Introduction
Osteoarthritis (OA) is the most common joint disease
and is the leading cause of physical disability in elderly people,
which results from progressive destruction of articular cartilage
(1). The most common symptoms are
joint pain and severe impaired mobility of the knee. This is due to
the knee being a weight-bearing joint. In Japan, a country
harboring one of the fastest aging societies in the world, the
incidence and prevalence of knee OA is increasing, with a rapid
increase exhibited in the elderly population, as with many other
developed countries (2). Therefore,
the management of knee OA, which requires extensive utilization of
healthcare resources, has become a major social and economic
topic.
Clinical management of knee OA usually includes
analgesic agents (such as nonsteroidal anti-inflammatory drugs and
selective cyclooxygenase-2 inhibitors) and intra-articular
injection of hyaluronan or corticosteroids (3). However, accumulating data suggests that
any of these pharmaceutical agents frequently produce insufficient
benefit with an associated risk of adverse reactions (4–6).
Therefore, it is necessary that OA patients have access to accepted
complementary and alternative approaches to pain management of
OA.
Glucosamine and N-acetyl-D-glucosamine (GlcNAc) are
amino sugars, in which the hydroxyl group of glucose at position 2
is substituted by an amino group and acetoamide group (7,8),
respectively. They are the key components of glycosaminoglycan
polymers (such as chondroitin sulfate and hyaluronan) contained in
articular cartilage (9). Since they
are believed to have a crucial role in the formation of
glycosaminoglycans in cartilage (10,11),
they have been widely used as alternative medicines or dietary
supplements for the treatment of OA. Previous clinical studies and
meta-analyses investigating the effect on OA have demonstrated the
potential benefit of glucosamine sulfate or hydrochloride in
relieving OA pain (12–14). In addition, oral administration of
GlcNAc has been shown to be effective in relieving symptoms of OA,
without generating any adverse reactions in human clinical studies.
Hatano et al (15) conducted
a randomized double-blind comparative study to evaluate the effects
of GlcNAc on OA in 67 untreated patients, who presented with mild
pain and discomfort in the knee. Subjects were divided into the two
groups and given 200 ml of normal soy milk or soy milk containing
1,250 mg of GlcNAc once-daily for 12 weeks. Assessments of
subjective symptoms and range of motion of the knee were performed.
The results indicated a significant improvement in knee joint pain
and the range of motion of the knee, beginning at 8 weeks after the
administration of GlcNAc compared with placebo. Furthermore, blood
chemistry and physical examination did not show any adverse
reactions of clinical importance.
However, to our knowledge, almost all previous
studies were performed using OA patients and no randomized clinical
trials have been conducted in healthy subjects. However, it is
difficult to assess the effect of dietary supplements on healthy
subjects that are without symptoms of OA using the conventional
methods, such as radiographs, the Western Ontario and McMaster
Universities Arthritis Index, Lequesne Index, or visual analog
scale. In a previous study, experimental OA models have revealed
that the early changes in the cartilage matrix are detectable by
biomarkers, which reflect the biochemical and metabolic changes in
the cartilage, before emerging the radiographic abnormality
energies (16). Magnetic resonance
imaging (MRI) studies have also revealed the correlation between
cartilage degeneration and biomarkers (17). Thus, in the present study, the effect
of GlcNAc administration on the cartilage biomarkers was
investigated, using healthy subjects without symptoms of OA. Doses
of 500 and 1,000 mg per day were administered due to findings from
previous studies, which demonstrated that GlcNAc at these doses can
relieve the symptoms of OA (15).
Furthermore, safety assessments of physical parameters, hematology,
blood biochemistry and urinalysis were conducted. In particular,
the effect of GlcNAc on the ratio of markers for type II collagen
degradation (type II collagen cleavage neoepitope, C2C) and
synthesis (carboxy-terminal propeptide of type II procollagen,
PIICP) was the predominant focus, due to previous findings which
demonstrated that analysis with the combination of markers for type
II collagen degradation and synthesis has a significant association
with the MRI-based changes of OA (18).
Materials and methods
Study design
A randomized, placebo-controlled, parallel-group
comparative study was designed to assess the efficacy and safety of
GlcNAc supplementation. The study was performed between October
2013 and August 2014, and involved four clinical service
organization centers under the control of five medical
investigators in Japan. The study protocol was approved by the
local Ethics Committee (Tana Orthopedic Clinic, Kanagawa, Japan),
and was conducted in accordance with the principles of the amended
Declaration of Helsinki and ‘Ethical Guidelines for Epidemiological
Research’ (recognized by the Japanese Government in 2008,
accessible from: http://www.mhlw.go.jp/general/seido/kousei/i-kenkyu/ekigaku/0504sisin.html).
Written informed consent was obtained from all participants prior
to enrollment in the study. The overall design of the study
consisted of a 16-week intervention period and 4-week
post-intervention period. The subjects, who accomplished full
clinical and laboratory examinations at the baseline, at weeks 4,
8, 12 and 16 during the intervention period, and at week 4 of
post-intervention period, were analyzed.
Subjects
Healthy male and female Japanese subjects
(male:female ratio, 33:43), aged 20–64 years, without symptoms of
arthritis (such as pain) in the knee 0 and I in Kellgren-Lawrence
grades (19) were included. Major
exclusion criteria were: Hyperuricemia with risk of gouty attack;
presence of rheumatoid arthritis that may cause joint pain;
previous surgical treatment of knee joint(s) or necessity for
during the test period; routine use of health foods for bone or
cartilage health (containing hyaluronic acid, GlcNAc, collagen
peptides, glucosamine and/or chondroitin sulfate) within 3 months
of inclusion; administration of prescribed medicines or
commercially available medicines more than three times a week;
intra-articular hyaluronic acid or corticosteroids within 12 months
of inclusion or during the test period; regular strenuous exercise
that may affect articular cartilage; a history of osseous or
articular diseases other than OA within the past 12 months;
patients with a history of cancer, hypertension, heart disease,
renal disease, thyroid dysfunction and hepatic disease; treatment
with warfarin prior to or during the study period; daily alcohol
intake of >60 g alcohol/day; potential for developing an allergy
to the test supplement; person judged unsuitable by the lifestyle
questionnaire; pregnant women; nursing mothers or women of
childbearing potential; participation in another clinical study;
and presence of any medical condition judged by the medical
investigator to preclude the subjects' inclusion in the study.
Intervention and subject
assignment
The test supplement contained green tea extract
powder and 500 or 1,000 mg of GlcNAc in a daily dose. GlcNAc was a
product of a commercial chitin hydrolysate, Marine Sweet 40 (Yaizu
Suisankagaku Industry Co., Shizuoka, Japan), containing 40.8% of
GlcNAc, 3.5% of N,N-diacetylchitobiose and dextrin as an excipient.
The placebo supplement contained green tea extract powder without
GlcNAc and was indistinguishable from the test supplement in taste,
flavor, appearance and packaging. All subjects were sequentially
assigned, based on random number tables, to the two GlcNAc
supplement groups (500 or 1,000 mg/day) and a placebo supplement
group. Following randomization, it was confirmed that the subjects
were divided almost equally, in terms of age and gender (Table I). The allocation table was kept by
an appointed person, who was not involved in the present study, and
concealed until the completion of intervention, follow-up and data
analysis from the subjects and the investigators, who recruited and
assessed the participants. All subjects were instructed to take the
supplements (dissolved in a cup of water) once a day, and
self-record in the study diary. Adherence rate to the intervention
was calculated based on the consumption record in the diary and
<80% adherence was considered a protocol violation.
 | Table I.Baseline characteristics of subjects
who completed the study. |
Table I.
Baseline characteristics of subjects
who completed the study.
|
|
| GlcNAc |
|
|---|
|
|
|
|
|
|---|
| Variables | Placebo (n=24) | 0.5 g/day (n=22) | 1.0 g/day (n=22) | P-value |
|---|
| Age (years) | 49.7±2.4 | 50.5±2.3 | 50.0±2.4 | 0.972 |
| Male/female
(number) | 10/14 | 10/12 | 10/12 | 0.956 |
| Body weight (kg) | 55.18±2.00 | 57.36±2.86 | 56.60±2.50 | 0.813 |
| Body mass index
(kg/m2) | 20.84±0.57 | 21.29±0.61 | 20.98±0.62 | 0.862 |
| Systolic blood
pressure (mmHg) | 119.8±2.1 | 118.1±2.5 | 115.7±2.1 | 0.447 |
| Diastolic blood
pressure (mmHg) | 72.5±1.7 | 72.9±1.7 | 72.6±1.6 | 0.984 |
| Pulse rate (beats
min−1) | 69.5±1.5 | 66.4±1.8 | 72.7±2.2 | 0.058 |
| Kellgren-Lawrence
grades (0/I/II–IV) |
|
|
|
|
| Right
knee (number) | 21/3/0 | 17/5/0 | 20/2/0 | 0.412 |
| Left knee
(number | 20/4/0 | 19/3/0 | 20/2/0 | 0.749 |
| C2C (ng/ml) | 260.02±13.26 | 241.59±9.80 | 244.00±13.43 | 0.512 |
| PIICP (ng/ml) | 52.09±2.46 | 45.20±2.27 | 43.74±1.91 | 0.022 |
| C2C/PIICP ratio | 5.26±0.34 | 5.67±0.40 | 5.95±0.51 | 0.503 |
Efficacy and safety assessment
Blood and urine samples were collected from fasting
subjects at the visit of four clinical service organization centers
by the standard methods, and analyzed by the same examination
laboratory. Sera for biomarker analysis were stored frozen and
analyzed at the same time after the follow-up period. The
biomarkers analyzed were C2C and PIICP. C2C was measured in sera
using a competitive inhibition enzyme-linked immunosorbent assay
(Collagen Type II Cleavage ELISA; IBEX Technologies, Inc.,
Mont-Royal, QC, Canada). Serum PIICP was measured using an ELISA
kit for Procollagen II C-terminal Propeptide (USCN Life Sciences,
Inc., Wuhan, China).
Tolerability and safety were assessed throughout the
study on the basis of the incidence and severity of
intervention-related adverse events and changes in physical
parameters, hematology, blood biochemistry and urinalysis.
Subjective pain assessment by visual analog scale (VAS) and
objective assessment of knee joints by the Japan Orthopaedic
Association (JOA) criteria were also performed.
Statistical analysis
Values are expressed as mean ± standard error.
Baseline data of subjects were compared with a placebo group and
two GlcNAc groups, using one-way analysis of variance for
continuous variables. The distributions of males and females, and
Kellgren and Lawrence grades were analyzed by the Pearson's
χ2 test. Biomarker and safety data were compared between
the placebo and GlcNAc groups using the unpaired Student's t-test
with the Holm adjustment along with changes from baseline (20). The Holm step-down adjustment was used
to correct for the multiple pairwise comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of subjects
A total of 227 subjects were assessed for
eligibility, of which 76 subjects fulfilled the eligibility
criteria and were randomly assigned into groups (Fig. 1). During the intervention, one female
in the GlcNAc group (500 mg/day) discontinued the study due to her
own request. The remaining 75 subjects completed the study, and the
adherence rate to the assigned dietary supplement was 94.1–100.0%.
Two subjects in the GlcNAc group (1,000 mg/day) were judged to have
violated the protocol due to a substantial increase in body weight
resulting from changes in dietary pattern and were excluded from
the analysis. Five subjects were also excluded for meeting the
exclusion criteria, including one subject in the placebo group due
to the onset of hyperuricemia; two subjects in the GlcNAc (500
mg/day) group due to the onset of hyperuricemia and a marked
increase of aspartate transaminase, alanine transaminase,
gamma-guanosine-5′-triphosphate; and two subjects in the GlcNAc
(1,000 mg/day) group due to a marked increase in body weight and
acute ankle sprain. The remaining 68 subjects were analyzed for
efficacy and safety. Table I
presents the baseline characteristics of subjects who completed the
study, including demographic characteristics (age, and distribution
of male and female subjects), physiological characteristics (body
height, body weight, body mass index, systolic blood pressure,
diastolic blood pressure and pulse rate) and distribution of
Kellgren and Lawrence grades. Within the groups of placebo and test
supplement (500 mg and 1,000 mg of GlcNAc/day), these parameters
were not significantly different. Levels of biomarkers for type II
collagen metabolism (C2C, PIICP and C2C/PIICP ratio) exhibited no
significant differences at the baseline in C2C, PIICP and C2C/PIICP
ratio between the placebo and GlcNAc groups, with the exception
that PIICP levels in the placebo group were significantly higher
than that of the GlcNAc (1,000 mg/day) group (P<0.05).
Effect on biomarkers
Fluctuations in the levels of C2C, PIICP and
C2C/PIICP at 8, 12 and 16 weeks during the intervention and 4-week
post-intervention are demonstrated in Fig. 2. Minor changes in the C2C levels of
the placebo and the two GlcNAc (500 mg and 1,000 mg/day) groups
(Fig. 2A) were observed. In
contrast, the PIICP levels in the placebo group were substantially
decreased, although the changes were not statistically significant,
at 12 and 16 weeks during the intervention, compared with the two
GlcNAc groups and returned to the same level as that of the two
GlcNAc groups at four weeks after the intervention (Fig. 2B). Moreover, the C2C/PIICP ratio was
marginally decreased at 16 weeks during the intervention in the
GlcNAc (500 mg/day) group when compared with the placebo and GlcNAc
(1,000 mg/day) groups (Fig. 2C).
 | Figure 2.Changes of C2C, PIICP and the ratio of
C2C and PIICP in subjects in the placebo and GlcNAc groups during
and after the intervention. (A) C2C and (B) PIICP were analyzed,
and (C) the ratio of C2C and PIICP was calculated using serum
samples collected from subjects in the placebo (n=24; open
circles), 500 mg/day GlcNAc (n=22; closed triangles) and 1,000
mg/day GlcNAc (n=22; closed squares) groups at baseline, weeks 8,
12 and 16 during the intervention period, and during week 4 of the
post-intervention period. Data are expressed as the mean ± standard
error. The unpaired t-test was used to evaluate
between-group differences. There were no significant differences
between groups. C2C, collagen type II cleavage; GlcNAc,
N-acetylglucosamine; PIICP, procollagen type II carboxy-terminal
propeptide; During, during the intervention; After, After the
intervention; Open circles, placebo; closed triangles, 500 mg/day
GlcNAc; closed squares, 1,000 mg/day GlcNAc. |
To further clarify the effect of GlcNAc
administration, subgroup analysis was performed using the subjects
with enhanced levels of type II collagen degradation and reduced
levels of type collagen synthesis in the articular cartilage.
Subjects with reduced levels of type II collagen degradation
(C2C<210 ng/ml) and enhanced levels of type collagen synthesis
(PIICP≥55 ng/ml) were excluded. Subgroup analysis was performed
using 39 subjects (baseline, C2C≥210 ng/ml and PIICP<55 ng/ml).
Their baseline characteristics are shown in Table II. Within the placebo and the two
GlcNAc groups, no significant differences in demographic
characteristics, physiological characteristics and distribution of
Kellgren and Lawrence grades were demonstrated. Levels of
biomarkers for type II collagen metabolism revealed no significant
differences in C2C, PIICP and C2C/PIICP ratio at the baseline
between the placebo and GlcNAc groups. Fig. 3A demonstrates that the C2C level was
decreased at 8 and 16 weeks during the intervention in the two
GlcNAc (500 mg and 1,000 mg/day) groups compared to the placebo
group. C2C levels in the GlcNAc (500 mg/day) group was
significantly decreased compared with the placebo group at 8
(P<0.05) and 16 (P<0.01) weeks during the intervention and 4
weeks after the intervention. In contrast, PIICP levels within the
placebo and two GlcNAc groups were not notably different (Fig. 3B). As a result, the C2C/PIICP ratio
was markedly decreased, although the changes were not statistically
significant, at 12 and 16 weeks during the intervention in the two
GlcNAc groups compared with the placebo group (Fig. 3C). These observations suggest that
the oral administration of GlcNAc likely improves cartilage
metabolism, predominantly by suppressing the degradation of type II
collagen in healthy subjects without symptoms of arthritis.
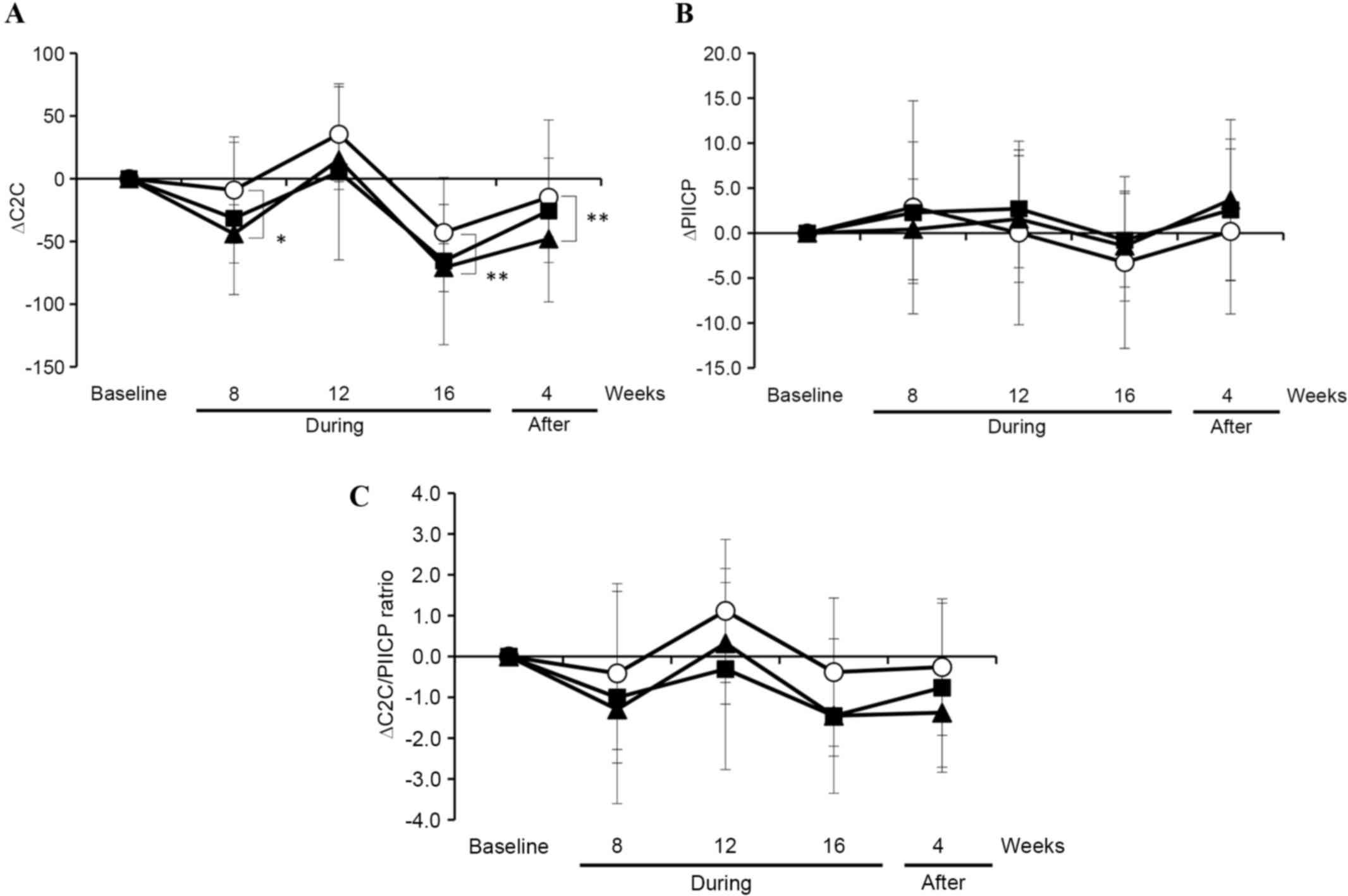 | Figure 3.Changes of C2C, PIICP and the ratio of
C2C and PIICP in subjects in the placebo and GlcNAc groups with
enhanced type II collagen degradation and lowed type collagen
synthesis in the articular cartilage during and after the
intervention. (A) C2C and (B) PIICP were analyzed, and (C) the
ratio of C2C and PIICP was calculated using serum samples collected
from 39 subjects (at the baseline, C2C≥210 ng/ml and PIICP<55
ng/ml) in the placebo (n=13; open circles), 500 mg/day GlcNAc
(n=12; closed triangles) and 1,000 mg/day GlcNAc (n=14; closed
squares) groups during and after the intervention. Data are
expressed as the mean ± standard error. The unpaired t-test
was used to evaluate between-group differences. Holm step-down
adjustment was used to correct for the multiple pairwise
comparisons. *P<0.05; **P<0.01. C2C, collagen type II
cleavage; GlcNAc, N-acetylglucosamine; PIICP, procollagen type II
carboxy-terminal propeptide; During, during the intervention;
After, After the intervention; Open circles, placebo; closed
triangles, 500 mg/day GlcNAc; closed squares, 1,000 mg/day
GlcNAc. |
 | Table II.Baseline characteristics of subjects
with ≥210 ng/ml of C2C and <55 ng/ml of PIICP. |
Table II.
Baseline characteristics of subjects
with ≥210 ng/ml of C2C and <55 ng/ml of PIICP.
|
|
| GlcNAc |
|
|---|
|
|
|
|
|
|---|
| Variables | Placebo (n=13) | 0.5 g/day (n=12) | 1.0 g/day (n=14) | P-value |
|---|
| Age (years) | 46.9±3.1 | 49.6±3.0 | 49.8±2.7 | 0.183 |
| Male/female
(number) | 6/7 | 5/7 | 8/6 | 0.715 |
| Body weight (kg) | 54.87±2.93 | 57.29±3.80 | 58.26±3.52 | 0.769 |
| Body mass index
(kg/m2) | 20.08±0.81 | 21.50±0.84 | 21.07±0.90 | 0.502 |
| Systolic blood
pressure (mmHg) | 121.5±2.4 | 116.3±3.1 | 114.7±2.1 | 0.149 |
| Diastolic blood
pressure (mmHg) | 74.4±2.6 | 73.5±2.3 | 72.0±2.1 | 0.754 |
| Pulse rate (beats
min−1) | 69.0±1.8 | 65.6±2.5 | 72.5±2.8 | 0.144 |
| Kellgren-Lawrence
grades (0/I/II–IV) |
|
|
|
|
| Right
knee (number) | 12/1/0 | 10/2/0 | 12/2/0 | 0.782 |
| Left
knee (number) | 12/2/0 | 11/1/0 | 12/2/0 | 0.852 |
| C2C (ng/ml) | 248.64±8.72 | 269.68±9.46 | 277.80±14.30 | 0.183 |
| PIICP (ng/ml) | 44.06±2.64 | 41.38±2.17 | 42.04±1.98 | 0.693 |
| C2C/PIICP
ratio | 5.90±0.40 | 6.77±0.49 | 6.91±0.62 | 0.339 |
Safety assessment
A total of 66 adverse events occurred in 12, 10 and
9 subjects receiving placebo, 500 mg/day GlcNAc and 1,000 mg/day
GlcNAc, respectively, and there was no significant difference in
the frequency among the three groups. Relatively frequent adverse
events reported included cold symptoms, gastric distress and pain
(head, low back pain, muscle, or knee); however, these events were
generally mild. There were no serious adverse events or deaths. No
adverse events were judged by the investigator to be related to the
intervention.
Routine physical and cardiovascular characteristics,
hematology and blood chemistry did not show any significant
abnormalities during the intervention and follow-up periods in all
the three groups (data not shown). Changes in subjective pain
assessment values by VAS and objective assessment of knee joints by
JOA criteria were not significantly different among the three
groups during the intervention and follow-up periods.
Discussion
In the present randomized, double-blinded,
placebo-controlled study, the effect of GlcNAc on serum biomarkers
of type II collagen metabolism using healthy subjects without
symptoms of arthritis (0 and I of K/L grades) was evaluated.
Previous studies have indicated the efficacy of GlcNAc for pain
relief and improvement of motion in knee OA subjects (15,21). In
addition, GlcNAc administration not only reduced subjective
symptoms but also improved the metabolism of type II collagen by
relatively increasing the synthesis of type II collagen compared to
degradation in knee OA subjects (22,23).
However, it remains to be elucidated how GlcNAc administration
exhibits this beneficial action on healthy individuals without
symptoms of OA. In the present study, there was no significant
change in the biomarkers for type II collagen degeneration and
synthesis during and post-intervention among the placebo and two
GlcNAc groups, using all subjects who completed the study. However,
the subgroup analysis, using subjects with enhanced type II
collagen degradation and lowed type II collagen synthesis (C2C ≥210
ng/ml and PIICP <55 ng/ml), indicated that the C2C/PIICP ratio
was markedly decreased at 12 and 16 weeks during the intervention
in the two GlcNAc groups compared with the placebo group. In
addition, significant suppression of type II collagen degradation
in the GlcNAc (500 mg/day) group was demonstrated when compared
with the placebo group, as evidenced by the changes of serum C2C
levels. However, GlcNAc administration did not significantly
increase the synthesis of type II collagen (serum PIICP), although
GlcNAc significantly increased the synthesis of type II collagen in
the subjects with arthritis (22,23).
Together these observations suggest that GlcNAc administration at
doses of 500 and 1,000 mg/day similarly suppresses type II collagen
degradation in the articular cartilage of healthy individuals
without symptoms of arthritis. Conventionally, pharmacotherapy of
OA is limited to the administration of anti-inflammatory agents for
pain relief and the intra-articular injection of hyaluronan for
supplementation of synovial fluid; there has been no effective
agent that regenerates articular cartilage. Thus, the present
results suggest that GlcNAc has the potential to improve the
metabolism of cartilage by suppressing the degradation of type II
collagen in the joints.
In regard to the action of orally administered
GlcNAc on OA, GlcNAc is reported to be incorporated into
glycosaminoglycans, proteoglycans and synovial fluid. A prior study
on the oral administration of radiolabeled GlcNAc into animals
indicated that radioactivity in the blood peaked at 4 h following
administration and then rapidly decreased; however, the residual
activity (24.7%) remained in the body even at 168 h
post-administration (24). Moreover,
autoradiograms revealed that the residual radioactivity was
distributed in the various glycosaminoglycan-containing tissues,
such as the skin, cartilage and eyes, suggesting that the
administered GlcNAc is utilized for the biosynthesis of
glycosaminoglycans. Notably, a previous study revealed that oral
administration of GlcNAc to the experimental cartilage injury model
demonstrated that injuries were significantly repaired by GlcNAc
administration, accompanied with the synthesis of
glycosaminoglycans and proteoglycans (25).
In addition, GlcNAc was also reported to stimulate
hyaluronan synthesis via the upregulation of hyaluronan synthase-2
in human articular chondrocytes (26). Hyaluronan is reported to inhibit
interleukin (IL-) 1β-induced matrix metalloproteinase-13 expression
and IL-1α-induced expression of a disintegrin and metalloproteinase
with thrombospondin motifs (ADAMTS)-4 through CD44 signaling in
arthritic chondrocytes (27,28). Moreover, GlcNAc inhibits the
IL-1β-induced gene expression and production of nitric oxide,
cyclooxygenase-2 and IL-6 in human articular chondrocytes (29). Based on these observations and the
present study, we hypothesize that GlcNAc exerts chondroprotective
and anti-inflammatory effects, possibly by suppressing the
degradation of type II collagen in the joints of healthy
individuals due to the inhibition of cartilage degrading enzymes
(such as the matrix metalloproteinases and ADAMTS) via the
upregulation of hyaluronan synthesis.
GlcNAc has been used safely in multiple clinical
trials; no adverse effects have been observed at >2,000
mg/kg/day of GlcNAc, based on previous chronic toxicity and
carcinogenicity studies (30). In
the present study, no serious adverse events after oral
administration of GlcNAc were exhibited. In contrast,
administration of glucosamine hydrochloride or sulfate may induce
insulin resistance and progression of diabetes, since glucosamine
has the potential to inhibit glucokinase in glucose metabolism.
GlcNAc has a lower affinity for glucokinase when compared with
glucosamine and therefore does not notably affect glucose
metabolism (31). Furthermore, a
previous study has demonstrated that 12-week administration of
GlcNAc (1,250 mg/day) does not affect the levels of blood glucose,
glycoalbumin and haemoglobin A1c (15). In the present study, blood glucose
levels were not significantly changed in the GlcNAc groups (500 and
1,000 mg/day). Thus, this suggests that GlcNAc may be safe to
administer as a supplement.
Glucosamine and GlcNAc are reported to clinically
alleviate knee OA; however, GlcNAc likely exhibits a potential
effect on the cartilage metabolism at a lower dose (500–1,000
mg/day) compared with glucosamine (1,500 mg/day), based on the
present and previous results (32).
In addition, the taste of GlcNAc is sweet and more appealing than
glucosamine, which is salty and bitter. To conclude, the present
study revealed that GlcNAc can be safely administered as an
appealing dietary supplement, and oral administration of GlcNAc may
improve the type II collagen metabolism of articular cartilage in
healthy subjects without symptoms of arthritis.
Acknowledgements
The authors would like to thank Mr. Takashi
Nakagawa, Ms. Kaori Yoshimura and Dr Tetsuro Yamamoto (Total
Technological Consultant Co., Ltd., Tokyo, Japan) for their helpful
discussion and statistical expertise in the preparation of this
manuscript.
References
|
1
|
Rabenda V, Manette C, Lemmens R, Mariani
AM, Struvay N and Reginster JY: Prevalence and impact of
osteoarthritis and osteoporosis on health-related quality of life
among active subjects. Aging Clin Exp Res. 19:55–60. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshimura N, Muraki S, Oka H, Mabuchi A,
En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, et
al: Prevalence of knee osteoarthritis, lumbar spondylosis, and
osteoporosis in Japanese men and women: The research on
osteoarthritis/osteoporosis against disability study. J Bone Miner
Metab. 27:620–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakasone Y, Watabe K, Watanabe K, Tomonaga
A, Nagaoka I, Yamamoto T and Yamaguchi H: Effect of a
glucosamine-based combination supplement containing chondroitin
sulfate and antioxidant micronutrients in subjects with symptomatic
knee osteoarthritis: A pilot study. Exp Ther Med. 2:893–899.
2011.PubMed/NCBI
|
|
4
|
Wieland HA, Michaelis M, Kirschbaum BJ and
Rudolphi KA: Osteoarthritis-an untreatable disease? Nat Rev Drug
Discov. 4:331–344. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rashad S, Revell P, Hemingway A, Low F,
Rainsford K and Walker F: Effect of non-steroidal anti-inflammatory
drugs on the course of osteoarthritis. Lancet. 2:11491989.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams ME, Lussier AJ and Peyron JG: A
risk-benefit assessment of injections of hyaluronan and its
derivatives in the treatment of osteoarthritis of the knee. Drug
Saf. 23:115–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderson JW, Nicolosi RJ and Borzelleca
JF: Glucosamine effects in humans: A review of effects on glucose
metabolism, side effects, safety considerations and efficacy. Food
Chem Toxicol. 43:187–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen JK, Shen CR and Liu CL:
N-acetylglucosamine: Production and applications. Mar Drugs.
8:2493–2516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Igarashi M, Kaga I, Takamori Y, Sakamoto
K, Miyazawa K and Nagaoka I: Effects of glucosamine derivatives and
uronic acids on the production of glycosaminoglycans by human
synovial cells and chondrocytes. Int J Mol Med. 27:821–827.
2011.PubMed/NCBI
|
|
10
|
Longas MO, Russell CS and He XY: Evidence
for structural changes in dermatan sulfate and hyaluronic acid with
aging. Carbohydr Res. 159:127–136. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shuster S, Black MM and McVitie E: The
influence of age and sex on skin thickness, skin collagen and
density. Br J Dermatol. 93:639–643. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: A randomised, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herrero-Beaumont G, Ivorra JA, Del Carmen,
Trabado M, Blanco FJ, Benito P, Martín-Mola E, Paulino J, Marenco
JL, Porto A, Laffon A, et al: Glucosamine sulfate in the treatment
of knee osteoarthritis symptoms: A randomized, double-blind,
placebo-controlled study using acetaminophen as a side comparator.
Arthritis Rheum. 56:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wandel S, Jüni P, Tendal B, Nüesch E,
Villiger PM, Welton NJ, Reichenbach S and Trelle S: Effects of
glucosamine, chondroitin, or placebo in patients with
osteoarthritis of hip or knee: Network meta-analysis. BMJ.
341:c46752010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hatano K, Hayashida K, Nakagawa S and
Miyakuni Y: Effects and safety of soymilk beverage containing
N-acetyl glucosamine on osteoarthritis. Jpn Pharmacol Ther.
34:149–165. 2006.
|
|
16
|
Qi C and Changlin H: Effects of moving
training on histology and biomarkers levels of articular cartilage.
J Surg Res. 135:352–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
King KB, Lindsey CT, Dunn TC, Ries MD,
Steinbach LS and Majumdar S: A study of the relationship between
molecular biomarkers of joint degeneration and the magnetic
resonance-measured characteristics of cartilage in 16 symptomatic
knees. Magn Reson Imaging. 22:1117–1123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cibere J, Zhang H, Garnero P, Poole AR,
Lobanok T, Saxne T, Kraus VB, Way A, Thorne A, Wong H, et al:
Association of biomarkers with pre-radiographically defined and
radiographically defined knee osteoarthritis in a population-based
study. Arthritis Rheum. 60:1372–1380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holm S: A simple sequentially rejective
multiple test procedure. Scand J Stat. 6:65–70. 1979.
|
|
21
|
Kajimoto O, Matahira Y, Kikuchi K,
Sakamoto A, Kajiya Y and Hirata H: Effects of milk containing
N-acetylglucosamine on osteoarthritis. J New Remedies Clin.
49:301–312. 2000.
|
|
22
|
Katsuno S, Sato K, Eguchi C, Yoshimura K,
Yamamoto T, Tomonaga A and Nagaoka I: Effects and safety of milk
beverage containing N-acetyl glucosamine on knee joint pain and
biomarkers of type II collagen metabolism. Jpn Pharmacol Ther.
38:435–445. 2010.
|
|
23
|
Yokoi K and Fujimoto Y: Effect of dietary
supplement containing N-acetylglucosamine on osteoarthritic pain
and collagen biomarkers. J New Remedies Clin. 62:1758–1768.
2013.
|
|
24
|
Shoji A, Iga T, Inagaki S, Kobayashi K,
Matahira Y and Sakai K: Metabolic disposition of
[14C]N-acetyglucosamine in rats. Chitin Chitosan Res. 5:34–42.
1999.
|
|
25
|
Tamai Y, Miyatake K, Okamoto Y, Takamori
Y, Sakamoto K and Minami S: Enhanced healing of cartilaginous
injuries by N-acetyl-d-glucosamine and glucuronic acid. Carbohydr
Polym. 54:251–262. 2003. View Article : Google Scholar
|
|
26
|
Shikhman AR, Brinson DC, Valbracht J and
Lotz MK: Differential metabolic effects of glucosamine and
N-acetylglucosamine in human articular chondrocytes. Osteoarthritis
Cartilage. 17:1022–1028. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Julovi SM, Ito H, Nishitani K, Jackson CJ
and Nakamura T: Hyaluronan inhibits matrix metalloproteinase-13 in
human arthritic chondrocytes via CD44 and P38. J Orthop Res.
29:258–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yatabe T, Mochizuki S, Takizawa M,
Chijiiwa M, Okada A, Kimura T, Fujita Y, Matsumoto H, Toyama Y and
Okada Y: Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1)
in human osteoarthritic chondrocytes. Ann Rheum Dis. 68:1051–1058.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shikhman AR, Kuhn K, Alaaeddine N and Lotz
M: N-acetylglucosamine prevents IL-1 beta-mediated activation of
human chondrocytes. J Immunol. 166:5155–5160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi M, Inoue K, Yoshida M, Morikawa
T, Shibutani M and Nishikawa A: Lack of chronic toxicity or
carcinogenicity of dietary N-acetylglucosamine in F344 rats. Food
Chem Toxicol. 47:462–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miwa I, Mita Y, Murata T, Okuda J, Sugiura
M, Hamada Y and Chiba T: Utility of
3-O-methyl-N-acetyl-D-glucosamine, an N-acetylglucosamine kinase
inhibitor, for accurate assay of glucokinase in pancreatic islets
and liver. Enzyme Protein. 48:135–142. 1994.PubMed/NCBI
|
|
32
|
Kongtharvonskul J, Anothaisintawee T,
McEvoy M, Attia J, Woratanarat P and Thakkinstian A: Efficacy and
safety of glucosamine, diacerein, and NSAIDs in osteoarthritis
knee: A systematic review and network meta-analysis. Eur J Med Res.
20:242015. View Article : Google Scholar : PubMed/NCBI
|

















