Introduction
Diabetes mellitus causes significant changes in
socioeconomic life and its prevalence is on the increase every
year. China has become the country with the highest number of
diabetic cases (1). Several studies
have shown that regular exercise can improve glycemic control and
increase insulin sensitivity in the patients with diabetes mellitus
(DM), along with reduction in body weight, control blood lipid and
blood pressure and risk of macrovascular and microvascular
complications (2). Out of the
different significant treatment methods, exercise has been given
increased attention (3).
Chronotropic response (CR) defined as the function
of the heart to increase its rate commensurate with the rise of the
body's metabolic demand during exercise. Not reaching a certain
extent is termed chronotropic incompetence (CI), which is also
known as an independent predictor of incidence of severe
cardiovascular events, and the all-cause mortality (4). It has been indicated that CI was
closely related to the impairment of cardiac autonomic nervous
function (5). Cardiac autonomic
nerves refer to the sympathetic and parasympathetic nerves that
administer circulation system including heart, lung and peripheral
blood vessels. Due to physiological or pathological factors, the
balance of coordination and confrontation is changed, which is
described as cardiovascular autonomic neuropathy (CAN) (6). It has been found that CAN, which can
cause painless myocardial infarction and sudden death, is one of
the important reasons for the increased mortality of type 2 DM
(T2DM) patients, even at the initial stage of T2DM (7).
In the present study, heart rate reserve rate
(HRRes) and heart rate value (rHR) were selected as the evaluation
criteria (4): i) rHR: The ratio of
maximum heart rate in exercise to predict maximum heart rate,
<85% was abnormal; and ii) HRRes: Heart rate reserve rate,
<80% was abnormal.
Heart rate recovery (HRR) refers to the difference
of peak heart rate (HRpeak) in exercise testing and heart rate
during convalescence after exercise ceased, is one of the common
indicators for assessing cardiac autonomic nerve function, thus
some scholars regarded it as the other evaluating indicator of CR
(8). In the past, the studies of HRR
mainly focused on coronary heart disease, heart failure and other
fields (9), minority of research was
on T2DM patients. Vagus nerve lesions mainly occur in the early
stage in T2DM patients, while abnormal HRR after exercise was
directly related to vagal nerve activity (4), which was a sign of reduced vagal
activity (10) also as independent
predictor of incidence of future cardiovascular events and
mortality in T2DM patients (11).
While there are various standards of cut-off point for assessing
abnormal HRR, the most generally accepted and employed by
specialists worldwide (4) was slow
walking after exercise testing, 1 min of HRR of <18 beats/min
(bmp) or 2 min of recovery of <42 bmp. If special circumstances
can be discovered, observation time could be extended until
abnormal manifestations or symptoms have disappeared. For general
observation for 6 min after test termination, this study may record
HRR of 1–6 min after movement as HRR1–6.
Exercise capacity directly reflects the level of
individual health status and self-care ability. During exercise,
the oxygen requirement of the body increases while the oxygen
uptake of the cells increases correspondingly. When the amount of
exercise raises to a certain degree, cell uptake of oxygen will
reach a plateau with no further increase i.e., to achieve the
maximal oxygen uptake (peak VO2), which reflects the
human maximum aerobic capacity and cardiopulmonary reserve
capacity. However, due to differences in body weight, peak
VO2 also has difference even if completed with the same
power load. So this study used peak VO2/kg to measure
the exercise ability of an individual (12).
A previous study on exercise therapy focused on the
control of blood glucose, blood pressure and the improvement of
metabolic parameters and body composition (13). However, to the best of our knowledge,
few studies have shown the effects of exercise therapy on CR, HRR
and exercise capacity in patients with T2DM. In the present study,
CR, HRR1–6 and the exercise capacity of T2DM patients
were determined by symptom-limited cardiopulmonary exercise testing
(CPET). The patients were given a 12-week exercise therapy
according to individual exercise prescription as formulated. The
impact of exercise training on the improvement of CI, and
regulation of the function of cardiac autonomic nerves and exercise
capability were investigated, exploring the possible mechanism, in
order to find new evidence of the mediation of individual exercise
treatment on CR, HRR of T2DM patients.
Patients and methods
Patients observation
Of the patients receiving cardiopulmonary
rehabilitation exercise therapy in the Center Hospital and
Rehabilitation Hospital of Xuzhou from October 2014 to October
2015, 30 cases were diagnosed with T2DM. The average age was
59.43±7.81 years, with 17 males and 13 females. There were 10 cases
with coronary heart disease and 7 cases with hypertension. All of
the patients were treated conventionally as before.
Inclusion criteria were as follows: Patients with a
clear history of T2DM, the diagnostic criteria of T2D references to
the Standards of Medical Care in Diabetes - 2010 of ADA (14); age 40–75 years; middle school
education level or higher; to be conscious and stable to cooperate
with the inspection; patients with no regular exercise history; no
use of β-blockers, atropine, nitrates and antiarrhythmic drugs in
48 h. All the patients signed informed consent.
Exclusion criteria were, patients with psychosis and
dyscinesia; patients with serious heart, brain, kidney disease not
yet stabilized; patients with non-sinus rhythm, atrial
fibrillation, atrial flutter, the conduction block and ventricular
rhythmia patients; cases with infection, acute metabolic disorders,
diabetic foot, diabetic retinopathy and other serious complications
of DM; patients with systolic blood pressure (SBP) l,200 mmHg
and/or diastolic blood pressure (DBP) ≥≥Pod pr (15) (1 mmHg=0.133 kPa), blood glucose
before exercising, diab/l or Hg=0.1/l (15); and poor adherence to exercise
therapy.
Methods
All subjects underwent symptom-limited CPET, with
K4b2 exercise cardiopulmonary function test and metabolic analysis
system from Cosmed (Rome, Italy) and CASE treadmill exercise
machine from GE Healthcare (Piscataway, NJ, USA). To formulate
individualized exercise prescription on the basis of test results
and to require a 12-week exercise training, equipment from Welch
Allyn Inc. (Skaneateles Falls, NY, USA), telemetric
electrocardiogram (ECG) monitor, finger pulse oximeter and related
fitness equipment from our department was used. The repeated
measure by symptom-limited CPET was undertaken 12 weeks later.
All subjects received symptom-limited
CPET
Exercise programs used were the modified Bruce
grading program. The termination criteria for exercise test was
according to the ACMS (15): i)
Patient requirements; ii) with the raise of exercise load, SBP has
decreased 10 mmHg comparing to baseline, accompanied with other
ischemic evidence; iii) the occurrence of moderate to severe
angina; iv) nervous system symptoms such as ataxia, dizziness and
close to syncope; v) signs of hypoperfusion: cyanosis, pale; vi)
serious arrhythmias: ventricular tachycardia, ventricular flutter
and ventricular fibrillation; vii) ST-segment of severe depression
in oblique type or horizontal type depressed ≥2 mm or ST-segment
elevation ≥Tmm; and viii) monitoring blood pressure difficult or
not reaching the target heart rate.
Before exercise, we measured and recorded blood
pressure, HRrest (bmp), resting ECG. In the test, blood pressure,
ECG, heart rate at the end of each grade, HRpeak and peak
VO2/kg (ml/kg/min) were recorded as required. The ECG
was recorded until 6 min after exercise or ST-segment returned to
resting state by the end. The following formulas were used to
calculate rhR, HRRes, HRR1–6:
rHR=(HRpeak)/(220–age)x100%HRRes=(HRpeak–HRrest)/(220–age–HRrest)x100%
The patients continued to walk slowly after the
exercise test was completed. While HRR1–6 refers to an
absolute difference between heart rate reaching peak during
exercise and heart rate at 1, 2, 3, 4, 5 and 6 min after movement
cessation, namely HRR value at each time-point by the end of
exercise test.
Individual exercise prescription was formulated.
According to Chinese Guidelines of Exercise Therapy in Diabetes
Mellitus (2) and exercise test and
exercise prescription guidelines by American College of Sports
Medicine to formulate individual exercise prescription. The general
principle of individual exercise prescription formulation is as
follows: (needs to be adjusted according to the individual
situation). i) Exercise mode: Aerobic exercise: Brisk walking,
bicycle; resistance movement: Elastic band resistance training,
fluid resistance movement; flexibility: Stretching of muscles or
ligaments; balance movement: Single foot standing, throwing and
catching a ball. ii) Movement time: Exercise time 30–60 min, the
duration of the effective heart rate reaching at least 20–30 min,
not including the warm-up before exercise and finishing after
exercise. iii) Exercise intensity: Heart rate reserve method.
Combining with perceived exertion (RPE) scale, peak
VO2/kg, that is, to achieve the RPE 13–14 level or the
selected exercise according to the intensity of 50–60% peak
VO2/kg. Target heart rate = (HRpeak - HRrest) ×
intensity% + HRrest. iv) Exercise frequency: 3–7 times/week. If the
amount of exercise each time is greater, it can be spaced over one
or two days, if the amount of each exercise is less and the body
allows, then adhere to 7 times/week.
Implementation of exercise
therapy
According to the individual situation of patients to
decide to wear telemetric ECG monitor or finger pulse oximeter and
to comply with exercise prescriptions. The rating of RPE has
reached 13–14 grades in the process of movement. Before and after
exercise test, blood glucose, blood pressure, and heart rate were
determined and attention was given to observe ECG, blood pressure
response and discomfort during movement.
To complete repeated symptom-limited
CEPT, calculation and statistics of indexes
The implementation methods of symptom-limited CPET
and the calculation of each index were the same as above. The
relevant physical parameters and biochemical indicators were
arranged as statistical information.
Statistical analysis
Data were entered into the database, using SPSS
statistical analysis software (SPSS, Inc., Chicago, IL, USA).
Quantitative data were expressed as mean ± SD, the income data were
under normality and homogeneity of variance test. According to the
data characteristics, in line with the conditions or data
conversion according with the conditions, before and after the
treatment itself compared using paired samples t-test. Pearson
correlation analysis was used for correlation analysis, if not
meeting the conditions the non-parametric test was selected,
correlation analysis was used by Spearman correlation analysis.
α=0.05 was regarded as test level, P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of body parameters and
biochemical indexes before and after treatment
After treatment, BMI, abdominal girth, FPG and HbAlc
were lower than pre-treatment. The difference was statistically
significant (P<0.01) (Table
I).
 | Table I.Changes of body parameters and
biochemical indexes before and after treatment. |
Table I.
Changes of body parameters and
biochemical indexes before and after treatment.
| Index | Before | After |
|---|
| BMI
(kg/m2) | 25.55±3.17 |
24.56±2.37b |
| Abdominal girth
(cm) | 93.10±9.74 |
91.65±9.23b |
| FPG (mmol/l) | 9.41±4.11 |
8.06±2.15a |
| HbAlc (%) | 8.25±1.78 |
7.45±1.07a |
Comparison of CR and HRR index before
and after treatment
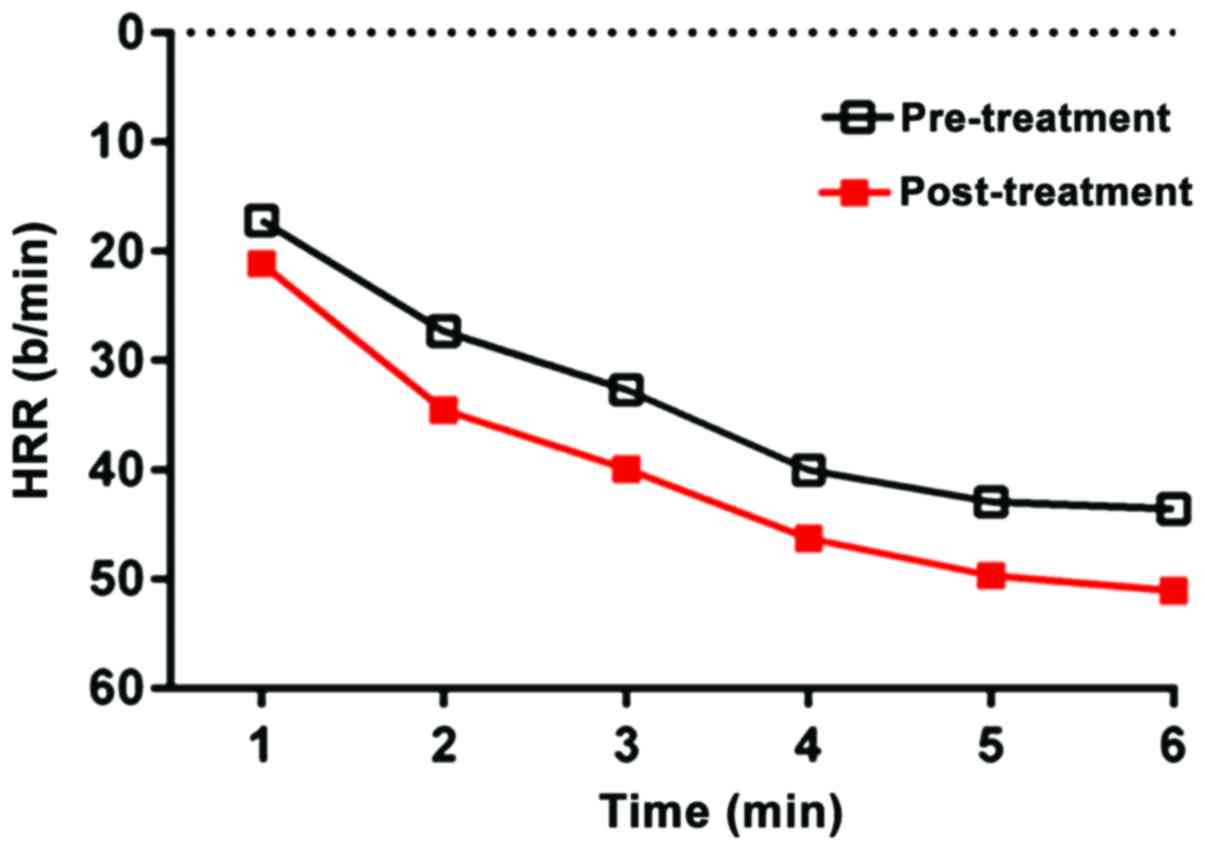
Following treatment, HRrest was decreased
significantly, whereas HRpeak, rHR, HRRes and HRR1–6
increased significantly (P<0.05) (Table II). The exercise test was completed
before and after treatment, and the 1–6 min HRR curve diagram is
shown in Fig. 1.
 | Table II.Comparison of CR, HRR1–6
before and after treatment. |
Table II.
Comparison of CR, HRR1–6
before and after treatment.
| Index | Before | After |
|---|
| HRrest (bmp) | 74.37±8.40 |
69.77±5.91b |
| HRpeak (bmp) | 129.23±16.32 |
140.50±13.41b |
| rHR | 0.81±0.11 |
0.88±0.07b |
| HRRes | 0.65±0.18 |
0.77±0.13b |
| HRR1
(bmp) | 17.17±7.98 |
20.47±9.01a |
| HRR2
(bmp) | 27.33±10.18 |
34.20±10.62a |
| HRR3
(bmp) | 32.67±9.86 |
39.93±11.49a |
| HRR4
(bmp) | 40.03±12.96 |
46.43±14.26a |
| HRR5
(bmp) | 42.90±13.18 |
49.93±13.58a |
| HRR6
(bmp) | 43.57±12.01 |
51.10±13.05a |
Comparison and correlation analysis of
exercise capacity indexes before and after treatment
After treatment, exercise time, peak
VO2/kg and peak VO2/kg were significantly
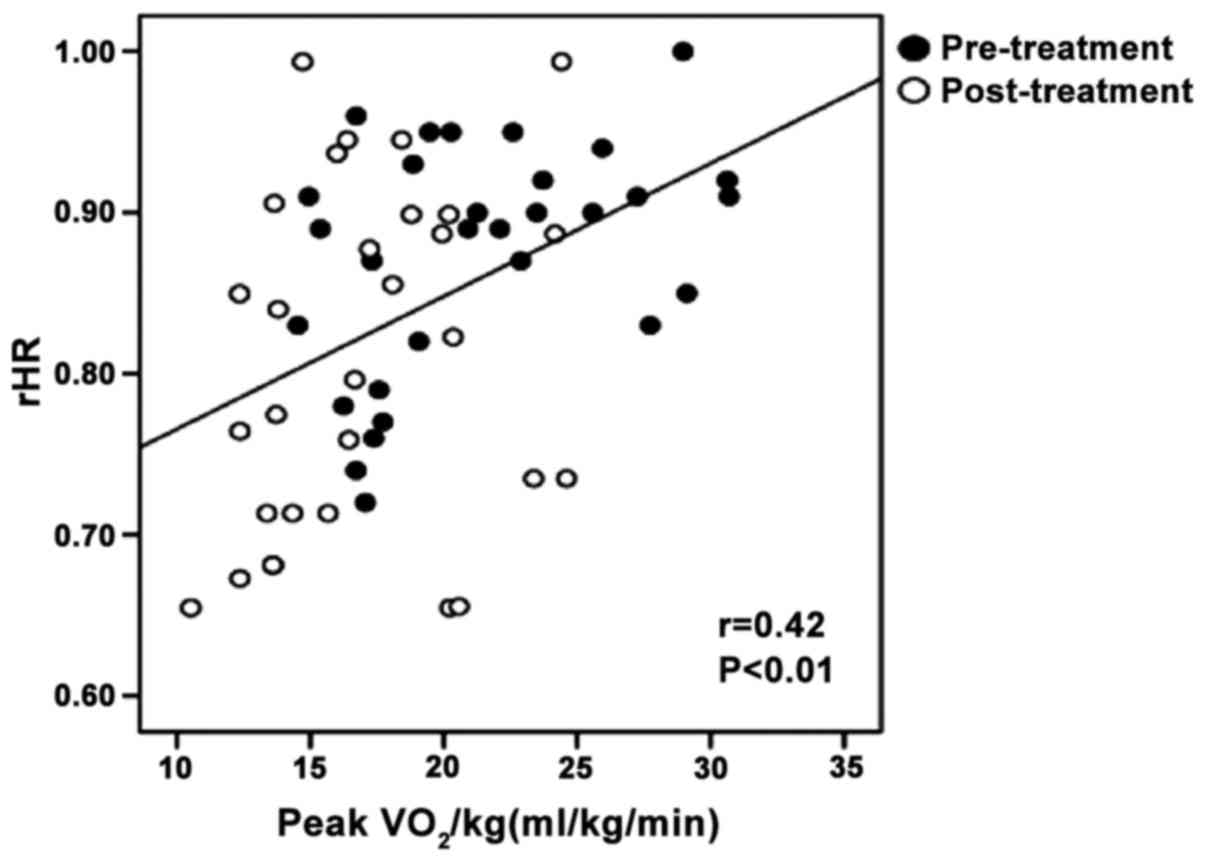
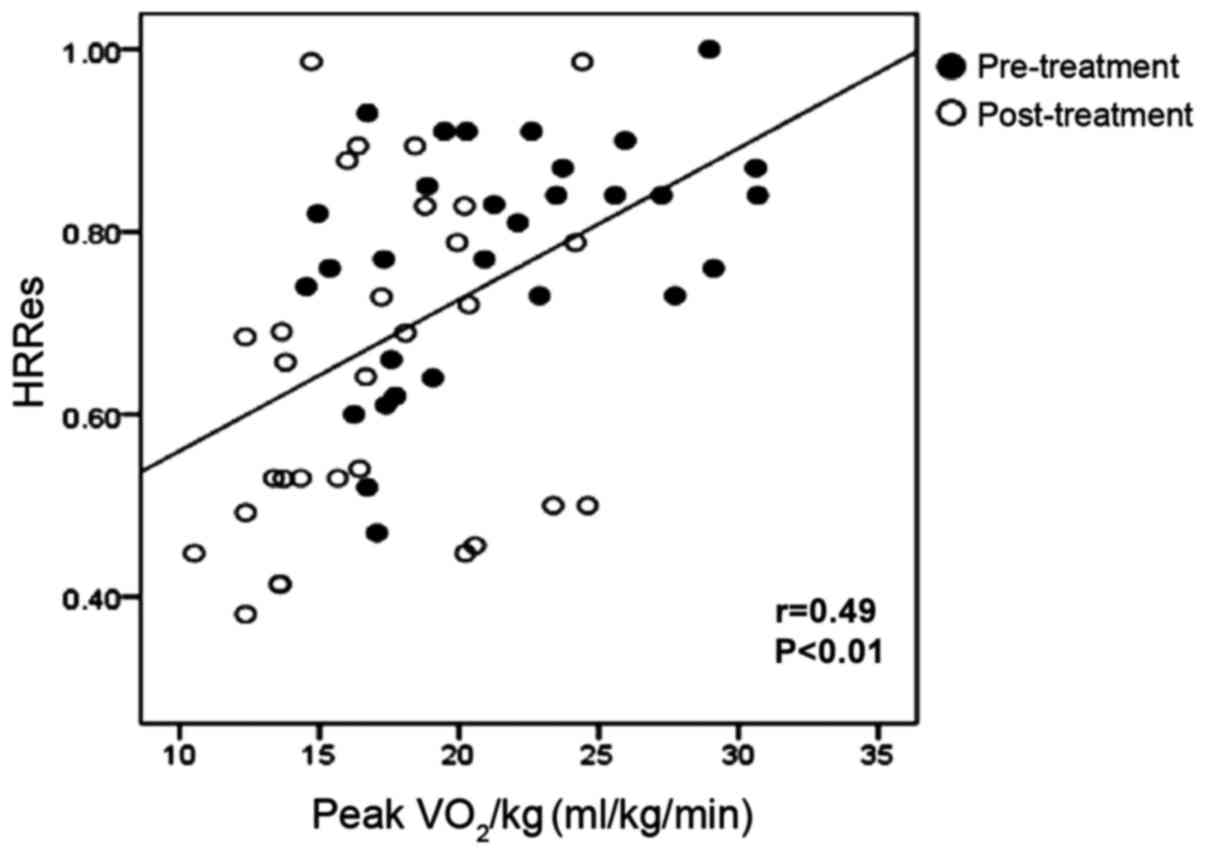
higher than pre-treatment (P<0.01) (Table III). Pearson correlation analysis
showed that Peak VO2/kg was positively correlated with
rHR, HRRes (r-value: 0.42 and 0.49, both P<0.01) (Figs. 2 and 3).
 | Table III.Comparison of exercise capacity
before and after treatment. |
Table III.
Comparison of exercise capacity
before and after treatment.
| Index | Before | After |
|---|
| Exercise time
(sec) | 703.10±196.47 |
873.80±177.79a |
| Peak
VO2/kg (ml/kg/min) | 16.99±3.96 |
21.40±4.94a |
| Peak
VO2/kg pred (%) | 70.97±11.82 |
89.17±13.68a |
Discussion
The heart rate at any time-point responses affects
the dynamic balance of distribution of the sympathetic nerve and
vagal tone in the autonomic nervous system. Under resting
conditions, heart rate receives double regulation of sympathetic
nerve and vagus nerve, predominantly vagus nerve (3). The sympathetic tone increased and/or
vagal tone decreased causing the heart rate to be adjusted to the
normal heart rate with a decrease of physical activity, which
showed the increase of HRrest. At the initial stage of exercise,
the vagus nerve was inhibited and the heart rate increased rapidly.
Sympathetic nerve excitability reached a peak in a few seconds.
After that, the heart rate increased slowly with the increase of
the concentration of catecholamine in the blood. The heart rate
during exercise could not be increased with the demands of the body
metabolism, or could not maintain stability in the performance of
CI (16). At the completion of
exercise with sympathetic nerve tension retreat and vagal
activation, a rapid decrease of heart rate, especially in the 30
sec after termination was induced (17). HRR of healthy adults after exercise
can be classified as the following three stages (18): i) Fast recovery period with short
duration, the activity of vagus nerve predominated just at the end
of movement; ii) then entered the slow recovery phase with longer
duration, performance for the sympathetic nerve activity recovered
gradually; and iii) accessed to the stable period with slight
fluctuation in the end, the sympathetic nervous tension and the
tension of the vagus nerve are in a relatively balanced state. The
HRR will slow down also the time will prolong if regulation is
impaired.
It has been found that the CI index in T2DM patients
with no metabolic syndrome was significantly lower than that of
patients with metabolic syndrome (19). In addition, autonomic nerve
dysfunction dominated by the sympathetic and vagal nerve exists in
T2DM patients with no cardiovascular disease (20,21). A
study of 1,341 T2DM patients showed that 35.7% with CI during the
exercise test and long-term follow-up showed that it was closely
related to the malignant endpoint events (22). In another study, 2,123 men (mean age,
47±6 years) underwent a complete health examination, and all
subjects in the first examination were excluded from coronary heart
disease and T2DM. After 5 years, the subjects underwent a secondary
check, which revealed 137 (4.4%) subjects suffering from T2DM
(23). The results suggested that CI
was associated with the incidence of T2DM during test in a healthy
male population, and was independent of other risk factors.
The delay of HRR is closely related to the incidence
of DM (24). A study on a group of
male T2DM patients with a follow-up lasting 14.9 years showed that
the HRR delay is an independent predictor of cardiovascular disease
and the occurrence of all-cause of death (24,25). HRR
has a certain correlation with myocardial ischemia in patients with
T2DM (26), showing that abnormal
HRR may decrease the exercise capacity of patients in exercise test
and it is related to the increase of all-cause mortality and
cardiovascular events risk (27).
For the selected 30 patients of T2DM before exercise
therapy, rHR (0.81±0.11), HRRes (0.65±0.18), HRR1
(17.17±7.98 bmp), and HRR2 (27.33±10.18 bmp) were
significantly lower than normal. The results are in agreement with
data from other reports showing abnormal CI, and HRR exist widely
in T2DM patients (25,26). Therefore, the treatment of CI,
abnormal HRR and other related manifestations which were caused by
autonomic nerve dysfunction in patients with T2DM is imminent.
By selecting coronary heart disease, hypertension,
T2D patients as the subjects, some studies have found short-term
individualized rehabilitation exercise on cardiac autonomic nerve
dysfunction with immediate effect and improvement of
parasympathetic nerve function was better than that of sympathetic
nerve function in exercise (28). In
a previous study, 4,503 T2DM patients were randomly divided into
the exercise and control groups, with the former being offered
lifestyle intervention and training, and the latter being given
conventional treatment and education (29). One year later, the results showed
that the exercise group lost weight, HRrest decreased, HRRes
increased, with an acceleration in HRR and the reduction in weight
and exercise capacity upgrade were independent influencing factors
for HRR improvement. By contrast, in the control group, these
indexes had no obvious change, thus HRR can be an important
indicator in assessing cardiovascular risk (29). Our study found that after 12 weeks of
exercise therapy, HRrest (74.37±8.40 vs. 69.77±5.91 bmp) decreased,
HRpeak (129.23±16.32 vs. 140.50±13.41 bmp), rHR (0.81±0.11 vs.
0.88±0.07) and HRRes (0.65±0.18 vs. 0.77±0.13) increased, and HRR
(HRR1: 17.17±7.98 vs. 20.47±9.01 bmp, HRR2:
27.33±10.18 vs. 34.20±10.62 bmp) were significantly higher than
before. Our results also showed that exercise can improve cardiac
autonomic nerve balance and regulation in T2DM patients. The vagus
nerve retreat in exercise early stage and full activation of the
sympathetic nerve activity in the later period of exercise leads to
an increase in heart rate. Additionally, sympathetic rapid retreat
resulted in a decrease in heart rate and served as a protective
mechanism for the heart and a fulfillment for the body's metabolic
demand.
T2DM is considered to be a disease of the lack of
exercise (body inertia) (30), with
more than 80% of T2DM being related to obesity and body inertia.
The study found that low exercise tolerance is an independent risk
factor for mortality in patients with T2DM (31). Since the 1960s, with the development
of rehabilitation medicine and the performance of rehabilitation
training for cardiovascular disease, exercise prescription began to
be valued. The Chinese Guideline for Exercise in Diabetes (12) recommended that the main content of
the exercise prescription includes exercise program, exercise
intensity, exercise timing, exercise duration and exercise
frequency. In addition, the exercise intensity is the core of
exercise prescription and each time of training should consists of
three parts, including preparation, basic activities and finishing
part.
Exercise could not only improve the risk factors of
diabetes attacks, glucose tolerance, fasting glucose damaged state,
prevent the occurrence of complications, the same can enhance
exercise capacity in patients with T2DM, improve complication
process and prognosis (12). The
exercise capacity directly reflects the level of individual health
status and self-care ability. Our investigation found that after 12
weeks of exercise training, with exercise test time was prolonged,
peak VO2/kg and peak VO2/kg pred increased,
suggesting that the exercise capacity of patients may be enhanced.
Previous findings have shown that peak VO2/kg in
patients with T2DM increased after exercise training may due to
strengthening of the major muscle groups caused by addition in the
uptake of oxygen, enhancement of heart diastolic function, increase
of left ventricular mass and the increase of muscle fiber and
capillary density (32).
It was demonstrated that the improvement of cardiac
autonomic nerve function in patients with T2DM was partly due to
the increase of exercise tolerance (33). The vagal activity was positively
correlated with exercise tolerance in healthy people and patients
with chronic diseases (34). Our
investigation also demonstrated that peak VO2/kg was
positively correlated with HRRes and rHR, showing that enhancement
of exercise capacity was closely related to the improvement of
autonomic nerve function.
Exercise training has been shown to increase insulin
receptor sensitivity, improve lipid metabolism, decrease blood
sugar and reduce weight of T2DM patients (12). In the present study, BMI decreased,
abdominal girth was reduced, as were FPG and HbAlc, as suggested by
previous studies (35,36).
The present study has demonstrated that exercise
training improved the CI and HRR delay in T2DM patients and promote
the adaptability of the cardiovascular system to exercise stress,
by regulating the function of cardiac autonomic nerves. In
addition, the training also improved the exercise capacity and
optimized the body metabolism index. Exercise therapy plays an
important role in the treatment of DM as one of ‘five carriages’.
This study is conducive to the promotion of exercise rehabilitation
effect and health education for the patients, which could improve
the exercise compliance.
The deficiency of this study was that the limitation
of clinical sources led to a low number of patients being enrolled
in the study. In future studies, we intend to continue to expand
the sample size for further study and analysis.
References
|
1
|
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J,
Shan Z, Liu J, Tian H, Ji Q, et al: China National Diabetes and
Metabolic Disorders Study Group: Prevalence of diabetes among men
and women in China. N Engl J Med. 362:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chinese Diabetes Society (CDS), . Chinese
guidelines of exercise therapy in diabetes mellitus. Chinese
Diabetes Society; Beijing: pp. 46–57. 2013
|
|
3
|
Sigal RJ, Kenny GP, Wasserman DH,
Castaneda-Sceppa C and White RD: Physical activity/exercise and
type 2 diabetes: a consensus statement from the American Diabetes
Association. Diabetes Care. 29:1433–1438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brubaker PH and Kitzman DW: Chronotropic
incompetence: causes, consequences, and management. Circulation.
123:1010–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dresing TJ, Blackstone EH, Pashkow FJ,
Snader CE, Marwick TH and Lauer MS: Usefulness of impaired
chronotropic response to exercise as a predictor of mortality,
independent of the severity of coronary artery disease. Am J
Cardiol. 86:602–609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ST: Effect of aerobic exercise on the
balance of cardiovascular autonomic nerve. Beijing Sport
University; pp. 6–9. 2006
|
|
7
|
Young LH, Wackers FJ, Chyun DA, Davey JA,
Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD,
Filipchuk N, et al: DIAD Investigators: Cardiac outcomes after
screening for asymptomatic coronary artery disease in patients with
type 2 diabetes: the DIAD study: a randomized controlled trial.
JAMA. 301:1547–1555. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaplan JM, Okin PM and Kligfield P: The
diagnostic value of heart rate during exercise electrocardiography.
J Cardiopulm Rehabil. 25:127–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin XF, Liu Z and Dong FY: Application and
research progress of heart rate recovery in the rehabilitation of
cardiovascular diseases. Chinese J Physical Med Rehabil.
35:498–500. 2013.(In Chinese).
|
|
10
|
Halon DA, Dobrecky-Mery I, Gaspar T,
Azencot M, Yaniv N, Peled N and Lewis BS: Heart rate recovery after
exercise and coronary atheroma in asymptomatic individuals with
type 2 diabetes mellitus: a study using 64-slice coronary CT
angiogpaphy. Int J Cardiol. 145:102–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chacko KM, Bauer TA, Dale RA, Dixon JA,
Schrier RW and Estacio RO: Heart rate recovery predicts mortality
and cardiovascular events in patients with type 2 diabetes. Med Sci
Sports Exerc. 40:288–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu L, Liu YN and Yu RJ: Clinical
pulmonary function. 1st. People's Health Publishing House; Beijing:
pp. 296–297. 2004
|
|
13
|
Colberg SR, Sigal RJ, Fernhall B,
Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL
and Braun B: American College of Sports Medicine; American Diabetes
Association: Exercise and type 2 diabetes: the American College of
Sports Medicine and the American Diabetes Association: joint
position statement. Diabetes Care. 33:e147–e167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
American Diabetes Association, . Standards
of medical care in diabetes - 2010. Diabetes Care. 33:(Suppl 1).
S11–S61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
American College of Sports Medicine
(ACSM), . ACSM's Guidelines for Exercise Testing and Prescription.
6th. Lippincott Williams & Wilkins; Philadelphia, PA: 2000
|
|
16
|
Orso F, Baldasseroni S and Maggioni AP:
Heart rate in coronary syndromes and heart failure. Prog Cardiovasc
Dis. 52:38–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lauer MS: Autonomic function and
prognosis. Cleve Clin J Med. 76:(Suppl 2). S18–S22. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldberger JJ, Le FK, Lahiri M,
Kannankeril PJ, Ng J and Kadish AH: Assessment of parasympathetic
reactivation after exercise. Am J Physiol Heart Circ Physiol.
290:H2446–H2452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao M, Chen W, Gong ZK, Han L and Zhang L:
Correlation between chronotropic incompetence and metabolic
equivalents in patients with type 2 diabetes mellitus and
concomitant metabolic syndrome. Panminerva Med. 57:115–119.
2015.PubMed/NCBI
|
|
20
|
Ho JS, Fitzgerald SJ, Barlow CE, Cannaday
JJ, Kohl HW III, Haskell WL and Cooper KH: Risk of mortality
increases with increasing number of abnormal non-ST parameters
recorded during exercise testing. Eur J Cardiovasc Prev Rehabil.
17:462–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Gao M and Chen W: Relationship
between heart rate recovery after exercises and mobility in type 2
diabetes patients. Acta Acad Med Xuzhou. 35:238–241. 2015.
|
|
22
|
Ho PM, Maddox TM, Ross C, Rumsfeld JS and
Magid DJ: Impaired chronotropic response to exercise stress testing
in patients with diabetes predicts future cardiovascular events.
Diabetes Care. 31:1531–1533. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jae SY, Kurl S, Laukkanen JA, Heffernan
KS, Choi YH and Park WH: Chronotropic response to exercise and risk
of type 2 diabetes in men. Eur Heart J. 34:58152013. View Article : Google Scholar
|
|
24
|
Jae SY, Carnethon MR, Heffernan KS,
Fernhall B, Lee MK and Park WH: Heart rate recovery after exercise
and incidence of type 2 diabetes in men. Clin Auton Res.
19:189–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng YJ, Lauer MS, Earnest CP, Church TS,
Kampert JB, Gibbons LW and Blair SN: Heart rate recovery following
maximal exercise testing as a predictor of cardiovascular disease
and all-cause mortality in men with diabetes. Diabetes Care.
26:2052–2057. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Georgoulias P, Demakopoulos N, Orfanakis
A, Xydis K, Xaplanteris P, Vardas P and Fezoulidis I: Evaluation of
abnormal heart-rate recovery after exercise testing in patients
with diabetes mellitus: correlation with myocardial SPECT and
chronotropic parameters. Nucl Med Commun. 28:165–171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Georgoulias P, Demakopoulos N, Valotassiou
V, Orfanakis A, Zaganides A, Tsougos I and Fezoulidis I: Long-term
prognostic value of heart-rate recovery after treadmill testing in
patients with diabetes mellitus. Int J Cardiol. 134:67–74. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Chen W and Gao M: Effect of
individualized exercise on cardiac autonomic nerve function. J
Chinese Modern Med. 16:27–31. 2014.(In Chinese).
|
|
29
|
Ribisl PM, Gaussoin SA, Lang W, Bahnson J,
Connelly SA, Horton ES, Jakicic JM, Killean T, Kitzman DW, Knowler
WC, et al: Look AHEAD Research Group: Lifestyle intervention
improves heart rate recovery from exercise in adults with type 2
diabetes: results from the Look AHEAD study. J Obes.
2012:3091962012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwarz PE, Lindström J,
Kissimova-Scarbeck K, Szybinski Z, Barengo NC, Peltonen M and
Tuomilehto J: DE-PLAN project: The European perspective of type 2
diabetes prevention: diabetes in Europe - prevention using
lifestyle, physical activity and nutritional intervention (DE-PLAN)
project. Exp Clin Endocrinol Diabetes. 116:167–172. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Church TS, LaMonte MJ, Barlow CE and Blair
SN: Cardiorespiratory fitness and body mass index as predictors of
cardiovascular disease mortality among men with diabetes. Arch
Intern Med. 165:2114–2120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Albright A, Franz M, Hornsby G, Kriska A,
Marrero D, Ullrich I and Verity LS: American College of Sports
Medicine position stand. Exercise and type 2 diabetes. Med Sci
Sports Exerc. 32:1345–1360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pagkalos M, Koutlianos N, Kouidi E,
Pagkalos E, Mandroukas K and Deligiannis A: Heart rate variability
modifications following exercise training in type 2 diabetic
patients with definite cardiac autonomic neuropathy. Br J Sports
Med. 42:47–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hedelin R, Bjerle P and Henriksson-Larsén
K: Heart rate variability in athletes: relationship with central
and peripheral performance. Med Sci Sports Exerc. 33:1394–1398.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reitman JS, Vasquez B, Klimes I and
Nagulesparan M: Improvement of glucose homeostasis after exercise
training in non-insulin-dependent diabetes. Diabetes Care.
7:434–441. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kraus WE and Levine BD: Exercise training
for diabetes: The ‘strength’ of the evidence. Ann Intern Med.
147:423–424. 2007. View Article : Google Scholar : PubMed/NCBI
|