Introduction
Eclampsia refers to the occurrence of generalized
convulsions in a woman during puerperium without an alternative
identifiable cause. Approximately 50% of all cases of eclampsia
occur after delivery, which is known as postpartum eclampsia
(1). Late onset postpartum eclampsia
(LPE) occurs >48 h after childbirth (2). Complications in eclampsia include acute
live, renal failure and respiratory complications. Mortality in
eclampsia is predominantly a result of intracerebral hemorrhage
(2). Mortality rates could be
lowered with antihypertensive therapy and anticonvulsants in
patients with seizures (2).
Reversible posterior encephalopathy syndrome (RPES), also termed
reversible posterior leukoencephalopathy, refers to a clinical and
radiological entity in which patients often present with headaches,
seizures and altered mental status, with reversible edematous
changes in the cortex, subcortical region and deep white matter
observed upon brain radioimaging (3). Eclampsia is one of the underlying
clinical conditions predisposing patients to RPES (4). The majority of RPES cases with late
postpartum onset present within 4 weeks after the delivery
(5,6). The present study reports a rare case of
LPE-associated RPES, which occurred at 5 weeks after delivery in a
15-year-old female patient.
Case report
A 15-year-old female patient complained of headache
and transient visual loss ~5 weeks after preterm delivery at 32
weeks of pregnancy. The patient had a fainting episode prior to
admission to Qilu Hosptal of Shandong University (Jinan, China) in
March 2012, with upward gaze deviation and tongue biting reported.
The patient had experienced hypertension during the pregnancy
without any headaches or convulsions, and presented mild leg edema,
paroxysmal abdominal pain and nausea during the pregnancy and post
partum. No previous major systemic disease and medicine use were
reported. The patient was admitted to the emergency room, where she
experienced two further generalized tonic-clonic seizures and
became somnolent. Intravenous diazepine (10 mg) was administered
immediately. The patient had a high blood pressure (200/110 mmHg),
which was successfully controlled by urapidil administration (100
mg per day), and presented a temperature of 38.3°C.
A neurological examination indicated a somnolent
state without any focal neurological signs. Laboratory blood tests
revealed leukocytosis (white blood cell count, 16,000
cells/mm3; normal range, 3,500–9,500
cells/mm3) with left-shifting. A head magnetic resonance
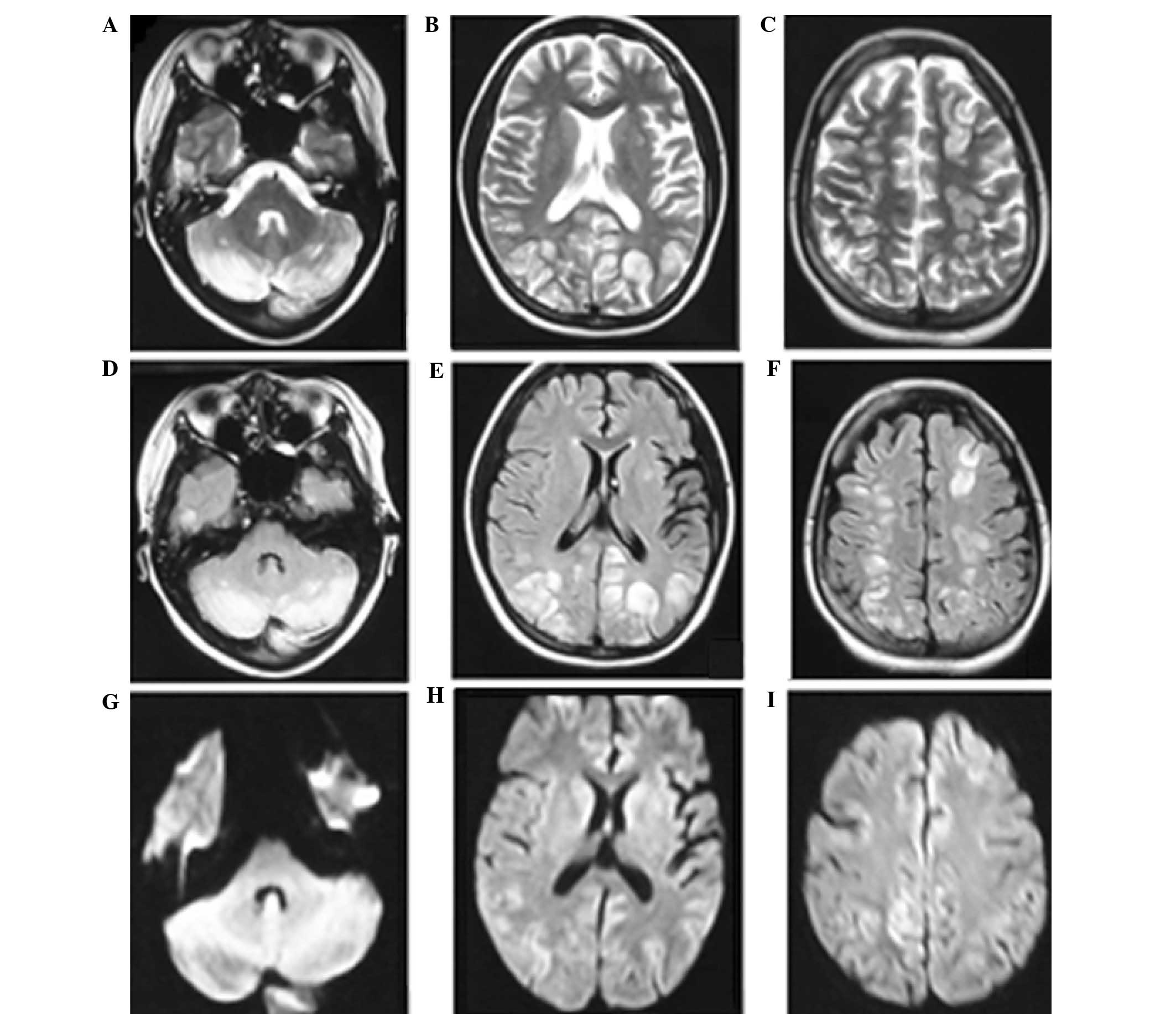
imaging (MRI) scan was performed, which indicated multiple foci of
high signal intensity on T2-weighted images and fluid-attenuated
inversion recovery (FLAIR) sequences in the cortical and
subcortical white matter bilaterally, predominantly in the
occipital, frontal and parietal lobes and cerebellum (Fig. 1). In addition, diffuse weighted
imaging (DWI) revealed no evident abnormalities (Fig. 1). Following admission, the results of
cardiovascular, respiratory, abdominal, neurological and
ophthalmologic examinations were normal. Laboratory tests were also
normal, including urinalysis, serum electrolytes, liver function,
glucose and lactic acid levels, coagulation profiles, serological
assessment (including the venereal disease research laboratory
test, and tests for thyroid hormones) and an autoimmune profile
(which involved assessments for antinuclear antibodies, double
strand DNA, antineutrophil cytoplasmic antibodies and
antiphospholipid antibodies). A lumbar puncture was also performed,
and the cerebrospinal fluid opening pressure, protein, glucose,
immunoglobulin and the number of cells were found to be normal.
On day 4 after hospital admission, the patient
received further head MRI and magnetic resonance
arteriography/magnetic resonance venography (MRA/MRV) scans. The
hyperintensity zones on T2-weighted images of the subcortex and
white matter in the cerebellum, and occipital, frontal and parietal
lobes were markedly decreased. The MRA scan revealed cerebral
vascular focal vasodilation and vasoconstriction features with a
string-of-beads appearance (Fig. 2),
predominantly in the vessels of the posterior circulation of the
brain. In addition, there was no intracranial sinus thrombosis;
however, the MRV indicated venous sinus dilation (Fig. 2). Based on the results of tests, the
diagnosis of reversible posterior encephalopathy induced by LPE was
suspected. The patient was administered cefoperazone (4 mg per
day), nimodipine (10–20 mg per day) and mannitol (250–500 ml per
day). The temperature and blood pressure were normalized following
treatment, and the patient experienced no further seizures during
the remaining of the hospital stay. On day 11 after admission, the
patient received further head MRI examination that indicated an
almost complete resolution of the previous abnormalities (Fig. 3). Subsequently, the patient was
discharged without any neurological sequela. The follow-up
neurological examination after 1 month showed no abnormalities.
During the two months follow-up examinations, no neurological
symptoms were found.
Discussion
The diagnosis of RPES associated with LPE in the
patient of the current study was based on the reversible clinical
course, the increasing of blood pressure, the detection of
vasogenic edema on a head MRI scan and the recent medical history
of childbirth. The differential diagnosis for frequent convulsions
in the puerperal period includes ischemic stroke, encephalitis,
meningitis, mitochondrial encephalopathy, Hashimoto's
encephalopathy, electrolyte or endocrine disturbances, vasculitis,
inflammatory demyelinating diseases and neoplastic diseases
(7). Laboratory assessments,
cerebrospinal fluid examination, reversal of the head MRI features
following treatment and good outcome without the use of
immunosuppressive treatment excluded the aforementioned
differential diagnoses. In addition, the patient only experienced a
mild headache during the puerperal period, which excluded the
possibility of reversible cerebral vasoconstriction syndrome, which
is characterized by severe or thunderclap headaches and reversible
constriction of cerebral arteries. Thus, the diagnosis of
LPE-associated RPES was established in the current patient.
LPE is distinguished from early onset postpartum
eclampsia by an onset that occurs >48 h after childbirth
(2). LPE may occur without any
pre-eclamptic prodromes. The delayed onset and the atypical
clinical presentation of LPE may lead to misdiagnosis. The patient
reported in the present study did not demonstrate typical
pre-eclamptic prodromes, although she experienced hypertension and
limb edema during her pregnancy, and presented with convulsions 5
weeks after the delivery. To the best of our knowledge, only one
patient reported in the literature experienced LPE with onset later
than 4 weeks postpartum (8).
LPE may manifest as RPES in which the most
frequently affected areas are the subcortical white matter in the
cerebral posterior circulation region (9). The less frequently affected areas
include the frontal lobe, temporal lobe and basal ganglia. The
characteristic radioimaging findings of RPES are reversible white
matter hyperintensities on FLAIR combined with normal DWI scans,
which indicate vasogenic edema (3,10). The
edema reported in RPES cases is typically completely reversible. In
certain cases, angiography may reveal a rosary bead-like
appearance, representing diffuse cerebral vasodilation and
vasoconstriction (3). The patient
reported in the present study presented diffuse symmetric vasogenic
edema in multiple regions, particularly the watershed zones of the
parietal, occipital and frontal lobes. In addition, MRA suggested
features of vasoconstriction or string-of-beads appearance, which
are reflective of brain hypoperfusion (3,10).
Notably, cerebral venous vasodilation was also observed in the
current patient, which has seldom been reported in the literature
(6,8). This abnormal venous vasodilation in
addition to the arterial vasoconstriction were completely reversed
following the aforementioned treatments, resulting in the patient's
recovery.
The mechanism of RPES induced by eclampsia has yet
to be fully elucidated. Increased cerebral vascular resistance and
vasospasm may be observed in patients with preeclampsia or
eclampsia (11). Thus, the generally
accepted hypertension or hyperperfusion hypotheses regarding the
mechanism of RPES development suggest that hypertension exceeds the
autoregulation limits of the brain, which leads to cerebral
autoregulatory vasoconstriction and subsequent brain edema
(12). However, watershed
hypoperfusion and reduced brain perfusion in the posterior brain
region have also been proposed by previous studies (13,14). At
present, the intrinsic mechanism of RPES is considered to be an
evolving systemic process with hypoperfusion/vasoconstriction and
the development of brain toxicity, involving immune system
activation, endothelial cell injury and inflammatory cytokine
responses (15,16). The aforementioned toxicity-associated
hypoperfusion/vasoconstriction leads to capillary bed injury and
vasogenic edema. In addition, hypertension in combination with
eclampsia further reduces brain perfusion and induces ischemia.
Thus, reducing the blood pressure of the patient can reduce
vasoconstriction, improve perfusion, reverse watershed edema and
subsequently improve clinical manifestations.
In conclusion, RPES associated with LPE is rare and
may be easily misdiagnosed. The present case indicates the
importance of considering the possibility of LPE without typical
preeclampsia symptoms even several weeks after delivery. In
addition, clinicians should be familiar with the artery
constriction features of RPES on head radioimaging scans. Early
diagnosis, adequate treatment and support are important for the
patient's outcome.
References
|
1
|
Douglas KA and Redman CW: Eclampsia in the
United Kingdom. BMJ. 309:1395–1400. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lubarsky SL, Barton JR, Friedman SA,
Nasreddine S, Ramadan MK and Sibai BM: Late postpartum eclampsia
revisited. Obstet Gynecol. 83:502–505. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartynski WS: Posterior reversible
encephalopathy syndrome, part 1: Fundamental imaging and clinical
features. AJNR Am J Neuroradiol. 29:1036–1042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Postma IR, Slager S, Kremer HP, de Groot
JC and Zeeman GG: Long-term consequences of the posterior
reversible encephalopathy syndrome in eclampsia and preeclampsia: A
review of the obstetric and nonobstetric literature. Obstet Gynecol
Surv. 69:287–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castrillo-Sanz A, Mendoza A,
Gutiérrez-Ríos R, Zamora MI, Morollón N, Rodríguez-Sanz MF and
Duarte J: Posterior reversible encephalopathy in a case of
late-onset eclampsia. Rev Neurol. 57:112–116. 2013.(In Spanish).
PubMed/NCBI
|
|
6
|
Pezzi M, Le Piane E, Giglio AM, Pagnotta
L, Scozzafava A, Tortorella V, Sergi A and Verre M: Posterior
reversible encephalopathy syndrome in late postpartum eclampsia.
Clin Ter. 166:68–71. 2015.PubMed/NCBI
|
|
7
|
Sibai BM: Diagnosis, prevention, and
management of eclampsia. Obstet Gynecol. 105:402–410. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Minnerup J, Kleffner I, Wersching H,
Zimmermann J, Schäbitz WR, Niederstadt T and Dziewas R: Late onset
postpartum eclampsia: It is really never too late-a case of
eclampsia 8 weeks after delivery. Stroke Res Treat. 2010:pii.
798616. 2010.PubMed/NCBI
|
|
9
|
Pizon AF and Wolfson AB: Postpartum focal
neurologic deficits: Posterior leukoencephalopathy syndrome. J
Emerg Med. 29:163–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koch S, Rabinstein A, Falcone S and
Forteza A: Diffusion-weighted imaging shows cytotoxic and vasogenic
edema in eclampsia. AJNR Am J Neuroradiol. 22:1068–1070.
2001.PubMed/NCBI
|
|
11
|
Qureshi AI, Frankel MR, Ottenlips JR and
Stern BJ: Cerebral hemodynamics in preeclampsia and eclampsia. Arch
Neurol. 53:1226–1231. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strandgaard S, Olesen J, Skinhoj E and
Lassen NA: Autoregulation of brain circulation in severe arterial
hypertension. Br Med J. 1:507–510. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartynski WS and Boardman JF: Catheter
angiography, MR angiography and MR perfusion in posterior
reversible encephalopathy syndrome. AJNR Am J Neuroradiol.
29:447–455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brubaker LM, Smith JK, Lee YZ, Lin W and
Castillo M: Hemodynamic and permeability changes in posterior
reversible encephalopathy syndrome measured by dynamic
susceptibility perfusion-weighted MR imaging. AJNR Am J
Neuroradiol. 26:825–830. 2005.PubMed/NCBI
|
|
15
|
Bartynski WS: Posterior reversible
encephalopathy syndrome, part 2: Controversies surrounding
pathophysiology of vasogenic edema. AJNR Am J Neuroradiol.
29:1043–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kofler J, Bartynski WS, Reynolds TQ,
Lieberman FS, Murdoch GH and Hamilton RL: Posterior reversible
encephalopathy syndrome (PRES) with immune system activation, VEGF
up-regulation and cerebral amyloid angiopathy. J Comput Assist
Tomogr. 35:39–42. 2011. View Article : Google Scholar : PubMed/NCBI
|