. 14:2262014.
|
|
2
|
Mihashi H and Okada M: Illustrated
medicinal plants of the world in colour. Hokuryūkan Co., Ltd.
Tokyo: 6661988.(In Japanese).
|
|
3
|
Abe M, Ozawa Y, Uda Y, Yamada F, Morimitsu
Y, Nakamura Y and Osawa T: Antimicrobial activities of diterpene
dialdehydes, constituents from myoga (Zingiber mioga
Roscoe), and their quantitative analysis. Biosci Biotechnol
Biochem. 68:1601–1604. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abe M, Ozawa Y, Morimitsu Y and Kubota K:
Mioganal, a novel pungent principle in myoga (Zingiber mioga
Roscoe) and a quantitative evaluation of its pungency. Biosci
Biotechnol Biochem. 72:2681–2686. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abe M, Ozawa Y, Uda Y, Yamada F, Morimitsu
Y, Nakamura Y and Osawa T: Labdane-type diterpene dialdehyde,
pungent principle of myoga, Zingiber mioga roscoe. Biosci
Biotechnol Biochem. 66:2698–2700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abe M, Ozawa Y, Uda Y, Morimitsu Y,
Nakamura Y and Osawa T: A novel labdane-type trialdehyde from myoga
(Zingiber mioga Roscoe) that potently inhibits human
platelet aggregation and human 5-lipoxygenase. Biosci Biotechnol
Biochem. 70:2494–2500. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyoshi N, Nakamura Y, Ueda Y, Abe M,
Ozawa Y, Uchida K and Osawa T: Dietary ginger constituents,
galanals A and B, are potent apoptosis inducers in Human T lymphoma
Jurkat cells. Cancer Lett. 199:113–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HW, Murakami A, Abe M, Ozawa Y,
Morimitsu Y, Williams MV and Ohigashi H: Suppressive effects of
mioga ginger and ginger constituents on reactive oxygen and
nitrogen species generation, and the expression of inducible
pro-inflammatory genes in macrophages. Antioxid Redox Signal.
7:1621–1629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho KH, Oh MS, Kim GH, Lee SH, Chung KS
and Kim AJ: Effects of Korean Zingiber mioga R. (Flower Buds
and Rhizome) extract on memory. J Korean Soc Food Sci Nutr.
43:1519–1526. 2014. View Article : Google Scholar
|
|
10
|
Shin JH, Lee SJ and Sung NJ: Effects of
Zingiber mioga, Zingiber mioga root and Zingiber
officinale on the lipid concentration in hyperlipidemic rats. J
Korean Soc Food Sci Nutr. 31:679–684. 2002. View Article : Google Scholar
|
|
11
|
Iwashita K, Kohji Y and Tsushida T: Mioga
(Zingiber mioga Rosc.) extract prevents 3T3-L1
differentiation into adipocytes and obesity in mice. Food Sci
Technol Res. 7:164–170. 2001. View Article : Google Scholar
|
|
12
|
Iwashita K, Wamaki K and Tsushida T: Mioga
(Zingiber mioga Rosc.) extract prevents 3T3-L1
differentiation into adipocytes and obesity in mice. Food Sci Tech
Int. 7:164–170. 2001.
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan SM, Sun RQ, Zeng XY, Choong ZH, Wang
H, Watt MJ and Ye JM: Activation of PPARα ameliorates hepatic
insulin resistance and steatosis in high fructose-fed mice despite
increased endoplasmic reticulum stress. Diabetes. 62:2095–2105.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qatanani M and Lazar MA: Mechanisms of
obesity-associated insulin resistance: Many choices on the menu.
Genes Dev. 21:1443–1455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X,
Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al: AMPK
phosphorylates and inhibits SREBP activity to attenuate hepatic
steatosis and atherosclerosis in diet-induced insulin-resistant
mice. Cell Metab. 13:376–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woo SL, Xu H, Li H, Zhao Y, Hu X, Zhao J,
Guo X, Guo T, Botchlett R, Qi T, et al: Metformin ameliorates
hepatic steatosis and inflammation without altering adipose
phenotype in diet-induced obesity. PloS One. 9:e911112014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiart C: Medicinal Plants of China, Korea,
and Japan: Bioresources for Tomorrow's Drugs and Cosmetics (1st).
CRC Press. Boca Raton, FL: 672012.
|
|
19
|
Kurobayashia Y, Sakakibara H, Yanai T,
Yajima I and Hayashi K: Volatile Flavor Compounds of Myoga
(Zingiber mioga). Agric Biol Chem. 55:1655–1657. 1991.
View Article : Google Scholar
|
|
20
|
Lee JW, Chon SU, Han SK, Choi DG and Ryu
J: Effects of antioxidant and flavor components of Zingiber
mioga Rosc. Han'guk Yakyong Changmul Hakhoe Chi. 15:203–209.
2007.(In Korean).
|
|
21
|
Jung S, Lee MS, Shin Y, Kim CT, Kim IH,
Kim YS and Kim Y: Anti-obesity and anti-inflammatory effects of
high hydrostatic pressure extracts of ginseng in high-fat diet
induced obese rats. J Funct Foods. 10:169–177. 2014. View Article : Google Scholar
|
|
22
|
de Luca C and Olefsky JM: Inflammation and
insulin resistance. FEBS Lett. 582:97–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H:
Chronic inflammation in fat plays a crucial role in the development
of obesity-related insulin resistance. J Clin Invest.
112:1821–1830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roytblat L, Rachinsky M, Fisher A,
Greemberg L, Shapira Y, Douvdevani A and Gelman S: Raised
interleukin-6 levels in obese patients. Obes Res. 8:673–675. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Heijden RA, Sheedfar F, Morrison
MC, Hommelberg PP, Kor D, Kloosterhuis NJ, Gruben N, Youssef SA, de
Bruin A, Hofker MH, et al: High-fat diet induced obesity primes
inflammation in adipose tissue prior to liver in C57BL/6j mice.
Aging (Albany NY). 7:256–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Borst SE and Conover CF: High-fat diet
induces increased tissue expression of TNF-alpha. Life Sci.
77:2156–2165. 2005. View Article : Google Scholar : PubMed/NCBI
|