Introduction
Ulcerative colitis (UC) is a chronic inflammatory
bowel disease affecting the mucosa and submucosa of the rectum,
which may extend to the entire colon, and is characterized by a
relapsing and remitting course (1).
The symptoms of UC commonly include bloody diarrhea, rectal
bleeding or rectal urgency. The severity of these symptoms is
typically related to the extent of mucosal inflammation and can be
observed by colonoscopy (2,3). The induction of remission and mucosal
healing are the goals of therapy. Aminosalicylates, azathioprine,
6-mercaptopurine and infliximab may be used for maintenance,
however, the above treatments do not adequately work in all
patients or are not well tolerated (4,5). There
remains an urgent need for novel therapeutic options to cure
UC.
Mesenchymal stromal cells (MSCs) can be easily
isolated and amplified from umbilical cord and other tissues. The
characteristics of MSCs result in extensive use in numerous
diseases, including tissue degeneration and refractory chronic
inflammatory diseases (6). In UC,
the inflammatory response is mediated by cytokines similar to the
Th2 response and different immunocytes (7). The involvement of T-cells, natural
killer (NK) cells and dendritic cells in UC pathophysiology has
been confirmed in a large recent genome-wide association study
(8). Recent data have indicated that
MSCs can restore tissues by their immunomodulatory function,
differentiation and paracrine effects. MSCs can modulate allogeneic
immune cell responses by affecting dendritic cells, T-lymphocytes
and NK cells (9). In addition, MSCs
appear to regulate the immune reaction in inflamed tissues by
affecting the formation and secretion of pro-inflammatory cytokines
and chemokines, such as prostaglandin E2 and interleukin (IL)-6
(10). MSCs may induce a cytokine
profile shift in the T helper (Th) 1/Th2 balance toward the
anti-inflammatory phenotype Th2, which is accompanied by an
increase of T regulatory lymphocytes and IL-10 (11,12).
Regarding the treatment of UC, it can be suggested
that MSCs are effective by their immune modulatory properties
coupled with a tissue repair capacity. Based on this consideration
and current findings, a clinical trial (trial registration no.
NCT01221428) was performed to investigate the safety and
therapeutic effect of MSCs derived from human umbilical cord on
moderate to severe UC.
Materials and methods
Study design
The present trial was a phase I/II, 24 month,
randomized controlled study conducted in patients with moderate to
severe UC. The study was conducted in accordance with the
Declaration of Helsinki and approved by the Ethical Committee of
the Affiliated Hospital of Qingdao University (Qingdao, China).
Each patient provided written informed consent prior to entering
the study. During the whole study, the investigators remained blind
to the treatment administered.
Patients
Between September 2011 and December 2012, all
patients were selected from The Affiliated Hospital of Qingdao
University for the treatment of UC. UC was diagnosed according to
current established clinical, endoscopic, radiologic and histologic
guidelines (13). The extent of UC
was evaluated based on the Mayo score classification (14,15).
Eligibility criteria for study entry included patients ≥18 years of
age, diagnosed with active UC with a Mayo score at baseline (range
between 8 and 10). Exclusion criteria included infectious
complications, toxic megacolon, previous colorectal surgery,
history of colorectal dysplasia or any malignancy, pregnancy and
any psychological illness.
Treatment
All patients were on stable doses of
aminosalicylates for ≥4 weeks prior to enrollment and all patients
continued their individual regimens throughout the trial. Patients
in group I were treated with MSC infusions twice besides the base
treatment, one via injection into the veins in the back of the
hand, and the other injected into the superior mesenteric artery by
interventional catheterization, with a 7 day interval. Patients in
group II were treated the same but with normal saline, which
had the same volume and appearance as the MSC solution. The volumes
of the parenteral solution of MSCs and normal saline for
intravenous injection in the two groups were 50 ml and the average
cell number was 3.8±1.6×107 (0.5×106
cells/kg; range, 2.3–4.7×107 cells), and the volumes of
the parenteral solution of MSCs and normal saline for mesenteric
artery injection by interventional catheterization in the two
groups were 10 ml and the cell number was 1.5×107. To
undergo MSC infusion, all patients were admitted to The Affiliated
Hospital of Qingdao University. During the follow-up, the dosage of
aminosalicylates administered were adjusted according to the
patients condition.
MSCs were provided by the Human
Umbilical Cord Mesenchymal Stem Cell Bank (Qingdao, China)
MSCs were cultured and expanded in a laminar flow
laboratory (designed according to Good Medical Practice) for four
passages to prepare stable final cell products that qualify for
aerobe, mycoplasma, hepatitis B virus, hepatitis C virus, human
immunodeficiency virus, Epstein-Barr virus, cytomegalovirus,
syphilis and endotoxin testing. Cells were stained with CD-PE and
CD-FITC (from the Human MSC Analysis kit; cat. no. 562245; BD
Biosciences, Franklin Lakes, NJ, USA) and then analyzed by flow
cytometry with a FACSCalibur flow cytometer (BD Biosciences). These
cells highly expressed cluster of differentiation (CD) 90 (89.37%),
CD105 (82.26%), CD73 (90.63%), and CD146 (65%) but not CD34
(0.23%), CD45 (0.02%) and Human Leukocyte Antigen-D Related (0.03%)
(Human MSC Analysis kit). The chromosomal karyotype of UC-MSC was
determined as normal using a Metascan Karyotyping System (Imstar,
Paris, France).
Clinical assessment and follow-up
The final evaluation prior to the first infusion of
MSC was used as baseline for all analyses. Laboratory assessments
of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR),
blood counts, liver enzymes, total protein, albumin, total
cholesterol, triacylglycerol, serum urea nitrogen, creatinine and
electrolytes were performed in the Clinical Laboratory of The
Affiliated Hospital of Qingdao University. All samples were handled
by the Clinical Laboratory of The Affiliated Hospital of Qingdao
University. Full Mayo scores and Inflammatory Bowel Disease
Questionnaire (IBDQ) scores (16,17) were
determined every 3 months in the first year after therapy, and then
every 6 months during the second year of follow-up. The Mayo
subscores for stool frequency and rectal bleeding were calculated
based on entries from patient diaries using the worst diary entry
from the 3 days before each study visit for each subscore. In all
patients who had undergone follow-up endoscopy, it was assessed
whether mucosal healing was achieved or not. Clinical response was
defined as a decrease from baseline in the total Mayo score of ≥3
points and ≥30%, with an accompanying decrease in the subscore for
rectal bleeding of at least 1 point or an absolute subscore for
rectal bleeding of 0 or 1. Clinical remission was defined as a
total Mayo score of ≤2 points, with no individual subscore
exceeding 1 point. Mucosal healing was defined as an absolute
subscore for endoscopy of 0 or 1. Tissue samples of colon were
fixed in 4% formalin solution overnight and embedded in paraffin by
the conventional method, then cut into 4 µm thick sections. The
sections were stained with hematoxylin and eosin for gross
histological examination using a fluorescence microscope.
Study objectives
The primary study endpoint was safety and a clinical
response documented by full Mayo scores and IBDQ scores at 3 months
following treatment completion, and the secondary endpoints were
safety and improvements of Mayo scores and IBDQ scores at 24
months.
Safety assessments
Patients were monitored continuously for adverse
events, including evaluations every 2 weeks during the follow-up
period. Other safety parameters (vital signs and clinical
laboratory parameters) were ascertained monthly.
Data collection
A data collection form was developed according to
the objectives of the present study. Training of researchers and
research assistants was performed during a pilot data collection
period and the case record form was standardized. Site visits by
internal and external auditors were performed regularly to assure
quality of the data and the clinical trial process.
Statistical analysis
All statistic analyses were performed using SPSS
version 15.0 software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Differences between the
means of the baseline values of group I and group II were analyzed
using Students t–test. Comparisons between time-dependent
changes at the time of baseline and different time points following
treatment were performed using repeated measure analysis of
variance and post hoc analysis with the Bonferroni correction.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
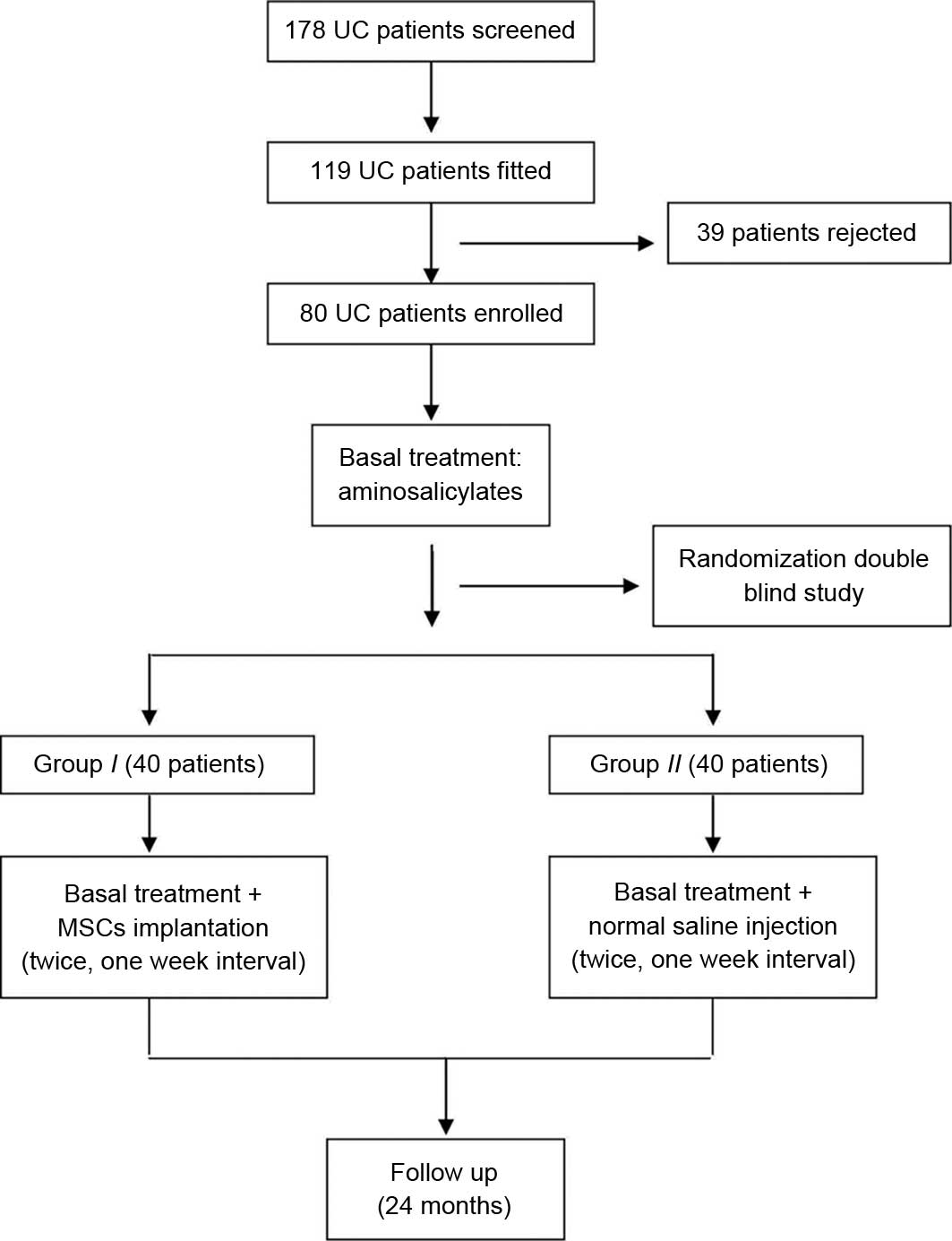
A total of 80 patients with UC were initially
enrolled in the study (Fig. 1) and
randomly divided into groups I and II. Four patients
in group II and six patients in group I withdrew from
the study following therapy because of intolerance of the
colonoscopy examination or lack of time to take part in the study.
In total, 70 patients completed the entire study and their data
were analyzed. Overall, there were 43 men and 27 women, with mean
age of 42.7±9.6 years (range, 18–52 years). The baseline
characteristics of all patients are presented in Table I. There were no significant
differences in clinical findings, laboratory examinations, Mayo
scores or IBDQ scores between the two groups prior to the trial. A
cancer screening test confirmed no cancer in all patients.
 | Table I.Baseline patient characteristics. |
Table I.
Baseline patient characteristics.
| Characteristic | Group I (n=34) | Group II
(n=36) |
|---|
| Gender,
male/female | 21/13 | 22/14 |
| Median age,
year | 42.9±23.1 | 43.7±28.7 |
| Duration of
disease, year | 5.6±4.2 | 6.1±4.9 |
| Extent of
disease |
|
|
| Total
colitis (%) | 24 (70.6) | 24 (66.7) |
|
Left-sided colitis (%) | 10 (29.4) | 12 (33.3) |
| Mayo
score at baseline | 8.9±3.2 | 8.5±3.8 |
| IBDQ at
baseline | 178.9±26.7 | 183.1±32.9 |
| CRP,
mg/l | 35.96±15.75 | 37.58±19.03 |
| ESR,
mm/h | 73±22.1 | 69±18.2 |
Safety evaluation
No clinical symptoms and no significant changes in
vital signs were observed during and after cell therapy. Compared
with group II, there were no evident adverse reactions
following MSC infusion in any of the patients in group I,
and no chronic side effects or lingering effects appeared during
the follow-up period.
Efficacy
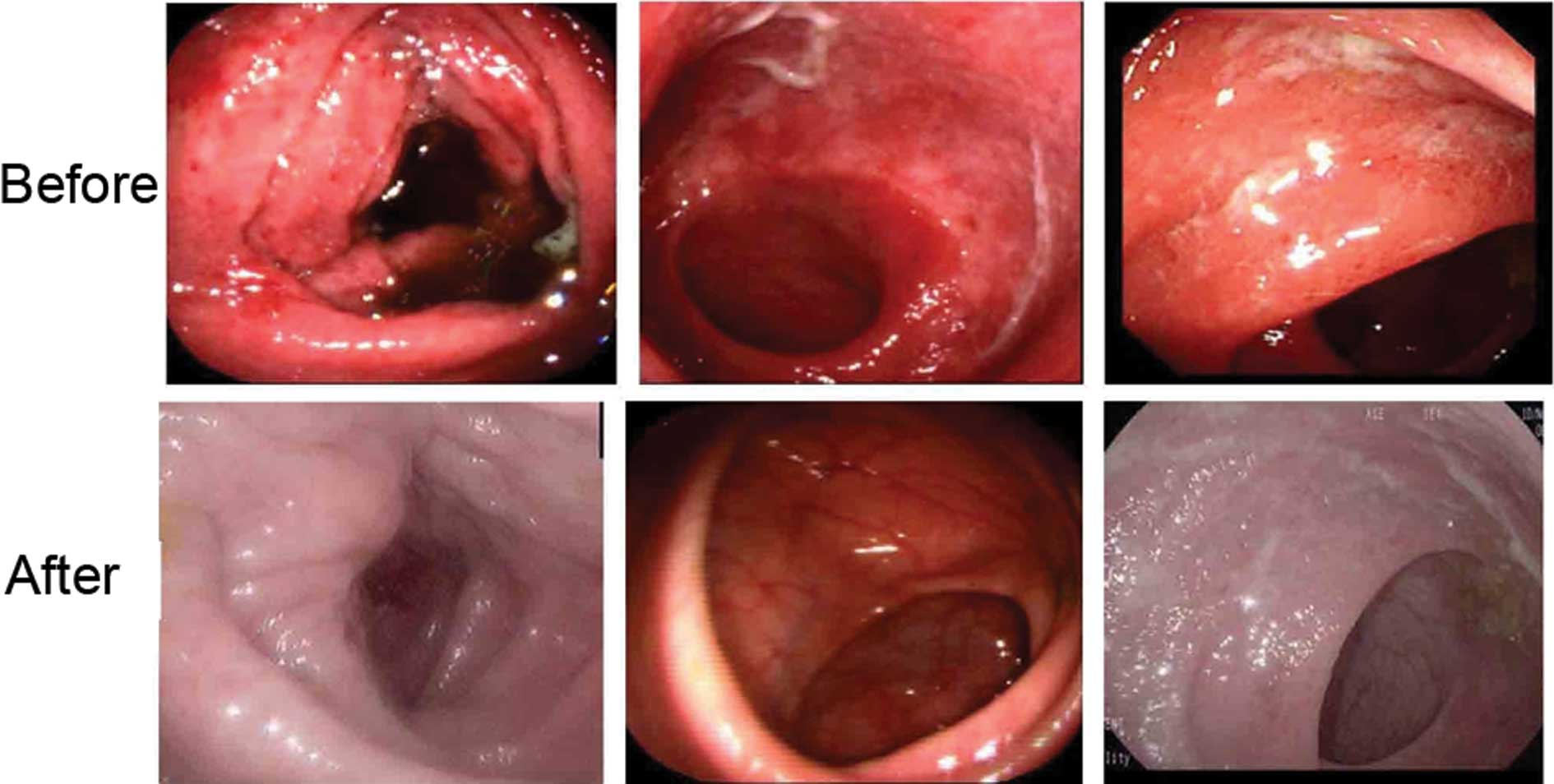
One month after therapy, 30/36 patients in group
I showed good response, such as remission of stomachache,
diarrhea and mucous bloody stool. All eligible patients underwent
endoscopy at baseline and every follow-up point after therapy. As
shown in Fig. 2, diffuse and deep
ulcer formation and severe inflammatory mucosa were observed prior
to therapy, but the findings were greatly improved in group
I following therapy.
Change in Mayo scores
Three months after therapy, the ratio of clinical
responses (≥3 point decrease in the Mayo score) or remission (Mayo
score 0 or 1) was significantly higher in group I compared
with group II (85.3 vs. 15.7%; P=0.007; Fig. 3A). The median Mayo score was improved
in 27 patients in group I at the third month after cell
therapy and reached the lowest level at six months, then sustained
or showed a little fluctuation during the entire follow-up period.
In group II, the median Mayo score of all patients
fluctuated during the entire follow-up period (Fig. 3B).
Change in histological assessment
scores
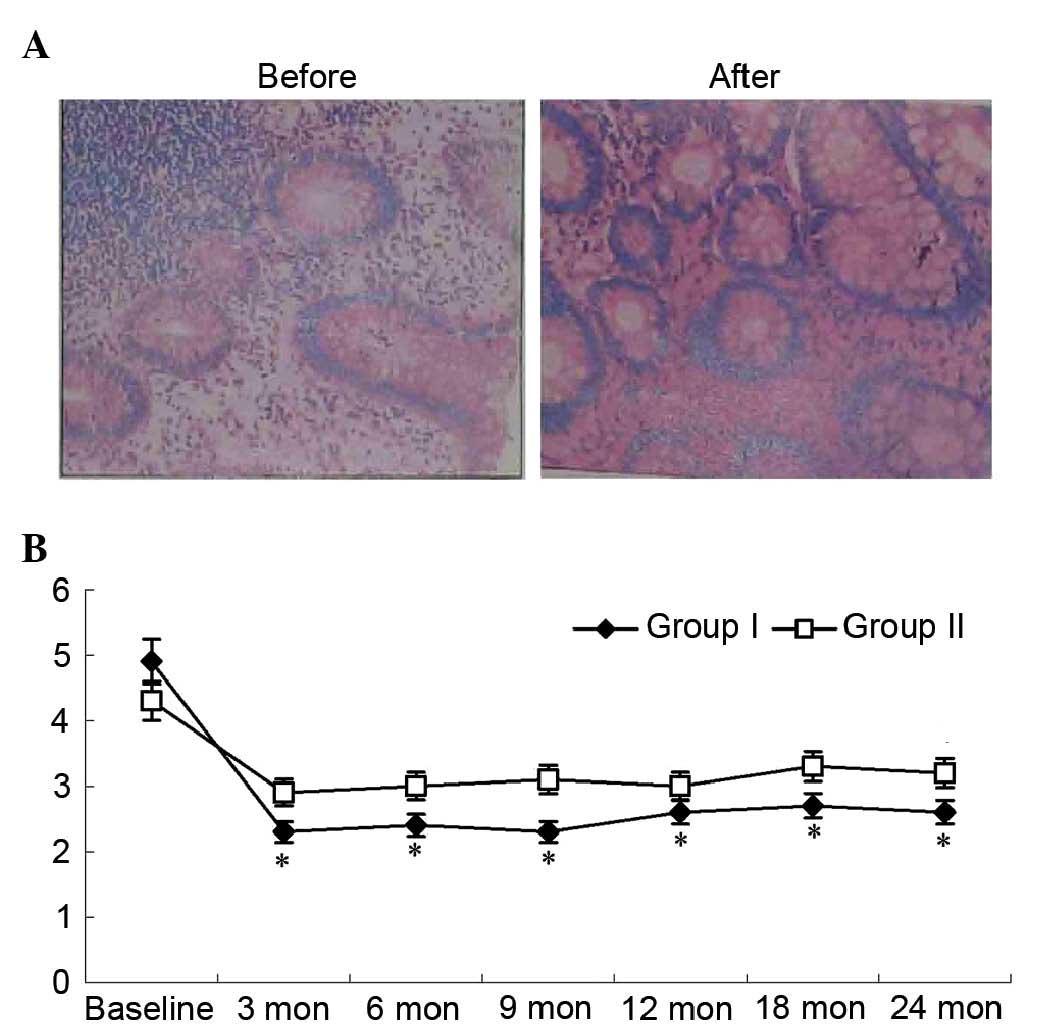
Histology scores from the colonic biopsies are
presented in Fig. 4. The median
histology score in group I decreased following cell therapy.
Histological findings observed at baseline, including abruption of
the surface layer, goblet cell depletion, crypt abscesses, gland
collapse and inflammatory cell infiltration, improved following
therapy. The median histology score in group I was
significantly lower compared with that in group II
(P<0.05; Fig. 4).
Change in IBDQ scores
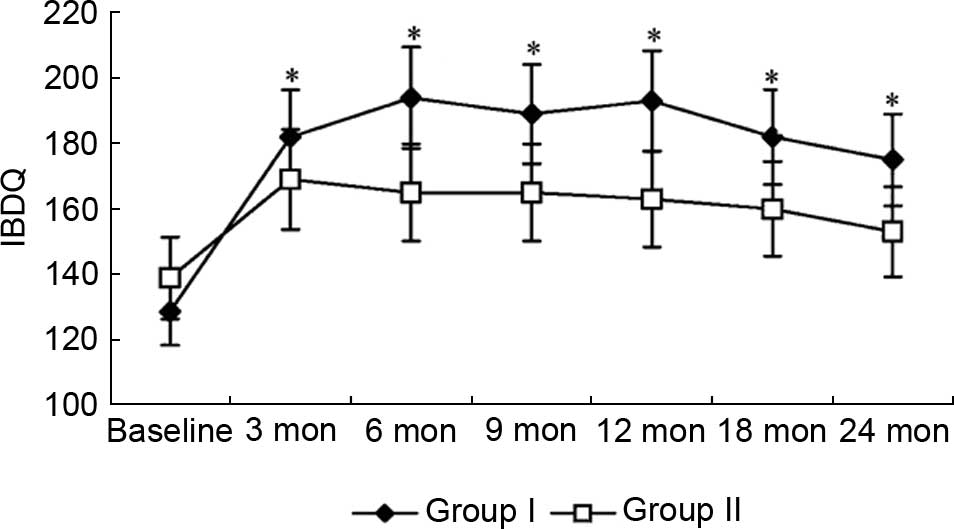
Inflammatory Bowel Disease Questionnaire (IBDQ)
scores were analyzed to evaluate any therapy-induced changes in
health-related quality of life. Fig.
5 shows changes in total IBDQ scores. The IBDQ scores in group
I were significantly improved compared with baseline (128.6
vs. 181.9; P=0.002) at the third month of the trial, and were
higher than group II from the sixth month of the trial. Although
IBDQ scores steadily increased in group II throughout the
trial, the change in scores failed to reach statistical
significance by the end of the trial (P>0.05).
Change in cytokine levels and blood
test
The mean plasma cytokine levels, including TNF-α,
IL-6 and IFN-γ, were not significantly different between the two
groups at baseline and during the follow-up period.
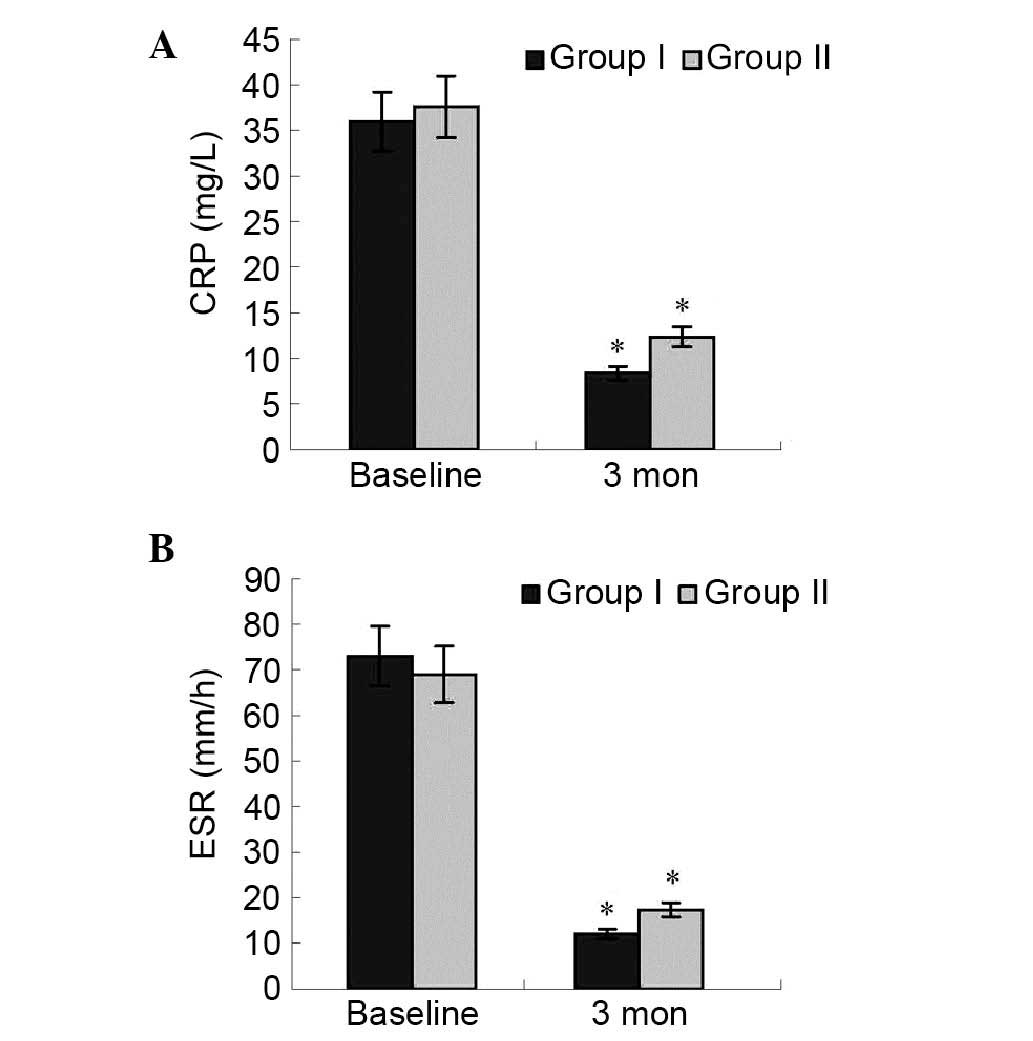
The changes in results of blood test between the two
groups was analyzed. In group I, CRP and ESR significantly
decreased at the third month (0.62 to 0.18 mg/l, P=0.03; 76 to 23
mm/h, P=0.02) compared with the baseline. There was no
significantly difference between two groups. Data are presented in
Fig. 6. There were no other
significant changes and no adverse events related to blood count,
hemoglobin and hematocrit, liver enzymes, total protein, albumin,
total cholesterol, triacylglycerol, serum urea nitrogen, creatinine
and electrolytes in group I compared with group II (data not
presented).
Discussion
One of the main targets of the clinical treatments
for UC is to repair the damaged colonic mucosa. MSCs have great
therapeutic potential in regenerative medicine due to their
differentiation capacity and their secretion of numerous bioactive
molecules (18). There are a number
of studies focused on the attractive regenerative properties of
MSCs, and evidence indicating that MSCs can promote regeneration of
injured tissue (19–22). MSCs have low immunogenicity and
display immunosuppressive proprieties (23), and can trigger the release of
anti-inflammatory cytokines (24).
Their capacity to suppress T cell activities and induce apoptosis
provides a rationale for applying these cells in UC therapy
(25,26).
In the present clinical trial, the safety and
therapeutic effect of MSCs derived from human umbilical cord were
investigated on patients with moderate to severe UC and followed up
for two years. The results showed that all evaluations, including
endoscopic findings, pathological findings, Mayo scores and IBDQ
scores, were markedly improved in group I following MSCs infusion
compared with baseline and group II. These results indicate that
MSC infusion is a safe and effective therapy for UC.
Mechanisms directing in vivo homing and
engraftment of MSCs are unclear and depend on complex interactions
between numerous signaling events. Several studies have
demonstrated the ability of MSCs to preferentially migrate to sites
of injury when infused in animal models (27–29).
After receiving appropriate signals during tissue inflammation,
MSCs could migrate to the lesions of the colon where they assisted
in recovery, displaying high therapeutic potential with regards to
tissue repair and/or the control of local inflammation (30). The expression of growth factors,
cytokines and extracellular matrix receptors by MSCs may drive this
process (31,32). In the present study, the endoscopic
findings, pathological findings and Mayo scores of patients in
group I were markedly improved compared with group II, indicating
that MSCs serve a role at the local sites.
MSCs can reduce colonic inflammation by
downregulating the production of inflammatory mediators by mucosal
immune cells, and by increasing the levels of the anti-inflammatory
cytokines (33). In UC, the
immunologic response is reflected by the imbalance in Th1 and Th2
cells, and thus the cytokine production at different stages of
disease (34). Intravenous treatment
with MSCs could increase the levels of the anti-inflammatory
cytokines IL-10 and IL-4, and decrease the levels of the
pro-inflammatory cytokine IL-6 (35,36).
Chatterjee et al (37)
demonstrated that high levels of IFN-γ produced by T cells in
contact with MSCs resulted in the activation of the
immunosuppressive effect of MSCs. Crucitti et al (38) assessed the therapeutic effect of MSCs
by measuring inflammatory cytokines such as TNF-α, IFN-γ, IL-6 and
IL-1β, and chemokines, such as macrophage inhibitory protein II,
which were significantly decreased in treated mice. Hengartner
et al (39) assessed the MSC
therapeutic effect by measuring the RNA expression of inflammatory
mediators such as TNF-α, IL-1β, cyclo-oxygenase 2, basic fibroblast
growth factor, hepatocyte growth factor and vascular endothelial
growth factor, all of which significantly decreased in MSC-treated
mice. In a study by Forte et al (40), human umbilical cord MSCs were used to
treat acute trinitrobenzene sulfonic acid-induced UC. In another
study, the inflammatory markers such as IL-17, IL-23, IFN-γ and
IL-6 were measured to assess the therapeutic efficacy of human
umbilical cord MSCs and were shown to be significantly decreased in
the treated mice (41). In the
present study, during the follow-up, the levels of cytokines in
group I, including TNF-α, IL-6 and IFN-γ, were not significantly
changed compared with group II. This may be due to the complexity
of the body; however, the exact mechanisms need to be
clarified.
Although the exact mechanisms underlying
MSC-mediated suppression of lymphocyte proliferation remain
essentially unknown, it is possible that MSCs can accelerate
apoptosis of active inflammatory cells. Akiyama et al
(42) showed that the systemic
infusion of MSC-induced T cell apoptosis via the Fas
ligand-dependent Fas pathway, reducing symptoms of dextran sulfate
sodium-induced colitis. MSCs modulate their micro-environment by
exerting powerful immunosuppressive effects (43,44).
These cells inhibit cell proliferation and the cytotoxic potential
of NK cells and CD8+ T cells (45). Furthermore, MSCs impair the
maturation, cytokine production and T cell stimulatory capacity of
dendritic cells. In addition, MSCs suppress the proliferation and
antibody production of B cells, inhibit the proliferation and
cytokine secretion of CD4+ T lymphocyte subsets, and
promote the expansion of regulatory T cell populations (46).
In the present clinical trial, there were no evident
adverse reactions following MSC infusion in any of the patients who
completed the study protocol, and no chronic side effects or
lingering effects appeared during the follow-up period. In
addition, the therapeutic effect of MSC infusion was sustained
during the entire follow-up period. However, the chronic effect of
MSC infusion in treating UC need to explore by an extended
follow-up period. In conclusion, MSC infusion may become a useful
and safe therapy for patients with UC.
Acknowledgements
The present study was supported by the Human
Umbilical Cord Mesenchymal Stem Cell Bank (Qingdao, China).
References
|
1
|
Chinnadurai R, Ng S, Velu V and Galipeau
J: Challenges in animal modelling of mesenchymal stromal cell
therapy for inflammatory bowel disease. World J Gastroenterol.
21:4779–4787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hiraoka S, Kato J, Moritou Y, Takei D,
Inokuchi T, Nakarai A, Takahashi S, Harada K, Okada H and Yamamoto
K: The earliest trough concentration predicts the dose of
tacrolimus required for remission induction therapy in ulcerative
colitis patients. BMC Gastroenterol. 15:532015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sameshima S, Koketsu S, Takeshita E,
Kubota Y, Okuyama T, Saito K, Ueda Y, Sawada T and Oya M: Surgical
resections of ulcerative colitis associated with dysplasia or
carcinoma. World J Surg Oncol. 13:702015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chande N, Tsoulis DJ and MacDonald JK:
Azathioprine or 6-mercaptopurine for induction of remission in
Crohns disease. Cochrane Database Syst Rev. 30:CD0005452013.
|
|
5
|
Naganuma M, Fujii T and Watanabe M:
Treatment strategy for refractory inflammatory bowel disease to
improve endoscopic lesions and long-term prognosis. Nihon Rinsho
Meneki Gakkai Kaishi. 35:99–106. 2012.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castro-Manrreza ME and Montesinos JJ:
Immunoregulation by mesenchymal stem cells: Biological aspects and
clinical applications. J Immunol Res. 2015:3949172015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seidelin JB, Coskun M, Kvist PH, Holm TL,
Holgersen K and Nielsen OH: IL-33 promotes GATA-3 polarization of
gut-derived T cells in experimental and ulcerative colitis. J
Gastroenterol. 50:180–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gordon H, Moller F Trier, Andersen V and
Harbord M: Heritability in inflammatory bowel disease: From the
first twin study to genome-wide association studies. Inflamm Bowel
Dis. 21:1428–1434. 2015.PubMed/NCBI
|
|
9
|
Liu W, Morschauser A, Zhang X, Lu X,
Gleason J, He S, Chen HJ, Jankovic V, Ye Q, Labazzo K, et al: Human
placenta-derived adherent cells induce tolerogenic immune
responses. Clin Transl Immunology. 3:e142014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Z, Zhou X, Li J, Meng Q, Cao H, Kang
L, Ni Y, Fan H and Liu Z: Mesenchymal stem cells reprogram host
macrophages to attenuate obliterative bronchiolitis in murine
orthotopic tracheal transplantation. Int Immunopharmacol.
15:726–734. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang H, He J, Teng X, Yu Y, Ye W, Hu Y
and Shen Z: Combined intrathymic and intravenous injection of
mesenchymal stem cells can prolong the survival of rat cardiac
allograft associated with decrease in miR-155 expression. J Surg
Res. 185:896–903. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi EW, Shin IS, Lee HW, Park SY, Park
JH, Nam MH, Kim JS, Woo SK, Yoon EJ, Kang SK, et al:
Transplantation of CTLA4Ig gene-transduced adipose tissue- derived
mesenchymal stem cells reduces inflammatory immune response and
improves Th1/Th2 balance in experimental autoimmune thyroiditis. J
Gene Med. 13:3–16. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bressler B, Marshall JK, Bernstein CN,
Bitton A, Jones J, Leontiadis GI, Panaccione R, Steinhart AH and
Tse F: Clinical practice guidelines for the medical management of
nonhospitalized ulcerative colitis: The Toronto consensus.
Gastroenterology. 48:1035–1058. 2015. View Article : Google Scholar
|
|
14
|
Suzuki Y, Uchiyama K, Kato M, Matsuo K,
Nakagawa T, Kishikawa H, Kimura N, Kasanuki J and Ino S: Potential
utility of a new ulcerative colitis segmental endoscopic index
combining disease severity and the extent of inflammation. J Clin
Gastroenterol. 49:401–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leong RW, Huang T, Ko Y, Jeon A, Chang J,
Kohler F and Kariyawasam V: Prospective validation study of the
International Classification of Functioning, Disability and Health
scorein Crohns disease and ulcerative colitis. J Crohns Colitis.
8:1237–1245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Travis SP, Schnell D, Feagan BG, Abreu MT,
Altman DG, Hanauer SB, Krzeski P, Lichtenstein GR and Marteau PR:
The impact of clinical information on the assessment of endoscopic
activity: Characteristics of the ulcerative colitis endoscopic
index of severity [UCEIS]. J Crohns Colitis. 9:607–616. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jharap B, Sandborn WJ, Reinisch W, DHaens
G, Robinson AM, Wang W, Huang B, Lazar A, Thakkar RB and Colombel
JF: Randomised clinical study: Discrepancies between
patient-reported outcomes and endoscopic appearance in moderate to
severe ulcerative colitis. Aliment Pharmacol Ther. 42:1082–1092.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mokarizadeh A, Delirezh N, Morshedi A,
Mosayebi G, Farshid AA and Dalir-Naghadeh B: Phenotypic modulation
of auto-reactive cells by insertion of tolerogenic molecules via
MSC-derived exosomes. Vet Res Forum. 3:257–261. 2012.PubMed/NCBI
|
|
19
|
Jung J, Moon JW, Choi JH, Lee YW, Park SH
and Kim GJ: Epigenetic alterations of IL-6/STAT3 signaling by
placental stem cells promote hepatic regeneration in a rat model
with CCl4-induced liver injury. Int J Stem Cells. 8:79–89. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu XC, Jin H, Zhang RY, Ding Y, Zeng X,
Lai BQ, Ling EA, Wu JL and Zeng YS: Donor mesenchymal stem
cell-derived neural-like cells transdifferentiate into
myelin-forming cells and promote axon regeneration in rat spinal
cord transection. Stem Cell Res Ther. 6:1052015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Nie L, Zhao H, Zhang W, Zhang YQ,
Wang SS and Cheng L: Conserved dopamine neurotrophic
factor-transduced mesenchymal stem cells promote axon regeneration
and functional recovery of injured sciatic nerve. PLoS One.
9:e1109932014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hyatt AJ, Wang D, van Oterendorp C,
Fawcett JW and Martin KR: Mesenchymal stromal cells integrate and
form longitudinally-aligned layers when delivered to injured spinal
cord via a novel fibrin scaffold. Neurosci Lett. 569:12–17. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gu H, Xiong Z, Yin X, Li B, Mei N, Li G
and Wang C: Bone regeneration in a rabbit ulna defect model: Use of
allogeneic adipose-derivedstem cells with low immunogenicity. Cell
Tissue Res. 358:453–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu W, Tang Y, Zhang Z, Zhang X, Yao Y, Fu
C, Wang X and Ma G: Inhibiting the mobilization of Ly6C (high)
monocytes after acute myocardial infarction enhances the efficiency
of mesenchymal stromal cell transplantation and curbs myocardial
remodeling. Am J Transl Res. 7:587–597. 2015.PubMed/NCBI
|
|
25
|
Gonçalves Fda C, Schneider N, Pinto FO,
Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP,
Cirne-Lima EO, Meurer L and Paz AH: Intravenous vs intraperitoneal
mesenchymal stem cells administration: What is the best route for
treating experimental colitis? World J Gastroenterol.
20:18228–18239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin Y, Lin L, Wang Q, Jin Y, Zhang Y, Cao
Y and Zheng C: Transplantation of human umbilical mesenchymal stem
cells attenuates dextran sulfate sodium-induced colitis in mice.
Clin Exp Pharmacol Physiol. 42:76–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marquez-Curtis LA and Janowska-Wieczorek
A: Enhancing the migration ability of mesenchymal stromal cells by
targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013:5610982013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu X, Wang W, Meng C, Yang S, Duan D, Xu
W, Liu X, Tang M and Wang H: Regulation of differentiation in
trabecular bone-derived mesenchymal stem cells by T cell activation
and inflammation. Oncol Rep. 30:2211–2219. 2013.PubMed/NCBI
|
|
29
|
Liu J, Chen J, Liu B, Yang C, Xie D, Zheng
X, Xu S, Chen T, Wang L, Zhang Z, et al: Acellular spinal cord
scaffold seeded with mesenchymal stem cells promotes long-distance
axon regeneration and functional recovery in spinal cord injured
rats. J Neurol Sci. 325:127–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu L, Chiu PW, Lam PK, Poon CC, Lam CC,
Ng EK and Lai PB: Effect of local injection of mesenchymal stem
cells on healing of sutured gastric perforation in an experimental
model. Br J Surg. 102:e158–e168. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang JX, Zhang N, Wang HW, Gao P, Yang QP
and Wen QP: CXCR4 receptor overexpression in mesenchymal stem cells
facilitates treatment of acute lung injury in rats. J Biol Chem.
290:1994–2006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen CP, Chen YY, Huang JP and Wu YH: The
effect of conditioned medium derived from human placental
multipotent mesenchymal stromal cells on neutrophils: Possible
implications for placental infection. Mol Hum Reprod. 20:1117–1125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Z, He X, He X, Chen X, Lin X, Zou Y,
Wu X and Lan P: Bone marrow mesenchymal stem cells ameliorate
colitis-associated tumorigenesis in mice. Biochem Biophys Res
Commun. 450:1402–1408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Strober W and Fuss IJ: Proinflammatory
cytokines in the pathogenesis of inflammatory bowel diseases.
Gastroenterology. 140:1756–1767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang C, Li J, Lin H, Zhao K and Zheng C:
Nasal mucosa derived-mesenchymal stem cells from mice reduce
inflammation via modulating immune responses. PLoS One.
10:e01188492015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gregorini M, Bosio F, Rocca C, Corradetti
V, Valsania T, Pattonieri EF, Esposito P, Bedino G, Collesi C,
Libetta C, et al: Mesenchymal stromal cells reset the scatter
factor system and cytokine network in experimental kidney
transplantation. BMC Immunol. 15:442014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chatterjee D, Marquardt N, Tufa DM,
Hatlapatka T, Hass R, Kasper C, von Kaisenberg C, Schmidt RE and
Jacobs R: Human umbilical cord-derived mesenchymal stem cells
utilize activin-A to suppress interferon-γ production by natural
killer cells. Front Immunol. 5:6622014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Crucitti A, Corbi M, Tomaiuolo PM, Fanali
C, Mazzari A, Lucchetti D, Migaldi M and Sgambato A: Laparoscopic
surgery for colorectal cancer is not associated with an increase in
the circulating levels of several inflammation-related factors.
Cancer Biol Ther. 16:671–677. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hengartner NE, Fiedler J, Schrezenmeier H,
Huber-Lang M and Brenner RE: Crucial role of IL1beta and C3a in the
in vitro-response of multipotent mesenchymal stromal cells to
inflammatory mediators of polytrauma. PLoS One. 10:e01167722015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Forte D, Ciciarello M, Valerii MC, De
Fazio L, Cavazza E, Giordano R, Parazzi V, Lazzari L and Laureti S:
Human cord blood-derived platelet lysate enhances the therapeutic
activity of adipose-derived mesenchymal stromal cells isolated from
Crohns disease patients in a mouse model of colitis. Stem Cell Res
Ther. 6:1702015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nam YS, Kim N, Im KI, Lim JY, Lee ES and
Cho SG: Negative impact of bone-marrow-derived mesenchymal stem
cells on dextran sulfate sodium-induced colitis. World J
Gastroenterol. 21:2030–2039. 2015.PubMed/NCBI
|
|
42
|
Akiyama K, Chen C, Wang D, Xu X, Qu C,
Yamaza T, Cai T, Chen W, Sun L and Shi S:
Mesenchymal-stem-cell-induced immunoregulation involves
FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell.
10:544–555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Selim AO, Selim SA, Shalaby SM, Mosaad H
and Saber T: Neuroprotective effects of placenta-derived
mesenchymal stromal cells in a rat model of experimental autoimmune
encephalomyelitis. Cytotherapy. 18:1100–1113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fayyad-Kazan H, Faour WH, Badran B,
Lagneaux L and Najar M: The immunomodulatory properties of human
bone marrow-derived mesenchymal stromal cells are defined according
to multiple immunobiological criteria. Inflamm Res. 65:501–510.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu H, Ding J, Wang J, Wang Y, Yang M,
Zhang Y, Chang F and Chen X: Remission of collagen-induced
arthritis through combination therapy of microfracture and
transplantation of thermogel-encapsulated bone marrow mesenchymal
stem cells. PLoS One. 10:e01205962015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu P, Bailey-Bucktrout S, Xi Y, Xu D, Du
D, Zhang Q, Xiang W, Liu J, Melton A, Sheppard D, et al: Innate
antiviral host defense attenuates TGF-β function through
IRF3-mediated suppression of Smad signaling. Mol Cell. 56:723–737.
2014. View Article : Google Scholar : PubMed/NCBI
|