Introduction
Blackhead analysis includes blackhead moles,
keratosis pilaris, hair follicle ichthyosis, keratosis follicularis
and trichostasis spinulosa (1). Pore
analysis was divided into three kinds of pore structures: Sweat
gland openings that are not visible to the naked eye; hair follicle
sebaceous gland openings that are visible to the naked eye; and
hair follicle sebaceous gland openings containing keratotic plug
that are visible to the naked eye (2). The main reasons for skin blackheads and
coarse pores were: Physique, heredity, age, gender, oily skin,
hormones affecting skin roughness, collagen density, sebaceous
gland distribution and secretion, light aging caused by ultraviolet
rays, and poor face-cleaning habits (3). Blackheads and coarse pores can
seriously impact facial beauty and reduce the self-confidence of
patients (4).
The traditional treatments of acne include
needle-lancing and topical drugs, such as retinoic acid and
salicylic acid (5). However,
conventional cleaning products are ineffective for certain
blackheads. Evidence suggests that Q-switched Nd:YAG and lattice
laser treatment for blackheads and coarse pores is effective, but
the improvement was limited and the effect was not lasting and
stable (6).
The aim of the present study was to determine the
mechanism of action of the 800 nm semiconductor laser on skin
blackheads and coarse pores. To the best of our knowledge, there is
no similar experimental study examining this type of laser
treatment to treat skin blackheads and coarse pores.
Materials and methods
Experimental animals
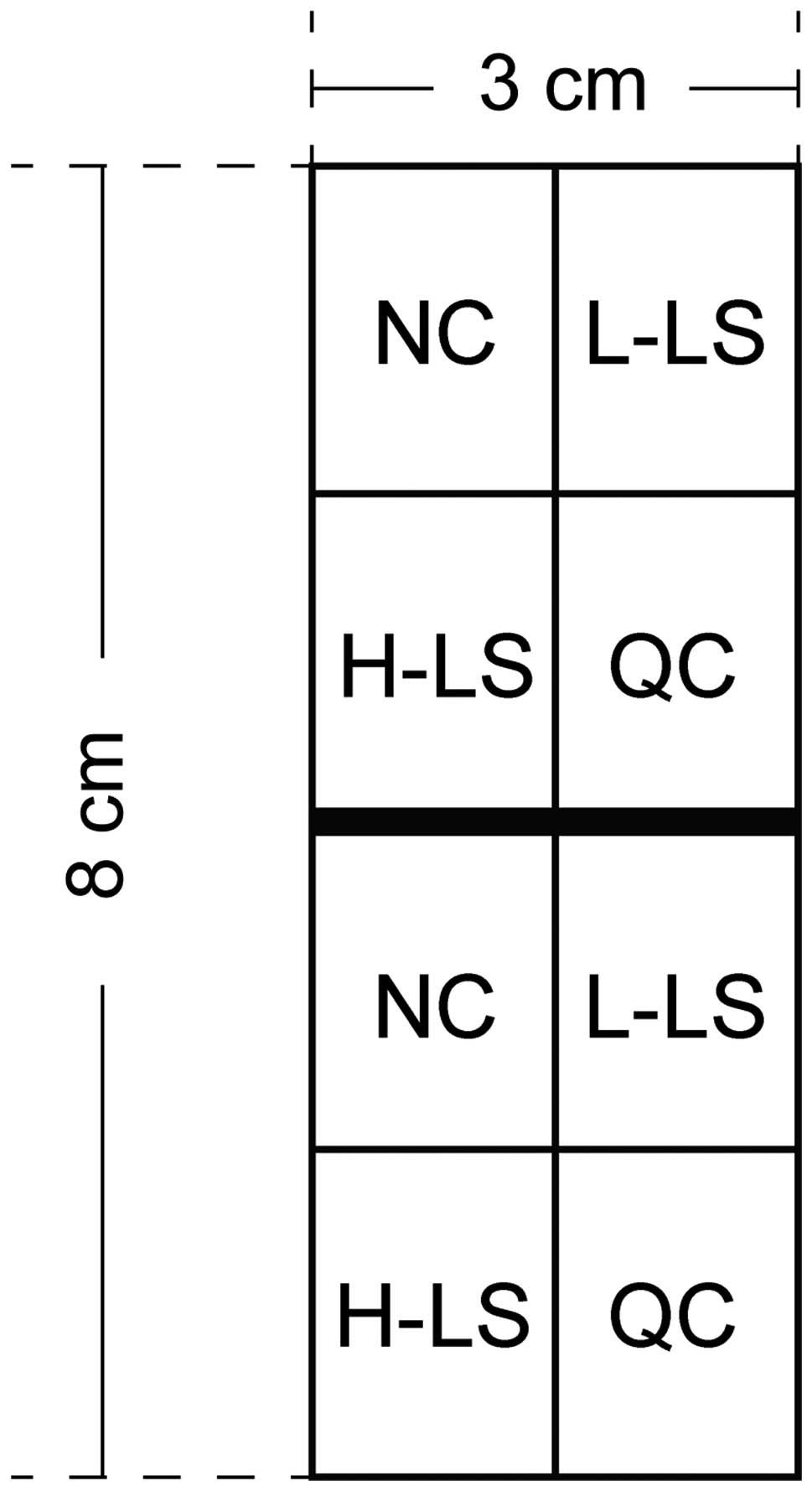
A total of 24 healthy purebred short-haired male
guinea pigs, weighing 350–400 g, were selected for the present
study. The animals were provided by the Shanghai Laboratory Animal
Center (Shanghai, China), batch no. SYXK 2015–0016. The guinea pigs
were fed routinely in an environment with temperatures of 20–25°C,
relative humidity of 55–60%, and light and dark cycle of 12 h each.
One week after adapting to the environment, the guinea pigs were
coated with 0.5 ml of coal tar suspension (Chongqing Jinrong
Chemical Co., Chongqing, China) evenly by injector once daily.
Treatment was continued for 14 days to form an experimental area of
8×3 cm on the back of the guinea pigs (Fig. 1).
Experimental groups
The subjects were divided into the following groups:
Normal control group (NC); low-dose laser treatment group (L-LS);
high-dose laser treatment group (H-LS); and Q-switched Nd:YAG
treatment group (QC).
Methods
A LightSheer Duet ET 800 nm semiconductor laser
(Lumenis Ltd., Yokneam, Israel) treatment was applied with an
energy density of 20–30 J/cm2 (L-LS group), 35–55
J/cm2 (H-LS group) and a Q-switched Nd:YAG mode. Spot
testing was completed on an area of molding, with a pulse width of
0.5 msec and a spot size of 7 mm. A single spot was arranged to
avoid repetition. During the treatment process, shading pockets
were used to cover eyes, with cold compression applied for 30 min
after treatment. A Nikon D800 SLR camera (Nikon, Tokyo, Japan) was
used to capture images before and after treatments. Samples were
taken at 1, 7 and 14 days after surgery. The applications of the
experimental method are shown in Fig.
1.
Observation of epidermis, dermis,
sebaceous gland changes, and hair follicle damage by hematoxylin
and eosin (H&E) staining
A solution of 4% lidocaine was injected locally in
the experimental area before skin-tissue cutting, iodine
disinfection and oppression hemostasis was performed. A 4%
paraformaldehyde fixation was used, along with phosphate-buffered
saline (PBS) flushing, gradient alcohol dehydration, xylene
transparence, paraffin wax soakage, embedding and a paraffin
section device (Beijing Liuyi Instrument Factory, Beijing, China)
to cut a 4-µm serial section. This was applied to plaster before
drying and performing H&E staining (Thermo Fisher Scientific,
Waltham, MA, USA). The sections were observed under a light
microscope (Olympus Corp., Tokyo, Japan).
Detection of the expression of
proliferating cell nuclear antigen (PCNA) of sebaceous gland cells
by immunohistochemical methods
Conventional xylene, gradient alcohol dewaxing, PBS
flushing, 50 µl peroxidase, room temperature blocking for 30 min,
distilled water washing of 5 min × 3 times, and 0.125% fresh
pancreatic enzyme dropping paraffin were all used as part of the
testing. A constant temperature box (Sanyo Electric Co., Osaka,
Japan) was set at 37°C for incubation for 20 min as 50-µl blocking
serum of goat was added and incubated at room temperature for 1 h.
Rabbit anti-PCNA monoclonal antibody (no. sc-7907) at a
concentration of 1:2,000) and internal resistance rabbit anti-GAPDH
monoclonal antibody (no. sc-25778) (both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at a concentration of 1:1,000
and incubated at 4°C overnight. PBS cleaning 5 min × 3 times; 50 BB
rat anti-rabbit bivalent antibody (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) at a concentration of
1:500 was added. Biotin labeling was incubated at room temperature
for 1 h with PBS rinsing for 5 min × 3 times, while 50 µl
horseradish peroxidase (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) was added and incubated at room
temperature for 1 h with PBS cleaning for 5 min × 3 times. The
samples were kept in the dark as 20 µl chromogenic solution
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) at a
concentration of 1:50 was added. Hematoxylin redyeing, ethyl
alcohol differentiation, gradient dehydration, xylene lucency and
neutral gum seal sheets were utilized. The results were observed by
a low-power lens.
Test sebaceous gland cell apoptosis
using TUNEL
Under the condition of conventional dewaxing and
rehydration, 20 mg/ml proteinase K (Chongqing Bofei Biochemical
Products Co., Ltd., Chongqing, China) without DNase was incubated
at 37°C for 20 min with PBS washing 5 min × 3 times. Hydrogen
peroxide of 3% was placed at room temperature for 10 min with PBS
washing 5 min × 3 times. TUNEL reaction mixture (Hoffmann-La Roche,
Basel, Switzerland) was prepared and added to create a 50 cr TUNEL
reaction mixture, followed by adjusting the thermostat to 37°C, and
wet box reacting for 60 min with PBS washing 5 min × 3 times. POD
transforming agent (50 µl) was added and incubated at 37°C in a wet
box for 30 min with PBS washing for 5 min × 3 times. Hematoxylin
redyeing, ethyl alcohol differentiation, gradient dehydration,
xylene lucency and neutral gum seal sheet were utilized. The
results were observed by a low-power lens.
Detection of protein expression of
caspase-3, Bax and Bcl-2 by western blot analysis
For cell total protein extraction, cell lysis liquid
P0013 (Beyotime Institute of Biotechnology, Jiangsu, China) at a
final concentration of 1 mM was used and blended thoroughly. It was
placed on ice and centrifuged at 10,000 for 20 min. The supernatant
was the extraction of the total protein. For the protein
concentration determination, a BCA protein concentration
determination kit P0009 (enhancement mode) (Beyotime Institute of
Biotechnology) was used to detect protein according to the
manufacturer's instructions. For the protein sample handling, we
used protein electrophoresis to calculate the volume. A
corresponding 5X loading buffer with a final concentration of 1X
loading buffer was added, and blending, heating, centrifugation and
electrophoresiswere conducted. We utilized sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE),
transmembrane protein (wet), sealing and the incubation of the
primary antibodies. To determine a pre-staining protein molecular
mark, a PVDF membrane was cut according to a target protein
molecular weight. This was placed in the antibody incubation box
with the following: A 1X TBST washing, rabbit anti-caspase-3
monoclonal antibody, no. sc-98785 at a concentration of 1:2,000,
and a rabbit anti-Bax monoclonal antibody, no. sc-25778 (both from
Santa Cruz Biotechnology, Inc.) at a concentration of 1:1,000.
Incubation of secondary antibody (the primary antibody was recycled
with 1X TBST washing before 5 ml of skim milk at a 5% concentration
and a corresponding species secondary antibody was added according
to the proportion of 1:5,000–1:20,000), and exposed
(chemiluminescence methI). The results were expressed by the gray
value ratio of the band.
Statistical analysis
SPSS 20.0 software (IBM, Armonk, NY, USA) was used
for statistical analysis. Measurement data were presented as mean ±
standard deviation. Comparison between groups was performed using
one-way ANOVA test followed by post hoc test (LSD). The comparison
in one group introduced variance analysis to repeat measurement
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
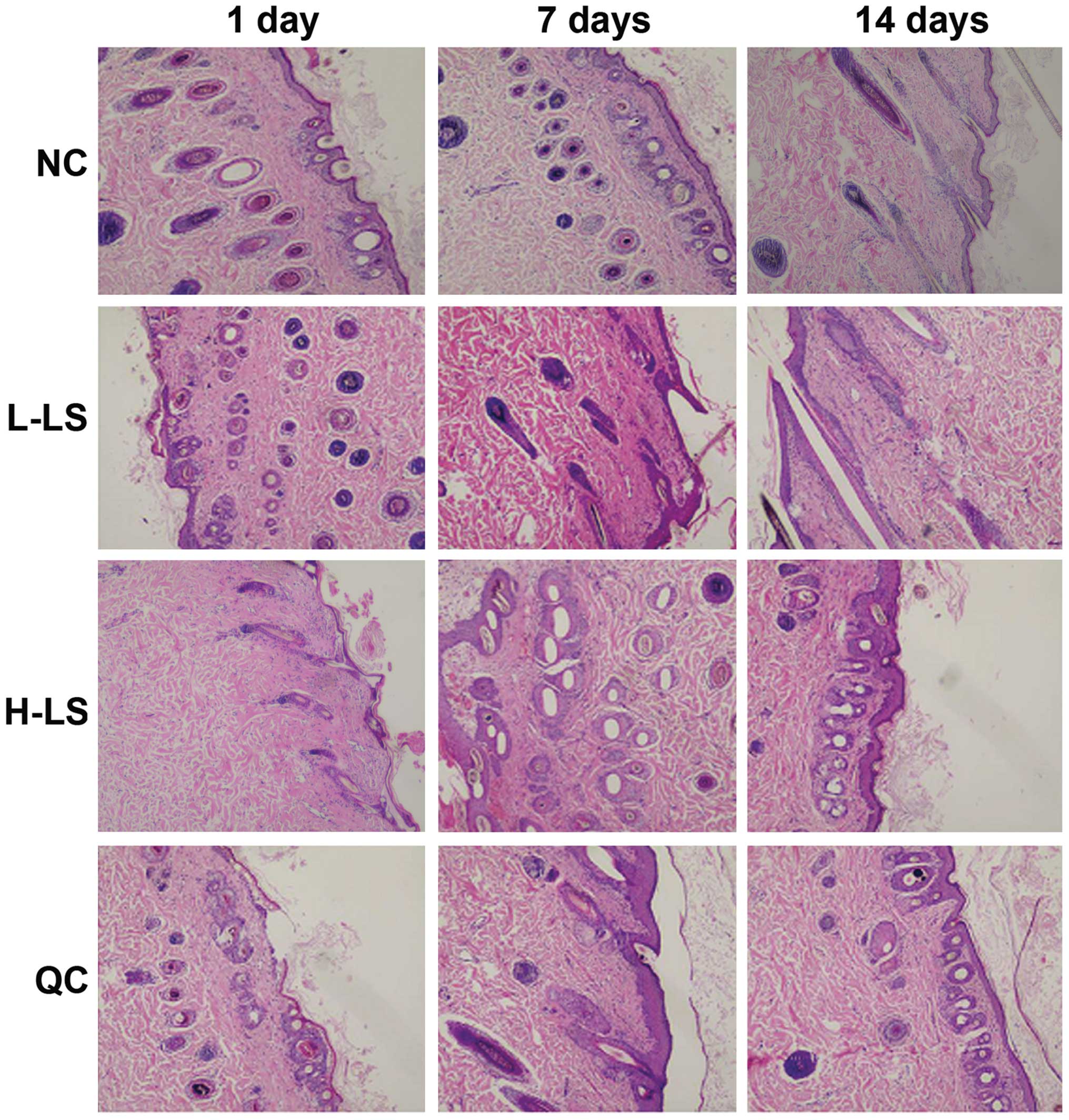
H&E staining results
Two to four cell layers of hair follicle epithelium
were evident in the NC group. Its follicular infundibulum did not
expand, while the hair follicle appeared quasicircular. The
mesenchyme did not have infiltration of inflammatory cells. In the
L-LS group, we observed hair follicle distortion and abundant
infiltration of inflammatory cells at 1 day. Hair follicle
retrogression and the separation between papilla and hair bulb were
seen at 7 days. At 14 days, the hair follicle epithelium appeared
thin, inflammatory cells were reduced and the hair follicle form
returned to normal. In the H-LS group, abundant inflammatory
necrosis under the hair follicle was visible, with the hair shaft
being heated for 7 days. This reduced hair follicle amounts at 14
days. In the QC group, the infiltration of inflammatory cells was
seen at 1 day, with hair follicle deformation at 7 days and partial
hair follicle inflammatory necrosis at 14 days (Fig. 2).
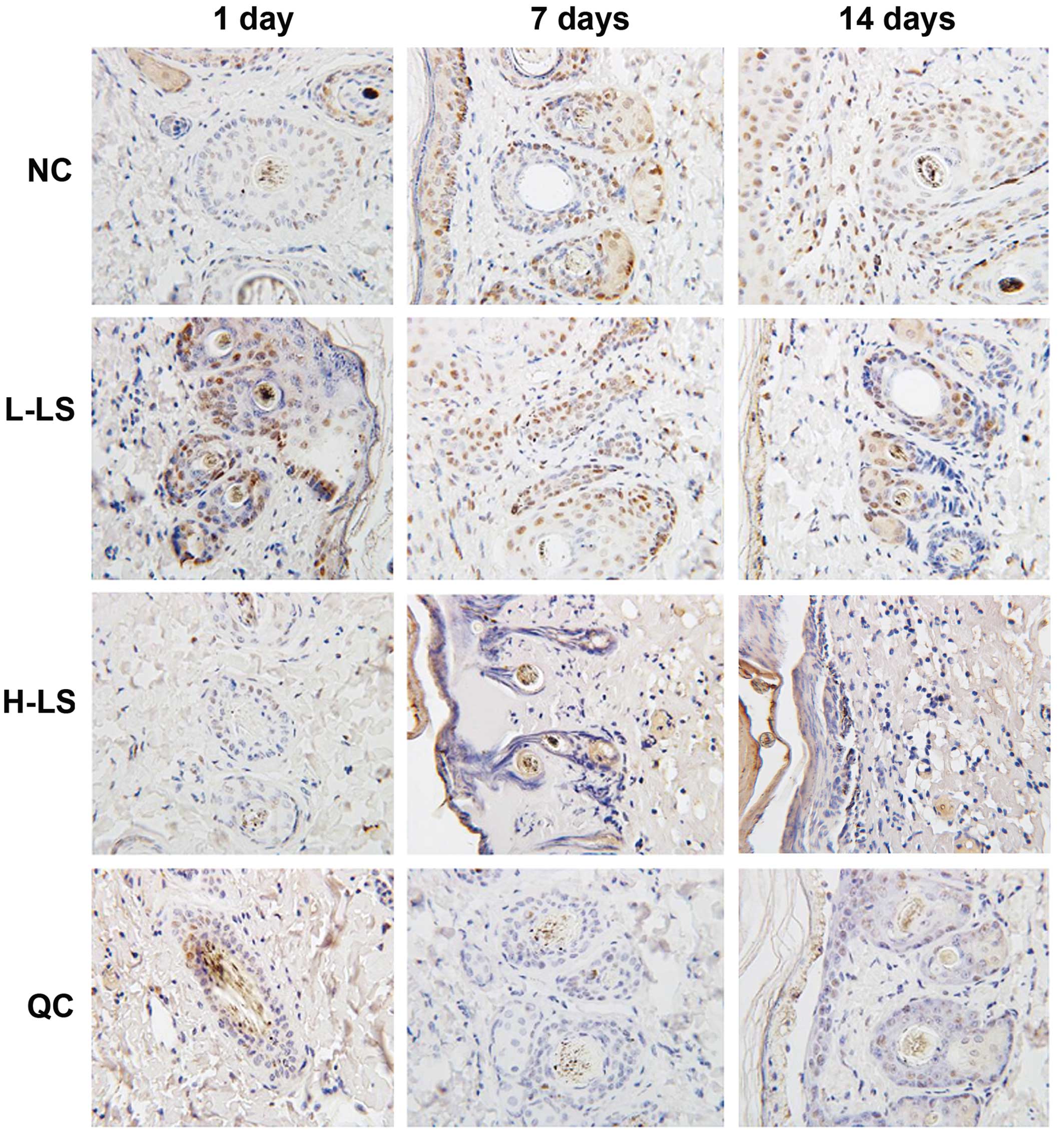
Immunohistochemistry
The expression levels of PCNA of the L-LS, H-LS and
QC groups were reduced with time. At the respective time points,
the NC group was highest, L-LS group and H-LS group were next
highest and the H-LSIup was lowest. The difference was
statistically significant (P<0.05) (Table I and Fig.
3).
 | Table I.Comparison of expression levels of
PCNA (%). |
Table I.
Comparison of expression levels of
PCNA (%).
| Groups | 1 day | 7 days | 14 days |
|---|
| NC | 32.6±5.5 | 33.5±5.4 | 32.7±5.3 |
| L-LS | 25.5±6.3 | 23.2±6.2 | 21.7±6.0 |
| H-LS | 11.2±4.7 | 8.6±4.2 | 6.3±4.3 |
| QC | 15.4±4.5 | 14.3±4.6 | 12.8±4.8 |
| F-value | 12.304 | 13.655 | 15.234 |
| P-value | <0.001 | <0.001 | <0.001 |
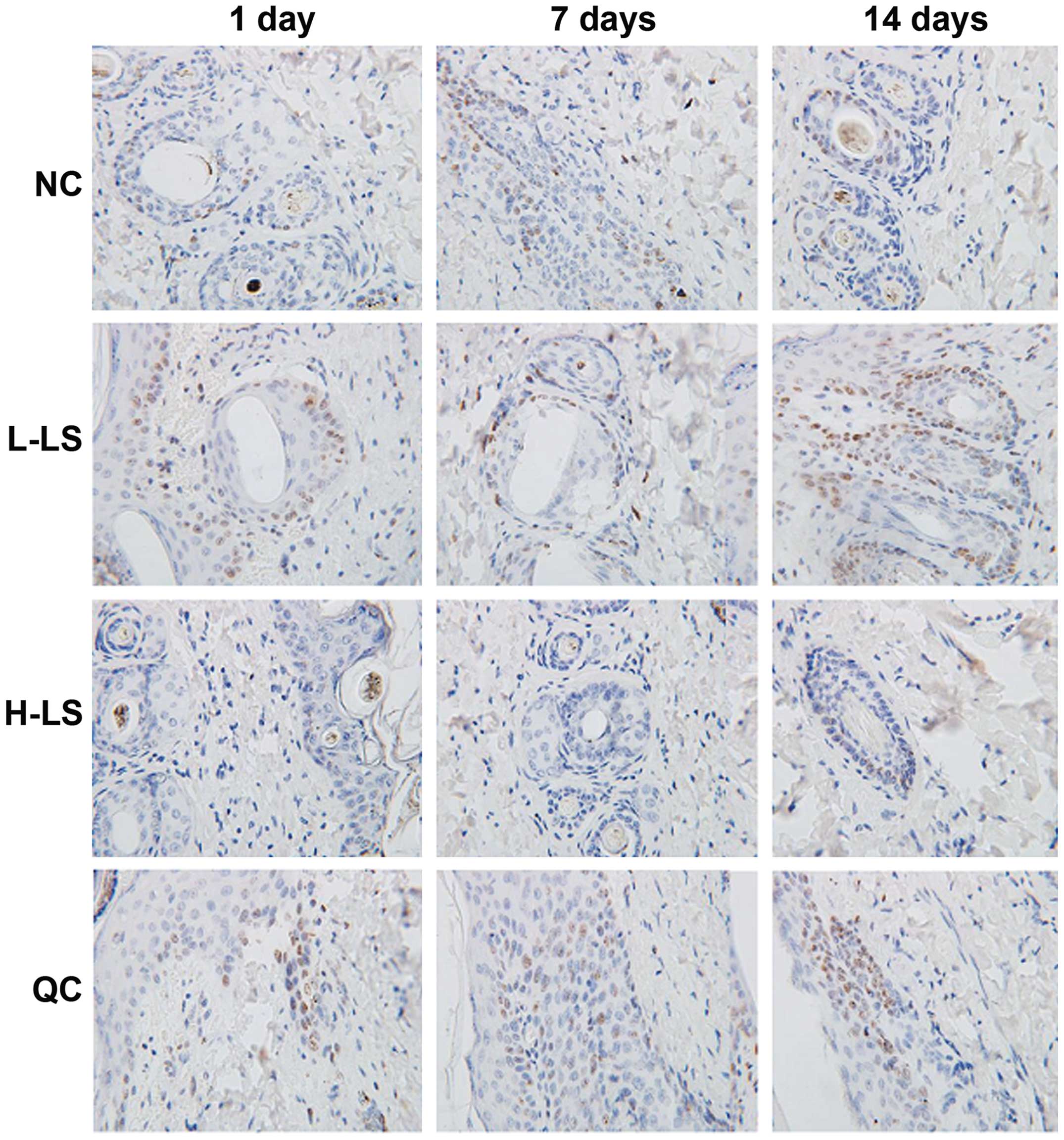
TUNEL method result
The apoptotic rate of the L-LS, H-LS and QC groups
increased with time extension. At the different time points, NC
group was lowest, the L-LS group and QC group was next and the H-LS
group was highest, with the difference being statistically
significant (P<0.05) (Table II
and Fig. 4).
 | Table II.Comparison of apoptotic rate. |
Table II.
Comparison of apoptotic rate.
| Groups | 1 day | 7 days | 14 days |
|---|
| NC | 0.5±0.1 | 0.4±0.1 | 0.6±0.2 |
| L-LS | 6.7±1.2 | 7.6±1.3 | 8.2±1.5 |
| H-LS | 13.6±3.4 | 15.8±3.5 | 17.2±3.6 |
| QC | 8.2±2.0 | 9.3±2.2 | 10.2±2.3 |
| F-value | 10.235 | 11.527 | 14.520 |
| P-value | <0.001 | <0.001 | <0.001 |
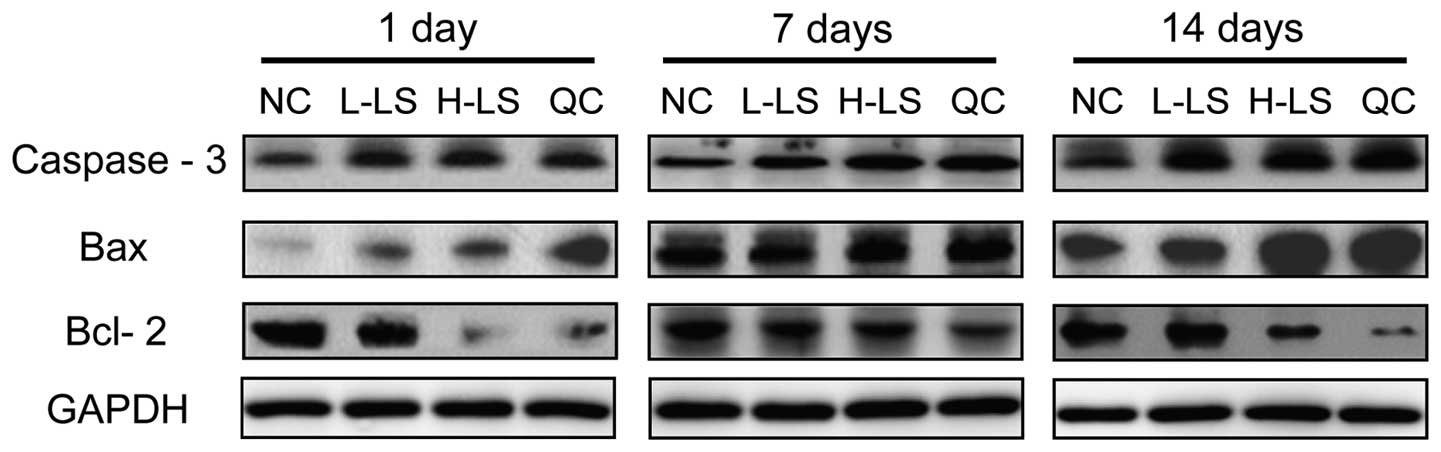
Western blot analysis
The protein expression level of caspase-3, Bax and
Bcl-2 of the L-LS, H-LS and QC groups increased with time. At the
respective time points, caspase-3 and Bax protein expression in the
NC group was lowest, the L-LS and QC groups were next lowest and
the H-LS group was highest. Bcl-2 protein expression in the NC
group was highest, the protein expression in the NC group was next
highest and the H-LS group was lowest. The difference was
statistically significant (P<0.05) (Table III and Fig. 5).
 | Table III.Protein expression level of caspase-3,
Bax and Bcl-2. |
Table III.
Protein expression level of caspase-3,
Bax and Bcl-2.
| Groups | Caspase-3 | Bax | Bcl-2 |
|---|
|
|
|
|
|
|---|
|
| 1 day | 7 days | 14 days | 1 day | 7 days | 14 days | 1 day | 7 days | 14 days |
|---|
| NC | 0.21±0.06 | 0.22±0.05 | 0.20±0.07 | 0.15±0.04 | 0.16±0.03 | 0.14±0.05 | 0.23±0.08 | 0.24±0.06 | 0.23±0.07 |
| L-LS | 0.42±0.10 | 0.46±0.12 | 0.50±0.14 | 0.36±0.10 | 0.40±0.13 | 0.43±0.11 | 0.16±0.07 | 0.18±0.06 | 0.20±0.09 |
| H-LS | 0.47±0.13 | 0.50±0.16 | 0.55±0.18 | 0.42±0.14 | 0.46±0.13 | 0.49±0.16 | 0.10±0.03 | 0.12±0.04 | 0.14±0.04 |
| QC | 0.44±0.20 | 0.48±0.21 | 0.52±0.24 | 0.40±0.21 | 0.43±0.22 | 0.45±0.23 | 0.14±0.05 | 0.16±0.06 | 0.18±0.05 |
| F-value | 8.632 | 9.203 | 9.624 | 9.032 | 9.421 | 9.637 | 9.562 | 9.624 | 9.758 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Discussion
The 800 nm semiconductor laser can be used in the
treatment of vascular diseases, such as nevus flammeus, and for
non-vascular diseases, such as virus infection, sebaceous gland
hyperplasia and stretch marks (7,8).
Evidence has confirmed that it has an effect on immunity
activation, acne formation reduction and hair follicle wall
maturity intervention (9). The
semiconductor laser takes advantage of many types of biological
effects on skin hair follicle tissue, such as thermal effects,
pressure effects, photochemical effects, light stimulation and
electromagnetic fields, leading to the release of cytokines and the
production of collagen (10). From
animal models, it has been found that semiconductor lasers can
significantly reduce the number of facial acne propionic acid
bacillus, with grease secretion decreasing significantly.
Additionally, the cytokines IL-1α receptor, TNF-α, melanocortin
receptor 1 and TGF-β1 can be reduced in tissues, so as to achieve
the effect of treating diseases (11).
A recent study also confirmed that semiconductor
lasers can induce the apoptosis of the hair follicles, which may be
associated with the therapeutic effect of the lasers (12). PCNA is the core element of the
eukaryotes replication complex, a driving factor in DNA polymerase
δ, which can bind with different replication-associated proteins
and coordinate the DNA replication process (13). As a factor of function conversion,
PCNA is involved in important cell events, such as DNA damage
repair, cell cycle control and apoptosis by different control
methods to interact with many cytokines (14). It is known that apoptosis may have
three main signal transduction pathways: Death receptor pathways of
apoptosis, mitochondrial pathways, and control pathways on which
the p53 gene depends. The p53 gene is a type of
tumor-inhibiting protein that is expressed by the
control-associated gene to induce apoptosis (15). The tumor-inhibiting factor in G1
contains the PIP box, which can promote apoptosis by interacting
with p53. Experimental results showed that ultraviolet rays can
promote the combination of ING1 and PCNA, and that ultraviolet ray
damage cell may be removed by apoptosis (15). Caspase-3 is a factor in performing
apoptosis, which can cause a cascade amplification effect of
downstream enzymes once triggered. Its excitation can lead
apoptosis to an apoptosis stage that is related to nuclear change
and is the most important downstream effect of protease (16). For the two signal transduction
pathways of conventional evolution in apoptosis, the component
ratio of Bcl-2 family members is the key factor of apoptosis
regulation, especially the Bcl-2/Bax ratio in the ‘molecular
switch’ that can trigger apoptosis (17). Bax and Bcl-2 regulate apoptosis by
forming homodimer or heterodimer, when Bax forms homodimer to
induce apoptosis, Bax and Bcl-2 may form a heterodimer to inhibit
apoptosis.
From the present study, we found that the expression
levels of PCNA of the L-LS, H-LS and QC groups reduced with time.
At the respective time points, the NC group was highest, the L-LS
and H-LS groups were next highest and the H-LS group was lowest.
The difference was statistically significant (P<0.05). It
suggested that low-dose laser treatment causes less damage to the
number of PCNA, which is beneficial in the recovery of hair
follicle regeneration capacity (18). The apoptotic rate of the L-LS, H-LS
and QC groups increased with time. At the respective time points,
the NC group was lowest, the L-LS and QC groups were next lowest
and the H-LS group was highest. The difference was statistically
significant (P<0.05). It suggested that low-dose laser treatment
can promote the apoptosis of hair follicle cells to some extent
(19). The protein expression of
caspase-3, Bax and Bcl-2 in the L-LS, H-LS and QC groups increased
with time. At the respective time points, caspase-3 and Bax protein
expression in the NC group was lowest, the L-LS and QC groups were
next lowest and the H-LS group was highest. Bcl-2 protein
expression in the NC group was highest, protein expression in the
NC group was in next highest and the H-LS group was lowest. The
difference was statistically significant (P<0.05).
In conclusion, the low-dose 800 nm semiconductor
laser is an effective treatment on skin blackheads and coarse
pores. It promotes hair follicle cell apoptosis without reducing
the expression of PCNA.
References
|
1
|
Ashique KT and Srinivas CR: Pen punching:
an innovative technique for comedone extraction from the well of
the concha. J Am Acad Dermatol. 73:e1772015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Handler MZ, Bloom BS and Goldberg DJ:
Energy-based devices in treatment of acne vulgaris. Dermatol Surg.
42:573–585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keyal U, Bhatta AK and Wang XL:
Photodynamic therapy for the treatment of different severity of
acne: a systematic review. Photodiagn Photodyn Ther. 14:191–199.
2016. View Article : Google Scholar
|
|
4
|
Demirci GT, Mansur AT and Gulec AT:
Comedones induced by vascular laser therapy. J Cutan Aesthet Surg.
9:38–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SJ, Seok J, Jeong SY, Park KY, Li K
and Seo SJ: Facial pores: definition, causes, and treatment
options. Dermatol Surg. 42:277–285. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levin MK, Ng E, Bae YS, Brauer JA and
Geronemus RG: Treatment of pigmentary disorders in patients with
skin of color with a novel 755 nm picosecond, Q-switched ruby, and
Q-switched Nd:YAG nanosecond lasers: a retrospective photographic
review. Lasers Surg Med. 48:181–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lapchak PA, Boitano PD, Butte PV, Fisher
DJ, Hölscher T, Ley EJ, Nuño M, Voie AH and Rajput PS: Transcranial
near-infrared laser transmission (NILT) profiles (800 nm):
systematic comparison in four common research species. PLoS One.
10:e01275802015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lekakh O, Mahoney AM, Novice K, Kamalpour
J, Sadeghian A, Mondo D, Kalnicky C, Guo R, Peterson A and Tung R:
Treatment of acne vulgaris with salicylic acid chemical peel and
pulsed dye laser: a split face, rater-blinded, randomized
controlled trial. J Lasers Med Sci. 6:167–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morton LM: The evolution of laser surgery
for acne and other scarring processes. Semin Cutan Med Surg.
33:169–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Been MJ and Mangat DS: Laser and face peel
procedures in non-Caucasians. Facial Plast Surg Clin North Am.
22:447–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Avci P, Gupta A, Sadasivam M, Vecchio D,
Pam Z, Pam N and Hamblin MR: Low-level laser (light) therapy (LLLT)
in skin: stimulating, healing, restoring. Semin Cutan Med Surg.
32:41–52. 2013.PubMed/NCBI
|
|
12
|
da Silva Neto Trajano L Alexsandra, da
Silva CL, de Carvalho SN, Cortez E, Mencalha AL, de Souza da
Fonseca A and Stumbo AC: Cell viability, reactive oxygen species,
apoptosis, and necrosis in myoblast cultures exposed to low-level
infrared laser. Lasers Med Sci. 31:841–848. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khalil MI, Ibrahim MM, El-Gaaly GA and
Sultan AS: Trigonella foenum (fenugreek) induced apoptosis in
hepatocellular carcinoma cell line, HepG2, mediated by upregulation
of p53 and proliferating cell nuclear antigen. Biomed Res Int.
2015.914645PubMed/NCBI
|
|
14
|
Melo RM, Martins YS, Luz RK, Rizzo E and
Bazzoli N: PCNA and apoptosis during post-spawning ovarian
remodeling in the teleost Oreochromis niloticus. Tissue Cell.
47:541–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li DW, Li GR, Zhang BL, Feng JJ and Zhao
H: Damage to dopaminergic neurons is mediated by proliferating cell
nuclear antigen through the p53 pathway under conditions of
oxidative stress in a cell model of Parkinson's disease. Int J Mol
Med. 37:429–435. 2016.PubMed/NCBI
|
|
16
|
Lossi L, Cocito C, Alasia S and Merighi A:
Ex vivo imaging of active caspase 3 by a FRET-based molecular probe
demonstrates the cellular dynamics and localization of the protease
in cerebellar granule cells and its regulation by the
apoptosis-inhibiting protein survivin. Mol Neurodegener. 11:342016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duan P, Hu C, Butler HJ, Quan C, Chen W,
Huang W, Tang S, Zhou W, Yuan M, Shi Y, et al: 4-Nonylphenol
induces disruption of spermatogenesis associated with oxidative
stress-related apoptosis by targeting p53-Bcl-2/Bax-Fas/FasL
signaling. Environ Toxicol. 18:14–15. 2016.
|
|
18
|
Tsai WC, Cheng JW, Chen JL, Chen CY, Chang
HN, Liao YH, Lin MS and Pang JH: Low-level laser irradiation
stimulates tenocyte proliferation in association with increased NO
synthesis and upregulation of PCNA and cyclins. Lasers Med Sci.
29:1377–1384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mun S, Cheon M, Kim SH, Choi N, Kim S, Yoo
Y and Lim S: The effect of laser diode irradiation on wound healing
of rat skin. J Cosmet Laser Ther. 15:318–325. 2013. View Article : Google Scholar : PubMed/NCBI
|