Introduction
Skin aging is a complex phenomenon that induces
numerous changes to the skin components (1). Aging is accompanied by various symptoms
such as wrinkles, dryness, darkening, pigmentation, decreased
dermal thickness and a loss of elasticity (2,3). Due to
the gradual aging of various populations worldwide, and the advance
of science associated with aging, a variety of anti-aging
procedures have been developed, such as photoprotection, cosmetics,
cosmeceuticals and antioxidants for prevention and treatment of
skin aging (4). The business of
anti-aging cosmetics, including cosmeceuticals, is growing rapidly
in the skin care market (5), and may
potentially benefit the care of wrinkles, elasticity, dermal
density and skin tone. However, although clinical experience
suggests an important role for topical anti-aging formulations,
such as eye cream and anti-wrinkle cream, further empirical studies
are required to investigate the underlying mechanisms and confirm
their effects.
Palmitoyl peptides, Silybum marianum (S.
marianum) seed oil and vitamin E are generally used
antioxidants for protecting and restoring the skin from damage that
may result in wrinkles and low elasticity (6–8), in
anti-aging cosmetics such as alpha and beta hydroxyl acids
(9) and kinetin (10). Palmitoyl peptides are the agents by
the action of which matrikines are transformed in palmitoyl
derivatives to increase transepidermal penetration (11,12).
Although matrikines are short chains of amino acids contributing to
tissues repair activity by facilitating collagen synthesis
(13), these are hydrophilic and
very weak to be absorbed across epidermis (11,12).
However, palmitoyl peptides, the modified formation of these small
peptides, have potential anti-aging functions (14). S. marianum seed oil contains
silymarin flavonoids including silibinin, silidianin and
silichrystin, which may exert antioxidative activity (15), and show potential anti-aging effects
as a cosmetic cream formulation by decreasing transepidermal water
loss and surface wrinkles (16).
Vitamin E has been demonstrated to be an antioxidant in numerous
studies (17–19). Additionally, cosmetic application of
vitamin E protects skin from ultraviolet (UV) damage, which may
exacerbate wrinkles, loss of elasticity and dehydration (20–22).
Creams, lotions or emulsions generally serve as vehicles for the
penetration and cutaneous absorption of vitamin E (21).
Although various formulations of anti-aging
cosmetics containing functional components are used and developed
extensively for relieving skin aging such as wrinkles, low
elasticity, low dermal density and photo damage (23), further clinical studies are required
to validate their effects on aged skin. The evaluation of
anti-aging products is considered appropriate to prove the effects
of the substances that improve skin wrinkles, elasticity, dermal
density and skin tone.
The aim of the present study was to evaluate the
anti-aging effects of cosmetic formulations, eye cream and facial
cream, containing palmitoyl peptides, S. marianum seed oil,
vitamin E and other functional ingredients on aged human skin after
4 weeks period of application, using skin bioengineering
techniques. The effects of the substances on wrinkles, elasticity,
dermal density and skin tone were determined.
Materials and methods
Subjects
This study complied with the principles of the
Declaration of Helsinki and Korean and was reviewed and approved by
the Institutional Review Board of Korea Institute for Skin and
Clinical Sciences (Seoul, Republic of Korea).
A total of 20 female volunteers (age, 30–65) were
selected on the basis of predetermined inclusion and exclusion
criteria. Inclusion criteria were as follows: Volunteers were
female and >30 years old; subjects voluntarily signed the
informed consent form; subjects were healthy without acute or
chronic physical diseases, including any skin diseases; and
subjects were available for follow-up during the testing period. A
person with any of the following factors was excluded from the
study: Pregnant, breast feeding or potentially pregnant; person who
had been treated with any external application containing steroids
for a skin disease treatment for >1 month; had participated in
the a similar test within the last 6 months; person with sensitive
or hypersensitive skin; person with skin abnormality on the test
site, including moles, acne, erythema, and dilated capillaries;
person who received any treatment on the test area within the last
6 months. Participants were withdrawn for the following reasons and
these were reported: Adverse events, such as itching or erythema at
the test area; hindrance of the evaluation due to a medical
treatment, application of another product, excessive sun exposure,
or excessive drinking or smoking during the test period; inability
to participate in a follow-up appointment during the test period
due to personal reasons; and person who did not comply with the
study directions without specific reason.
Adverse events, including erythema, edema, scaling,
itching, stinging, burning, tightness, prickling and other
abnormalities, were visually examined and described on the case
report form at every visit. The records included the degree of
symptoms and whether these were mild, moderate or severe. Each
subject's attendance was also recorded. Whether the participant was
excluded from the study due to withdrawal was also noted. If a
subject was unable to continue in the study, she signed an
abandonment consent form.
Preparation and application of test
materials
The facial cream and eye cream were freshly prepared
for this study. The facial cream contains 1% palmitoyl oligopeptide
and palmitoyl tetrapeptide-7 (BulkActives, Keelung City, Taiwan),
1% S. marianum seed oil (Botanic Innovations LLC, Spooner,
WI, USA), 1% vitamin E (BulkActives), 1% xylitylglucoside, xylitol,
and anhydroxylitol (Seppic S.A., Puteaux, France), 1% Rosmarinus
officinalis leaf extracts (Flavex Naturextrakte GmbH,
Rehlingen-Siersburg, Germany), 3% jojoba oil (Biocosmethic,
Bonnelles, France), 3% avocado oil (Biocosmethic) and 1% squalane
(BulkActives). The eye cream contains 1% palmitoyl oligopeptide and
palmitoyl tetrapeptide-7 (BulkActives), 1% hesperidin methyl
chalcon, dipeptide-2 (Sederma, Le Perray-en-Yvelines, France), 1%
S. marianum seed oil (Botanic Innovations LLC), 1%
Hordeum vulgare extracts (Presperse Corporation, Somerset,
NJ, USA), 1% sodium hyaluronate (Jinwoo Bio, Inc., Seoul, Republic
of Korea), 1% glycosphingolipid (Wha Costech Inc., Gyeonggi-Do,
Seoul, Republic of Korea), 1% vitamin E (BulkActives), 3% jojoba
oil (Biocosmethic), 1% jojoba esters (International Flora
Technologies, Ltd., Chandler, AZ, USA), 1% squalane (BulkActives)
and 1% acacia wax (Hangzhou Reb Technology Co., Ltd., Hangzhou,
China).
Following facial washing, subjects applied the eye
cream around eyes and face cream on the facial area twice per day,
morning and night. Except for the test materials supplied by the
institution, subjects were prohibited from using other products
that may affect the results during the trial period. These other
products included eye cream, functional cosmetics against aging and
treatments such as masks or massages.
Evaluation of wrinkle improvement
All clinical analyses were conducted after cleansing
face with same cleanser (Cleansing Foam; Anna Holtz Skin Care,
Incheon, Republic of Korea) and resting in a controlled temperature
and humidity room (temperature, 22±1°C; humidity, 45±5%) for 30
min. All measurements were conducted prior to any test material
application and subsequently after 2 and 4 weeks of
application.
For evaluation of wrinkle improvement, a PRIMOS Lite
3D Face and Skin Scanner Analyzing System (GFMesstechnik GmbH,
Berlin, Germany) was utilized. The outer corner of the right eye
was measured three consecutive times with the PRIMOS Lite after
placing subjects' face onto a special PRIMOS face-held-equipment
(GFMesstechnik GmbH) and focusing the outer corner of eye on a same
pattern of the PRIMOS Lite to prevent the test area from moving.
The images adjusted to the same position each time by applying 3D
matching and were analyzed with the PRIMOS Lite software (version
5.6E; GFMesstechnik GmbH). The measurement variable Ra (average of
all heights and depths to the reference plane) was used for wrinkle
analysis as the most common surface roughness index worldwide,
which represents the maximal mathematical average of the profile
within the entire measurement range. The Ra value decreases with a
lower depth of wrinkles, indicating that skin wrinkles were
improved.
Evaluation of skin elasticity
improvement
For evaluation of skin elasticity improvement, the
DermaLab USB elasticity probe (Cortex Technology ApS, Hadsund,
Denmark) was applied. After attaching the probe to the skin with
tape, the left cheek under the eye was measured only once for
prevention of skin fatigue caused by repeated measurement. The
DermaLab USB elasticity probe quantifies skin changes and restoring
forces in accordance with inhalation of skin and the duration of
the inhalation, and the results were analyzed using DermaLab USB
analysis software, version 1.09 (Cortex Technology ApS). Young's
modulus (E) was used for elasticity analysis, which is the value
representing the difference in forces to raise surface skin as much
as 1.5 mm, the distance between two infrared sensing wires within
the probe. Its unit of measure is the mega pascal (MPa). Young's
modulus (E) increases with a higher elasticity, indicating that
skin elasticity was improved.
Evaluation of dermal density
improvement
For evaluation of dermal density improvement, an
ultrasonographic DUB® SkinScanner (Tpm Taberna Pro
Medicum GmbH, Lüneberg, Germany) was applied. After applying
ultrasonography gel, 3 cm from the outer corner of left eye was
measured by using the probe at a right angle with skin and pressing
skin with same pressure. The range of analysis was set in limits
from epidermis to upper subcutaneous fat layer. The value increases
with a higher density of dermis, indicating that dermal density was
improved.
Evaluation of skin tone
improvement
For evaluation of skin tone improvement, a CR-2600D
spectrophotometer (Konica Minolta, Inc., Tokyo, Japan) was used.
The right cheek was measured three consecutive times, and the
average value was determined. The L* and a* of three measurement
values were determined as a measure of skin tone. L* indicates
brightness, a* indicates red and b* indicates yellow.
Evaluation of abnormal skin
response
During the trial period, medical doctors (Dr Kyu
Joong Ahn and Dr Hyung Jin Hahn) determined using a visual
inspection whether subjects had any visual dermatological
side-effects (including erythema, edema and scaling) or not. We
indicated the degree of symptoms and reported the results. A survey
was conducted to ask subjects about abnormal skin responses.
Statistical analysis
The data were analyzed using paired t-tests with
SPSS 17.0 software for Windows (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
result.
Results and Discussion
General characteristics and abnormal
skin responses of subjects
No subjects discontinued their participation due to
lack of effectiveness or adverse events. The average age of the 20
female subjects was 45.60±8.18 years (data not shown). On average,
the subjects used 80–98% of the quantity of test materials expected
(data not shown).
There were no abnormal responses, including allergic
contact dermatitis or irritant contact dermatitis, following
application of the test material on the subjects, and the survey
answered by subjects resulted in no special abnormal skin response
for this trial period (data not shown).
Evaluation of wrinkle improvement
The results of the evaluation of the facial wrinkles
using the wrinkle value (Ra) obtained using the PRIMOS Lite are
shown in Table I and Fig. 1.
 | Table I.Changes in skin wrinkle values (Ra)
after applying test materials. |
Table I.
Changes in skin wrinkle values (Ra)
after applying test materials.
| Application
period | Wrinkle values | P-value |
|---|
| Pre-application |
42.58±12.09 |
|
| 2 weeks |
40.04±11.32 | 0.043a |
| 4 weeks | 36.59±8.15 | 0.001b |
The evaluation results of the wrinkle value (Ra)
showed a 5.97% decrease after 2 weeks of the test material
application and a 14.07% reduction after 4 weeks of application in
comparison of pre-application. The results were statistically
significant (P<0.05).
The representative 3D images before application and
after 4 weeks of the test material application were shown in
Fig. 2. The outer corner of the
right eye was measured three consecutive times with the PRIMOS
Lite. Collectively, these suggest that the facial cream and eye
cream tested in this study contribute to the improvement of skin
wrinkles.
Evaluation of skin elasticity
improvement
Next, we have determined the skin elasticity
improvements with the DermaLab USB elasticity probe (Table II and Fig. 3).
 | Table II.Changes in skin elasticity after
applying test materials. |
Table II.
Changes in skin elasticity after
applying test materials.
| Application
period | Skin elasticity
(MPa) | P-value |
|---|
| Pre-application | 12.85±2.55 |
|
| 2 weeks | 13.73±2.36 | 0.045a |
| 4 weeks | 13.98±1.67 | 0.013a |
The evaluation results of skin elasticity showed
6.85 and 8.79% increase after 2 and 4 weeks of test material
application, respectively, in comparison with pre-application skin.
The results were statistically significant (P<0.05) and suggest
that the facial cream and eye cream contribute to improve skin
elasticity.
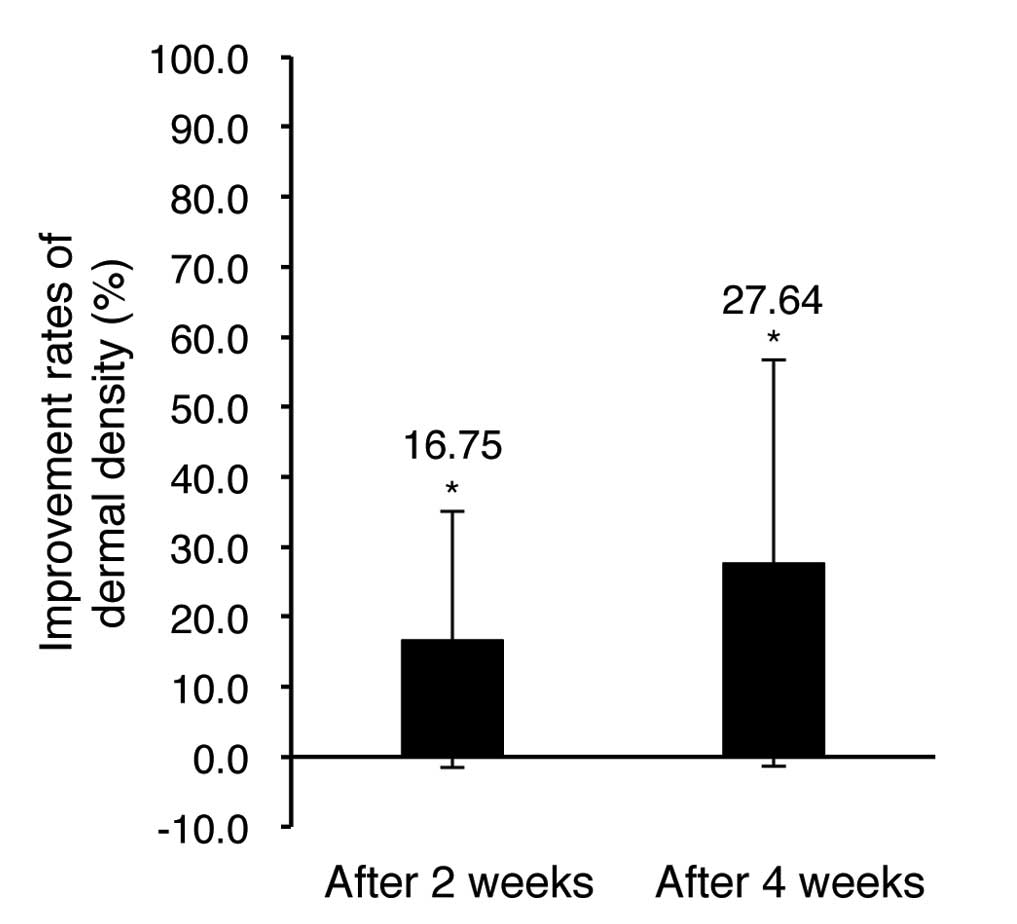
Evaluation of dermal density
improvement
The dermal density improvements were evaluated using
the DUB® SkinScanner (Table
III and Fig. 4).
 | Table III.Changes in dermal density after
applying test materials. |
Table III.
Changes in dermal density after
applying test materials.
| Application
period | Dermal density | P-value |
|---|
| Pre-application | 42.87±7.35 |
|
| 2 weeks | 50.05±9.81 | 0.001a |
| 4 weeks |
54.72±14.67 | 0.000b |
The dermal density evaluation results showed 16.75
and 27.64% increase after 2 and 4 weeks of test material
application, respectively, in comparison of pre-application. The
results were statistically significant (P<0.05) and indicate
that the facial cream and eye cream tested here improves dermal
density.
Evaluation of skin tone
improvement
The evaluation results of skin tone (L* and a*
values) improvement obtained with the spectrometer are shown in
Tables IV and V and Figs. 5
and 6.
 | Table IV.Changes in L* value after applying
test materials. |
Table IV.
Changes in L* value after applying
test materials.
| Application
period | L* value | P-value |
|---|
| Pre-application | 64.34±2.97 |
|
| 2 weeks | 65.44±2.27 | 0.007a |
| 4 weeks | 65.72±2.71 | 0.001a |
 | Table V.Changes in a* value after applying
test materials. |
Table V.
Changes in a* value after applying
test materials.
| Application
period | a* value | P-value |
|---|
| Pre-application | 10.61±2.11 |
|
| 2 weeks |
9.50±1.76 | 0.001a |
| 4 weeks |
8.23±2.16 | 0.000b |
As shown in Table IV
and Fig. 5, the evaluation results
of the L* value indicating skin brightness showed a 1.70% increase
after 2 weeks of the test material application and a 2.14% increase
after 4 weeks of application in comparison of pre-application. The
results were statistically significant (P<0.05). As shown in
Table V and Fig. 6, the evaluation results of a* value
indicating erythema (redness) showed 10.46 and 22.43% reduction
after 2 and 4 weeks of application, respectively, in comparison
with pre-application. The results were statistically significant
(P<0.05). These suggest that the facial cream and eye cream
examined here help improve skin tone.
Cosmetic formulations containing palmitoyl peptides,
S. marianum seed oil, vitamin E and other functional
ingredients showed anti-aging effect in the improvement of facial
wrinkles, elasticity, dermal density and skin tone on human aged
skin. Following a 4-week period of application, skin wrinkles were
decreased by 14.07% and elasticity was increased by 8.79% in
comparison with pre-application. Dermal density showed a 27.63%
increase and skin tone indicated a 2.14% increase in L* value
indicating skin brightness and a 22.39% reduction in the a* value
indicating erythema (redness) after 4 weeks of application.
Thus, formulations containing palmitoyl peptides,
S. marianum seed oil, vitamin E and other functional
ingredients may have some anti-aging effects on aging skin over a
4-week application period.
Acknowledgements
This study was supported by a grant of the Korean
Health Technology R&D Project (grant no. HN13C0075),
administered by the Ministry of Health & Welfare of the
Republic of Korea.
References
|
1
|
Uitto J and Bernstein EF: Molecular
mechanisms of cutaneous aging: Connective tissue alterations in the
dermis. J Investig Dermatol Symp Proc. 3:41–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Rigal J, Escoffier C, Querleux B,
Faivre B, Agache P and Lévêque JL: Assessment of aging of the human
skin by in vivo ultrasonic imaging. J Invest Dermatol. 93:621–625.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Domyati M, Attia S, Saleh F, Brown D,
Birk DE, Gasparro F, Ahmad H and Uitto J: Intrinsic aging vs.
photoaging: A comparative histopathological, immunohistochemical,
and ultrastructural study of skin. Exp Dermatol. 11:398–405. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCullough JL and Kelly KM: Prevention and
treatment of skin aging. Ann N Y Acad Sci. 1067:323–331. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Farris PK: A review of the science behind
the claims. Cosmetic Dermatol. 16:59–70. 2003.
|
|
6
|
Chirita RI, Chaimbault P, Archambault JC,
Robert I and Elfakir C: Development of a LC-MS/MS method to monitor
palmitoyl peptides content in anti-wrinkle cosmetics. Anal Chim
Acta. 641:95–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feher P, Vecsernyes M, Fenyvesi F, Varadi
J, Kiss T, Ujhelyi Z, Nagy K and Bacskay I: Topical application of
Silybum marianum extract. Arad Med J. 14:5–8. 2011.
|
|
8
|
Nachbar F and Korting HC: The role of
vitamin E in normal and damaged skin. J Mol Med (Berl). 73:7–17.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stiller MJ, Bartolone J, Stern R, Smith S,
Kollias N, Gillies R and Drake LA: Topical 8% glycolic acid and 8%
L-lactic acid creams for the treatment of photodamaged skin. A
double-blind vehicle-controlled clinical trial. Arch Dermatol.
132:631–636. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rattan SI and Clark BF: Kinetin delays the
onset of ageing characteristics in human fibroblasts. Biochem
Biophys Res Commun. 201:665–672. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lintner K: Promoting ECM production
without compromising barrier. Ann Dermatol Venereol.
129:1S1052002.
|
|
12
|
Mas-Chamberlin C, Lintner K, Basset L,
Adhoute H and Revuz J: Relevance of antiwrinkle treatment of a
peptide: 4 months clinical double blind study vs excipient. Ann
Dermatol Venereol. 129:1S4562002.
|
|
13
|
Maquart FX, Siméon A, Pasco S and
Monboisse JC: Regulation of cell activity by the extracellular
matrix: The concept of matrikines. J Soc Biol. 193:423–428.
1999.(In French). PubMed/NCBI
|
|
14
|
Robinson LR, Fitzgerald NC, Doughty DG,
Dawes NC, Berge CA and Bissett DL: Topical palmitoyl pentapeptide
provides improvement in photoaged human facial skin. Int J Cosmet
Sci. 27:155–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kshirsagar A, Ingawale D, Ashok P and
Vyawahare N: Silymarin: A Comprehensive Review. Phcog Rev.
3:126–134. 2009.
|
|
16
|
Rasul A and Akhtar N: Anti-aging potential
of a cream containing milk thistle extract: Formulation and in vivo
evaluation. African Journal of Biotechnology. 11:1509–1515.
2014.
|
|
17
|
Packer L and Valacchi G: Antioxidants and
the response of skin to oxidative stress: Vitamin E as a key
indicator. Skin Pharmacol Appl Skin Physiol. 15:282–290. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Black HS, Lenger WA, Gerguis J and Thornby
JL: Relation of antioxidants and level of dietary lipid to
epidermal lipid peroxidation and ultraviolet carcinogenesis. Cancer
Res. 45:6254–6259. 1985.PubMed/NCBI
|
|
19
|
Chow CK: Increased activity of pyruvate
kinase in plasma of vitamin E-deficient rats. J Nutr.
105:1221–1224. 1975.PubMed/NCBI
|
|
20
|
Djerassi D, Machlin KJ and Nocka C:
Vitamin E: biochemical function and its role in cosmetics. Drug
Cosmet. 1:29–34. 1986.
|
|
21
|
Furuse K: Vitamin E: Biological and
clinical aspects of topical treatment. Cosmetics Toiletries.
102:99–116. 1987.
|
|
22
|
Horio T and Okamoto H: Oxygen
intermediates are involved in ultraviolet radiation induced damage
of Langerhans cells. J Invest Dermatol. 88:699–702. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Binic I, Lazarevic V, Ljubenovic M, Mojsa
J and Sokolovic D: Skin aging: Natural weapons and strategies. Evid
Based Complement Alternat Med. 2013:8272482013. View Article : Google Scholar : PubMed/NCBI
|