Introduction
Hashimoto's thyroiditis (HT) was first discovered by
Hakaru Hashimoto in 1912. It is now recognized as the most common
autoimmune disease (1), and the most
frequent cause of hypothyroidism (2). Diagnosis of HT is based on thyroid
dysfunction, an enlarged thyroid gland with a diffusely
hypoechogenic pattern by ultrasound examination, and detection of
serum thyroid peroxidase (TPO) and thyroglobulin (TG) antibodies,
of which TPO is more important (3).
It is now believed that HT is a predominately T cell-mediated
autoimmunity (4,5). TPO-specific T cells alone are able to
induce thyroid destruction, leading to hypothyroidism (6). It has been reported that
CD4+CD25+ T cell depletion increases the
incidence of autoimmune thyroid disease (AITD) (7), indicating that the imbalance between
effector T cells and regulatory T cells (Treg) may be a key factor
in the pathogenesis of AITD. Clinical statistics have also
demonstrated that intrathyroidal Treg cells were decreased in
patients with AITD, contributing to the incomplete regulation of
autoreactive T cells and immune tolerance in AITD (8,9). In
addition, T helper 17 (Th17) cells is also one subset of CD4+ T
cells that may be associated with the pathogenesis of AITD
(10,11). A newly identified subset of T cells,
known as follicular helper T (Tfh) cells, has also been reported to
have an important role in autoimmune disease (12). As a crucial transcription factor for
the pathogenesis of autoimmunity, signal transducer and activator
of transcription (STAT)3 has a key role in Th17 differentiation
(13). Pathogenic Th17 responses in
mice are restrained by Tregs and this function is dependent on the
role of STAT3 (14). Balance and
homeostasis between these two subsets are modulated by STAT3
(15), indicating the essential role
of STAT3 in the immunological mechanisms of autoimmune diseases,
including AITD. In addition to STAT3, STAT1 also has a critical
role in the signal transduction pathway of interferon-gamma (IFN-γ)
and may be a novel target for anti-inflammatory treatment.
Flavonoids, a plant-derived food, are considered to
exert anti-inflammatory effects (16). As one of the most common flavonoids,
luteolin is present in numerous edible plants and plants used in
traditional Chinese medicine. Luteolin has been shown to possess
anti-inflammatory activity both in vitro and in vivo
(17–20). Previous studies have demonstrated
that Tyr705 activation/phosphorylation of STAT3 is markedly
inhibited by luteolin (21,22), and luteolin has also been shown to
inhibit the phosphorylation of STAT1 (23). In addition, as an anti-inflammatory
medication, luteolin has been proven to be effective against other
autoimmune diseases, including multiple sclerosis (24,25) and
experimental autoimmune encephalomyelitis (26). Therefore, the present study focused
on the effects of luteolin on experimental autoimmune thyroiditis
(EAT) and the possible mechanisms associated with STAT1 and STAT3
were discussed.
Materials and methods
Animals
A total of 30 female 8-week-old C57BL/6 mice
weighing 20.35±0.86 mg were purchased from Model Animal Research
Center of Nanjing University (Nanjing, China). Prior to the study,
the mice were housed in a clean-grade animal breeding center with
an indoor temperature of 20–24°C and humidity of 50–70%, under
alternate dark/light cycles. Tap water and laboratory feed were
available ad libitum. All procedures were performed in
accordance with the guidelines outlined by the Animal Research
Ethics Committee of Jinling Hospital (Nanjing, China).
Chemicals and reagents
Luteolin was purchased from the National Institute
for the Control of Pharmaceutical and Biological Products (Jilin,
China). Luteolin (20 mg/ml) was dissolved in DMSO and stored at
−20°C. Fetal bovine serum (FBS), Dulbecco's modified Eagle's medium
(DMEM), penicillin and streptomycin were obtained from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Mouse T4 and
TNF-α enzyme-linked immunosorbent assay (ELISA) kits were purchased
from ExCell (Shanghai, China). Antibodies used in western blot and
immunohistochemistry were as follows: Rabbit monoclonal
phospho-STAT3 antibodies (Tyr705; #9145), rabbit monoclonal
phospho-STAT1 antibodies (Tyr701; #9167), rabbit monoclonal STAT3
antibodies (#9139), rabbit monoclonal STAT1 antibodies (#14994) and
rabbit COX2 antibodies (#12282), and were all purchased from Cell
Signaling Technology, Inc. (Beverly, MA, USA). Rabbit GAPDH
antibodies (#BS60630) and horseradish peroxidase (HRP)-conjugated
goat anti-rabbit antibodies (#BS10043) were purchased from Bioworld
Technology, Inc. (Nanjing, China).
In vivo study
Establishment of an EAT model and treatment with
luteolin
Mice were divided into four groups: Luteolin (n=10),
dexamethasone (Dex; n=5; positive control), Tg (n=10), and control
(n=5). For the induction of autoimmune thyroiditis, 100 µg porcine
Tg (pTg; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) was
emulsified in 100 µl Freund's complete adjuvant (CFA;
Sigma-Aldrich; Merck Millipore) and was subcutaneously injected
into each mouse (except the control) on day 0. A second
subcutaneous injection was administered on day 14 using the same
amount of pTg in incomplete Freund's adjuvant (IFA; Sigma-Aldrich;
Merck Millipore). Following the second immunization, Luteolin and
Dex-treated mice were given daily intraperitoneal injections of
luteolin (10 mg/kg/day) and dexamethasone (5 mg/kg/day; both
Sigma-Aldrich; Merck Millipore), respectively, whereas TG mice were
administered PBS instead. After 7 days of treatment, all mice were
sacrificed by cervical dislocation following pentobarbital
anesthesia (50 mg/kg, i.p.). Blood samples and thyroid tissues were
obtained. Sera were stored at −80°C. Thyroid tissues were fixed in
4% paraformaldehyde solution, sectioned, and hematoxylin and eosin
(H&E) staining and immunohistochemistry (IHC) were performed
for histopathological examination. Mononuclear cell infiltration
index was scored as follows: 0, no infiltration; 1, interstitial
accumulation of cells between two or three follicles; 2, one or two
foci of cells at least the size of one follicle; 3, extensive
infiltration, 10–40% of total area; 4, extensive infiltration,
40–80% of total area; and 5, extensive infiltration >80% of
total area.
Detection of serum T4 and antibodies against
pTg
Serum T4 was assayed using ELISA according to
manufacturer's instructions. Antibodies against pTg were detected
by ELISA. Briefly, flat-bottomed 96-well plates (Costar 3590;
Corning, Inc., Corning NY, USA) were coated overnight at 4°C with
100 µl pTg (#T1126; Sigma-Aldrich; Merck Millipore) diluted to 100
µg/ml in PBS, and then washed twice with PBS with 0.05% Tween 20
(PBST). Free protein binding sites were blocked by adding 1% bovine
serum albumin (BSA) for 2 h at 37°C. Following washing with PBST,
the sera from individual mice were diluted 1:1,600 in PBS with 1%
BSA and incubated overnight at 4°C. Following extensive washing of
the plates, HRP-conjugated goat anti-mouse IgG (1030-05; Southern
Biotech, Birmingham, AL, USA), diluted 1:5,000 in PBS with 1% BSA,
was added and the plates were incubated for 1 h at 37°C and
subsequently washed. The substrate, 50 µl/well
tetramethylbenzidine, was added for 20 min and the reaction was
terminated with 50 µl/well 2 NH2SO4, after
which the optical density was measured at 450 nm.
In vitro study
Cell culture and treatment
RAW264.7 mouse macrophage cell line was obtained
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells were maintained in DMEM supplemented with
10% FBS and antibiotics (100 U/ml penicillin and 100 U/ml
streptomycin). In brief, cells were grown in 6-well plates and
stimulated by human IFN-γ (10 ng/ml) overnight. Cells were treated
with luteolin (20 µmol/l) for 6 h the next day.
Western blot analysis
Western blotting was performed to detect the
expression of COX2, an anti-inflammatory marker, and STAT1 and
STAT3 transcription factors, which are downstream of the
interleukin (IL)-6 signaling pathway. Cells were washed with PBS,
harvested and lysed using radioimmunoprecipitation assay buffer.
Protein concentrations were determined using a bicinchoninic
protein kit according to the manufacturer's instructions. Protein
(50 µg) of each sample was resolved using 10% SDS-PAGE, then
transferred to a PVDF membrane. The membrane was blocked with 5%
BSA for 2 h at room temperature, then washed with TBST (1:1,000)
three times. Phospho-STAT3 (Tyr705; #9145), phospho-STAT1 (Tyr701;
#9167), total STAT3 (#9139), total STAT1 (#14994), COX2 (#12282)
and GAPDH antibodies (#BS60630) were used at a dilution of 1:1,000
and incubated at 4°C overnight, followed by HRP-conjugated goat
anti-rabbit antibodies (#BS10043) at a dilution of 1:20,000 for 80
min at room temperature. Detection of HRP-conjugated antibodies was
performed using an ECL Plus Blotting Reagent and a Quality One
documentation system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cytokine assay
TNF-α concentrations were measured in the
supernatants of cultured RAW264.7 cells using a sandwich ELISA kit
(#EM008-48; ExCell, Shanghai, China) according to the
manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software, version 6.0 (GraphPad Software, Inc., La Jolla, CA,
USA). Data was analyzed using the t-test and one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Luteolin inhibits lymphocytic
infiltration in thyroids of EAT mice
Anatomical observation demonstrated that 4/10 mice
manifested with a goiter (Fig. 1A),
indicating the occurrence of EAT. H&E examination of thyroid
glands demonstrated that 43±5.7% TG mice exhibited infiltration of
immune cells between follicles, whereas the incidences of Luteolin
and Dex-treated mice are 23±5.7 and 17±11.5%, respectively. The
infiltration index of the thyroid sections also indicated
significantly decreased infiltration of lymphocytes in the luteolin
and Dex-treated mice, compared with the TG-treated mice (P<0.05,
Fig. 1B and C).
Luteolin reduces plasma T4 levels,
while increases plasma anti-pTG levels
T4 concentrations and anti-pTG antibodies were
evaluated via ELISA assays. The results demonstrated that all three
groups with EAT (Dex, Luteolin and TG) exhibited increased anti-TG
antibodies compared with control mice, and luteolin and Dex
treatment both significantly increased antibody levels compared
with the TG group (P<0.05; Fig.
2A). Serum T4 concentrations in TG mice were mildly elevated,
compared with the other groups, but the difference was not
significant (P>0.05; Fig.
2B).
Luteolin inhibits STAT3
phosphorylation in thyroid glands
The effect of luteolin on the phosphorylation of
STAT3 (Y705) in thyroid sections was evaluated by IHC.
Phosphorylated STAT3 expression was significantly increased in TG
mice compared with the control (P<0.05), whereas luteolin and
Dex treatment markedly inhibited this alteration (Fig. 2C and D).
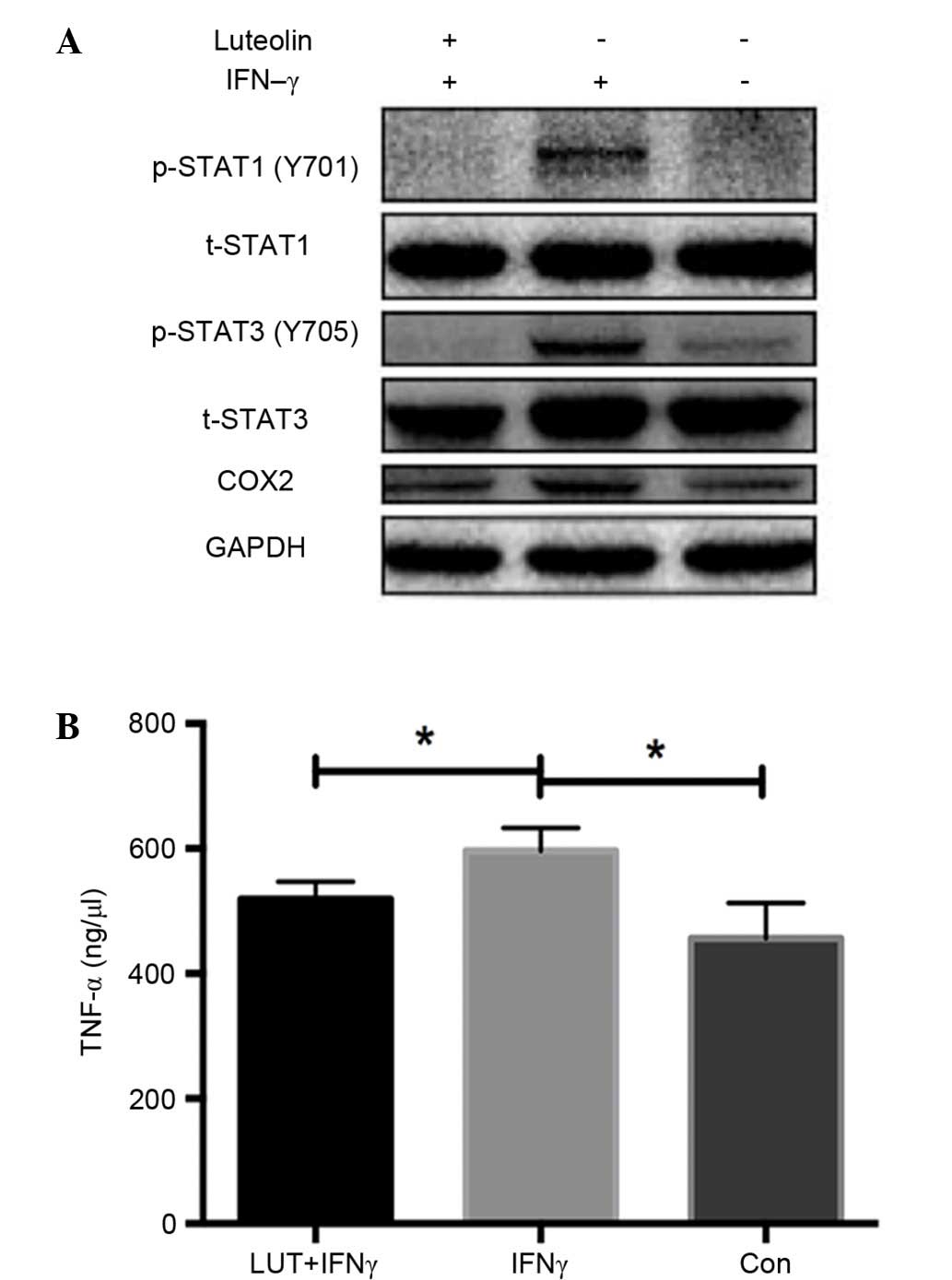
Luteolin inhibits the expression of
COX2 and phosphorylation of STAT1 and STAT3
Western blot analysis of RAW264.7 macrophage cell
line demonstrated that luteolin markedly inhibited the increased
expression of COX2, phosphorylated (p)-STAT1 (Y701) and p-STAT3
(Y705) induced by IFN-γ treatment, whereas total STAT1 and STAT3
remained unchanged. These findings demonstrated the
anti-inflammatory effect of luteolin by inhibiting the STAT1 and
STAT3 signaling pathway in vitro (Fig. 3A).
Luteolin reduces TNF-α secretion in
the RAW264.7 cell line
TNF-α concentrations were measured in the
supernatants of RAW264.7 cells using ELISA kits. TNF-α
concentration levels were significantly increased when treated with
IFN-γ, whereas they were markedly decreased after treatment with
luteolin (P<0.05; Fig. 3B).
Discussion
STAT3 has an important role in T cell-mediated
immunity, including the proliferation (27) and migration (28) of T cells, differentiation into Th17
cells (29), and balance between
Treg cells and Th17 cells (30,31).
Moreover, STAT3 and its downstream SOCS3 gene polymorphism are
associated with AITD susceptibility and IL-6 secretion (32–34).
Cytokines, such as IL-6, are important in the pathogenesis of AITD
due to their functions in recruiting inflammatory cells in the
thyroid, upregulating some inflammatory molecules and interfering
in the production of thyroid hormones (35). It has been demonstrated that
IL-6-STAT3 signaling has a crucial role in dendritic cell
differentiation during T cell-mediated immune responses in
vivo (36). Thyroid follicular
epithelial cells are able to synthesize and secrete large
quantities of IL-6 (37), which
further promotes the development of autoimmune responses.
Therefore, it is theoretically reasonable to target IL-6/STAT3 to
intervene in the early stage of autoimmune thyroiditis in order to
explore novel therapeutic strategies for HT. Previous studies have
shown that luteolin has potent anti-inflammatory effects in
vitro and in vivo (38,39) and
the mechanisms involved include the activation of NF-κB, which
leads to the expression of IL-6 and COX-2 (18,40).
Activator protein-1 (AP-1) is also an important transcription
factor associated with immune responses. Expression of IL-6 is
induced by AP-1 and NF-κB (41).
Jang et al (41) found that
luteolin was able to reduce LPS-induced IL-6 expression by
inhibiting JNK and AP-1 pathways both in vitro and in
vivo, and the mice treated with luteolin exhibited decreased
plasma and hippocampal IL-6 levels.
HT, which is also known as chronic lymphocytic
thyroiditis, is the most common autoimmune disease. There is
usually a long latency period before hypothyroidism occurs
(42). Therefore, early intervention
may theoretically prevent the development of the disease and
maintain the normal structure and function of the thyroid glands.
Thus, the present study aimed to explore the anti-inflammatory
effects of luteolin on autoimmune thyroiditis and the mechanisms
involved. A classical C57BL/6 mouse model of EAT was established.
As a result, 4/10 mice exhibited goiter symptoms and infiltration
of mononuclear cells into the thyroid glands. C57BL/6 mice are
known to have a relative low incidence of EAT (43), which is consistent with the present
findings. The effects of luteolin on EAT were subsequently
evaluated. Mice treated with luteolin demonstrated significantly
reduced infiltration of lymphocytes compared with TG mice. As an
intracellular inhibitor of IL-6/STAT1 and STAT3 signaling pathway,
luteolin significantly inhibited the phosphorylation of STAT1 and
STAT3 in thyroid glands, as identified by H&E examination.
Anti-Tg antibodies were also elevated in the three
EAT groups, as compared with the control; however, the treatment of
luteolin and Dex appeared to further increase the antibodies.
Although the mechanisms remain unknown, clinical data has shown
that thyroid antibodies are elevated shortly after
131iodine treatment for hyperthyroidism (44). The mechanisms involved require
further investigation. In addition, serum T4 levels were slightly
elevated in TG mice, which may be due to the thyroid damage caused
by thyroiditis. Luteolin appears to reduce the release of T4 into
the blood; however, no statistical significance was detected.
Western blot analysis of RAW264.7 cells demonstrated
that luteolin exerted anti-inflammatory effects by significantly
inhibiting the IFN-γ-induced increase of COX2 and p-STAT1 (Y701)
and p-STAT3 (Y705) expression, whereas total STAT1 and STAT3
remained unchanged. COX-2 is an inducible enzyme (45), which is highly expressed in cells
involved in the inflammatory response including
monocytes/macrophages and mast cells. Cytokine TNF-α detection in
supernatants also demonstrated that luteolin exhibited
anti-inflammatory effects by significantly reducing TNF-α secretion
in vitro.
Additional experiments are required to elucidate the
anti-inflammatory mechanisms of luteolin, which may include the
IL-6/STAT1 and STAT3 pathways discussed in the present study or
other pathways. Even if the immunosuppressive effects are mediated
solely through the IL-6 pathway, additional proteins or molecules
involved in the process remain to be discovered.
In conclusion, treatment with luteolin exhibited a
significant immunosuppressive effect by attenuating lymphocytic
infiltration and the destruction of the thyroid epithelia in
thyroid glands, which is likely to have occurred via the inhibition
of IL-6/STAT1 and STAT3 signaling pathway in the glands. The
present study provides evidence for a promising novel therapeutic
strategy for the early intervention of autoimmune thyroiditis.
Further investigation is required to fully elucidate the mechanisms
involved.
References
|
1
|
McLeod DS and Cooper DS: The incidence and
prevalence of thyroid autoimmunity. Endocrine. 42:252–265. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vaidya B and Pearce SH: Management of
hypothyroidism in adults. BMJ. 337:a8012008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caturegli P, De Remigis A and Rose NR:
Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun
Rev. 13:391–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chistiakov DA: Immunogenetics of
Hashimoto's thyroiditis. J Autoimmune Dis. 2:12005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McLachlan SM and Rapoport B: Autoimmune
hypothyroidism: T cells caught in the act. Nat Med. 10:895–896.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quaratino S, Badami E, Pang YY, Bartok I,
Dyson J, Kioussis D, Londei M and Maiuri L: Degenerate
self-reactive human T-cell receptor causes spontaneous autoimmune
disease in mice. Nat Med. 10:920–926. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saitoh O and Nagayama Y: Regulation of
Graves' hyperthyroidism with naturally occurring CD4+ CD25+
regulatory T cells in a mouse model. Endocrinology. 147:2417–2422.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakano A, Watanabe M, Iida T, Kuroda S,
Matsuzuka F, Miyauchi A and Iwatani Y: Apoptosis-induced decrease
of intrathyroidal CD4+ CD25+ regulatory T cells in autoimmune
thyroid diseases. Thyroid. 17:25–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang SH and Baker JR: The role of
apoptosis in thyroid autoimmunity. Thyroid. 17:975–979. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi Y, Wang H, Su Z, Chen J, Xue Y, Wang
S, Xue Y, He Z, Yang H, Zhou C, et al: Differentiation imbalance of
Th1/Th17 in peripheral blood mononuclear cells might contribute to
pathogenesis of Hashimoto's thyroiditis. Scand J Immunol.
72:250–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Cai W, Gu R, Zhang Y, Zhang H, Tang
K, Xu P, Katirai F, Shi W, Wang L, et al: Th17 cell plays a role in
the pathogenesis of Hashimoto's thyroiditis in patients. Clin
Immunol. 149:411–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen
J, Tang X, Xu H, Lu L and Wang S: Increased frequency of follicular
helper T cells in patients with autoimmune thyroid disease. J Clin
Endocrinol Metab. 97:943–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harris TJ, Grosso JF, Yen HR, Xin H,
Kortylewski M, Albesiano E, Hipkiss EL, Getnet D, Goldberg MV,
Maris CH, et al: Cutting edge: An in vivo requirement for STAT3
signaling in TH17 development and TH17-dependent autoimmunity. J
Immunol. 179:4313–4317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaudhry A, Rudra D, Treuting P, Samstein
RM, Liang Y, Kas A and Rudensky AY: CD4+ regulatory T cells control
TH17 responses in a Stat3-dependent manner. Science. 326:986–991.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Durant L, Watford WT, Ramos HL, Laurence
A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F and
O'Shea JJ: Diverse targets of the transcription factor STAT3
contribute to T cell pathogenicity and homeostasis. Immunity.
32:605–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HP, Son KH, Chang HW and Kang SS:
Anti-inflammatory plant flavonoids and cellular action mechanisms.
J Pharmacol Sci. 96:229–245. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
López-Lázaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xagorari A, Papapetropoulos A, Mauromatis
A, Economou M, Fotsis T and Roussos C: Luteolin inhibits an
endotoxin-stimulated phosphorylation cascade and proinflammatory
cytokine production in macrophages. J Pharmacol Exp Ther.
296:181–197. 2001.PubMed/NCBI
|
|
19
|
Kritas SK, Saggini A, Varvara G, Murmura
G, Caraffa A, Antinolfi P, Toniato E, Pantalone A, Neri G, Frydas
S, et al: Luteolin inhibits mast cell-mediated allergic
inflammation. J Biol Regul Homeost Agents. 27:955–959.
2013.PubMed/NCBI
|
|
20
|
Ziyan L, Yongmei Z, Nan Z, Ning T and
Baolin L: Evaluation of the anti-inflammatory activity of luteolin
in experimental animal models. Planta Med. 73:221–226. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Selvendiran K, Koga H, Ueno T, Yoshida T,
Maeyama M, Torimura T, Yano H, Kojiro M and Sata M: Luteolin
promotes degradation in signal transducer and activator of
transcription 3 in human hepatoma cells: An implication for the
antitumor potential of flavonoids. Cancer Res. 66:4826–4834. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parker-Athill E, Luo D, Bailey A, Giunta
B, Tian J, Shytle RD, Murphy T, Legradi G and Tan J: Flavonoids, a
prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose
IL-6/MIA associated autism. J Neuroimmunol. 217:20–27. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rezai-Zadeh K, Ehrhart J, Bai Y, Sanberg
PR, Bickford P, Tan J and Shytle RD: Apigenin and luteolin modulate
microglial activation via inhibition of STAT1-induced CD40
expression. J Neuroinflammation. 5:412008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Theoharides TC: Luteolin as a therapeutic
option for multiple sclerosis. J Neuroinflammation. 6:292009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Theoharides TC, Kempuraj D and Iliopoulou
BP: Mast cells, T cells and inhibition by luteolin: Implications
for the pathogenesis and treatment of multiple sclerosis. Adv Exp
Med Biol. 601:423–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beeton C, Pennington MW, Wulff H, Singh S,
Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA
and Chandy KG: Targeting effector memory T cells with a selective
peptide inhibitor of Kv1. 3 channels for therapy of autoimmune
diseases. Mol Pharmacol. 67:1369–1381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takeda K, Kaisho T, Yoshida N, Takeda J,
Kishimoto T and Akira S: Stat3 activation is responsible for
IL-6-dependent T cell proliferation through preventing apoptosis:
Generation and characterization of T cell-specific Stat3-deficient
mice. J Immunol. 161:4652–4660. 1998.PubMed/NCBI
|
|
28
|
McLoughlin RM, Jenkins BJ, Grail D,
Williams AS, Fielding CA, Parker CR, Ernst M, Topley N and Jones
SA: IL-6 trans-signaling via STAT3 directs T cell infiltration in
acute inflammation. Proc Natl Acad Sci USA. 102:9589–9594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L, Ivanov II, Spolski R, Min R,
Shenderov K, Egawa T, Levy DE, Leonard WJ and Littman DR: IL-6
programs T(H)-17 cell differentiation by promoting sequential
engagement of the IL-21 and IL-23 pathways. Nat Immunol. 8:967–974.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kimura A and Kishimoto T: IL-6: Regulator
of Treg/Th17 balance. Eur J Immunol. 40:1830–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nishihara M, Ogura H, Ueda N, Tsuruoka M,
Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, et
al: IL-6-gp130-STAT3 in T cells directs the development of IL-17+
Th with a minimum effect on that of Treg in the steady state. Int
Immunol. 19:695–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xiao L, Muhali FS, Cai TT, Song RH, Hu R,
Shi XH, Jiang WJ, Li DF, He ST, Xu J and Zhang JA: Association of
single-nucleotide polymorphisms in the STAT3 gene with autoimmune
thyroid disease in Chinese individuals. Funct Integr Genomics.
13:455–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kotkowska A, Sewerynek E, Domańska D,
Pastuszak-Lewandoska D and Brzeziańska E: Single nucleotide
polymorphisms in the STAT3 gene influence AITD susceptibility,
thyroid autoantibody levels and IL6 And IL17 secretion. Cell Mol
Biol Lett. 20:88–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan R, Yang J, Jiang P, Jin L, Ma J, Huang
R, Ma N and Jiang F: Genetic variations in the SOCS3 gene in
patients with Graves' ophthalmopathy. J Clin Pathol. 68:448–452.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ajjan R and Weetman A: Cytokines in
thyroid autoimmunity. Autoimmunity. 36:351–359. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park SJ, Nakagawa T, Kitamura H, Atsumi T,
Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, et al:
IL-6 regulates in vivo dendritic cell differentiation through STAT3
activation. J Immunol. 173:3844–3854. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weetman A, Bright-Thomas R and Freeman M:
Regulation of interleukin-6 release by human thyrocytes. J
Endocrinol. 127:357–361. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kotanidou A, Xagorari A, Bagli E, Kitsanta
P, Fotsis T, Papapetropoulos A and Roussos C: Luteolin reduces
lipopolysaccharide-induced lethal toxicity and expression of
proinflammatory molecules in mice. Am J Respir Crit Care Med.
165:818–823. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen CY, Peng WH, Tsai KD and Hsu SL:
Luteolin suppresses inflammation-associated gene expression by
blocking NF-kappaB and AP-1 activation pathway in mouse alveolar
macrophages. Life Sci. 81:1602–1614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen CC, Chow MP, Huang WC, Lin YC and
Chang YJ: Flavonoids inhibit tumor necrosis factor-alpha-induced
up-regulation of intercellular adhesion molecule-1 (ICAM-1) in
respiratory epithelial cells through activator protein-1 and
nuclear factor-kappaB: Structure-activity relationships. Mol
Pharmacol. 66:683–693. 2004.PubMed/NCBI
|
|
41
|
Jang S, Kelley KW and Johnson RW: Luteolin
reduces IL-6 production in microglia by inhibiting JNK
phosphorylation and activation of AP-1. Proc Natl Acad Sci USA.
105:7534–7539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hutfless S, Matos P, Talor MV, Caturegli P
and Rose NR: Significance of prediagnostic thyroid antibodies in
women with autoimmune thyroid disease. J Clin Endocrinol Metab.
96:E1466–E1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maron R and Cohen IR: H-2K mutation
controls immune response phenotype of autoimmune thyroiditis.
Critical expression of mutant gene product in both thymus and
thyroid glands. J Exp Med. 152:1115–1120. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Einhorn J, Fagraeus A and Jonsson J:
Thyroid antibodies after 131I treatment for hyperthyroidism. J Clin
Endocrinol Metab. 25:1218–1224. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Crofford LJ: COX-1 and COX-2 tissue
expression: Implications and predictions. J Rheumatol Suppl.
49:15–19. 1997.PubMed/NCBI
|