Introduction
Osteoarthritis develops due to the progressive
destruction of articular cartilage. It is the most common joint
disease and the leading cause of physical dysfunction and pain in
elderly people (1–3). In osteoarthritis, knee joints are
particularly impaired, as they are weight-bearing joints. Previous
results in experimental osteoarthritis models have indicated that
changes in the chemical and metabolic properties of cartilage
matrix are detectable at the early stages of arthritis (4). Thus, various molecular markers have
been developed to measure cartilage metabolism in joint disorders
(5–9). Such biomarkers are used to evaluate the
actions of disease-modifying drugs and chondroprotective
supplements on the cartilage as they specifically reflect
alterations in cartilage metabolism (10).
Type II collagen is a major constituent of cartilage
and represents 90–95% of the cartilage collagens (7). Thus, components of type II collagen
have been utilized as biomarkers for cartilage metabolism. A
C-terminal crosslinking peptide (CTX-II) is cleaved during
degradation of type II collagen (11), whereas a neoepitope, either C2C or
C1,2C/Col 2 3/4Cshort, is generated by intrahelical
cleavage at the C terminus of the 3/4 piece of degraded type II
collagen (12,13). Thus, CTX-II and C2C or C1,2C are both
used as markers for type II collagen degradation. By contrast, a
C-terminal type II procollagen propeptide (PIICP), which is cleaved
during the processing of newly synthesized type II procollagen, can
be used as a marker for type II collagen synthesis (14). In addition, along with type II
collagen, aggrecan is one of the most abundant proteins of the
cartilage matrix, and aggrecan synthesis can be measured using
antibodies against epitope CS846, which is located on the
chondroitin sulfate chains (15).
Notably, in the cartilage of patients with osteoarthritis aggrecan
levels are significantly increased (16). Furthermore, cartilage oligometric
matrix protein (COMP), a member of the thrombospondin family of
glycoproteins, is predominantly found in the extracellular matrix
of cartilage, tendons and ligaments, and it has been demonstrated
that serum levels of COMP are increased in individuals with
osteoarthritis (17). Thus, CS846
and COMP may also be utilized as markers of cartilage metabolism
(18,19).
Various nutritional supplements, including
glucosamine, chondroitin and collagen, are used to promote joint
health. They are also specifically used to treat or prevent
cartilage disorders, such as including osteoarthritis (20,21).
Glucosamine suppresses the degradation and stimulates the synthesis
of glycosaminoglycans, such as proteoglycans (22,23).
Glucosamine also suppresses the expression of collagen-degrading
enzymes, such as matrix metalloproteinases (MMPs) and stimulates
type II collagen synthesis in chondrocytes (24,25).
Thus, studies have suggested that glucosamine may exert
chondroprotective action in cartilage disorders by maintaining
proteoglycan and type II collagen levels in the articular cartilage
(26–28).
A previous open-label (unblended) study by the
current authors investigating individuals with knee osteoarthritis
indicated that administration of proteoglycan extracted from salmon
nasal cartilage alleviates the symptoms of knee osteoarthritis,
possibly by reducing the degradation of type II collagen, as
evidenced by a reduction in the C2C/PIICP ratio (29). Notably, salmon nasal cartilage
proteoglycan has the potential to induce the proliferation of
chondrocytes and the production of proteoglycans by chondrocytes
in vitro (29,30) and also exhibits anti-inflammatory
action in vivo in arthritic mice (31). Thus, it was proposed that the
administration of salmon nasal cartilage proteoglycan may improve
the symptoms of knee osteoarthritis, due to its chondroprotective
and anti-inflammatory actions.
In the present study, a randomized double-blind
placebo-controlled clinical trial was conducted to evaluate the
chondroprotective action of salmon nasal proteoglycan on cartilage
metabolism. The effect of oral administration of proteoglycan (10
mg/day) on cartilage metabolism was investigated in subjects with
knee joint discomfort by analyzing serum levels of markers for type
II collagen (C1,2C and PIICP), aggrecan (CS846) and COMP. The
results indicated that administration of salmon nasal cartilage
proteoglycan may suppress the degradation of type II collagen, as
evidenced by the significant reduction of C1,2C level, in the
cartilage of subjects with severe or constant joint discomfort.
Materials and methods
Subjects
Inclusion criteria were as follows: i) Japanese male
or female aged 35–75 years; ii) radiographic severity of knee
joints grade 0 or 1, based on Kellgren and Lawrence grades
(32) (grade 0, no radiographic
features of osteoarthritis; grade 1, doubtful joint space narrowing
and possible osteophytic lipping) without the diagnosis of
osteoarthritis by orthopedists; iii) one knee joint with visual
analog scale (VAS) of ≥20 mm (33),
based on at least one of four VAS subscales, including subscale I
(degree of knee pain evaluated by VAS) of the Japan Knee
Osteoarthritis Measure (JKOM) (33)
and three VAS subscales (pain at rest, pain at walking and pain at
going up and down stairs). Each VAS subscale was scored from 0 to
100 mm, where 0 mm indicates ‘no pain at all’ and 100 mm indicates
‘the most severe pain ever experienced’.
Exclusion criteria were as follows: i) Total VAS
subscale of ankle, elbow, shoulder and hip joints being greater
than the subscale of knee joint (the subscale I of JKOM); ii) a
score of ≥30 points on subscale I (pain on walking) of the Japanese
Orthopedic Association criteria (34), which indicates that patients can walk
≥1 km without apparent pain; iii) a diagnosis of gout/hyperuricemia
or rheumatoid arthritis; iv) surgical treatment of joint(s)
performed or required; v) clinical history of bone or cartilage
disorders including fracture and distortion within one year prior
to enrollment; vi) routine use of dietary supplements containing
proteoglycans, hyaluronic acid, N-acetyl glucosamine, glucosamine,
chondroitin sulfate, collagen peptides or any other constituents of
the test supplement within three months prior to enrollment; vii)
hypersensitivity or allergy to the test component; viii) diagnosis
or current medication of disorders including malignancies,
hypertension (atherosclerosis), cardiac, renal, thyroid, lung and
hepatic disorders, or cerebral infarction; ix) routine use of
external medicine including poultices and taking prescribed
medicine (>3 days/week); x) intra-articular injections of either
corticosteroids or hyaluronic acid within one year prior to
enrollment; xi) excessive exercise, which places loads on the
joints; xii) daily drinking of >60 g alcohol/day; xiii) pregnant
women, nursing mothers or women intending to have children in the
future; xiv) participation in any other clinical studies within one
month prior to enrollment; and xv) the presence of any clinical
conditions judged by the medical investigator to preclude the
participation of subjects in the study.
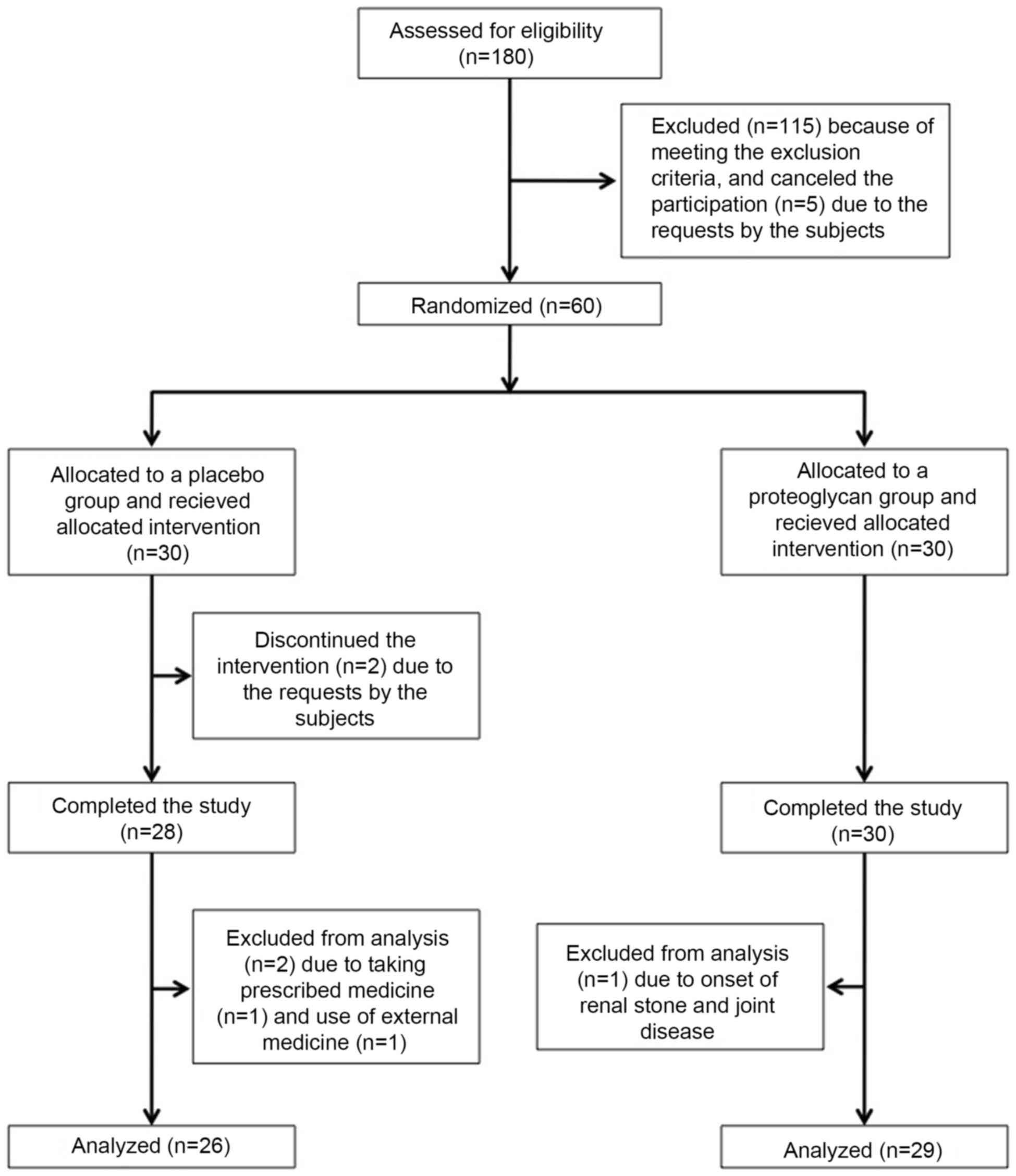
Following assessment of 180 subjects for
eligibility, 115 subjects were excluded based on the exclusion
criteria and 5 subjects declined to participate of their own
volition. Finally, 60 Japanese adults (26 males and 34 females;
aged 36–71 years; mean age, 52.4±1.1 years) with knee discomfort
but without a diagnosis of knee osteoarthritis (Kellgren and
Lawrence grades 0–1) (32) were
enrolled as eligible subjects. The research co-coordinators created
an allocation table and randomly assigned the eligible subjects to
receive a salmon nasal proteoglycan-containing capsule (n=30,
proteoglycan group) or a placebo capsule (n=30, placebo group;
Fig. 1). The allocation table was
sealed, and all research staff and participants were blinded to the
allocation during the test period. Following completion of the
study, the allocation table was made available for analysis of the
data. During the intervention, two subjects in the placebo group
discontinued the study of their own volition (one for a long-term
business trip, and the other for the treatment of right knee joint
pain). A further three subjects (two subjects in the placebo group
and one subject in the proteoglycan group) were excluded by the
medical investigator, due to the onset of renal stone and joint
disease, taking prescribed medicine including inhaled steroids and
the use of external medicine (non-steroidal anti-inflammatory
drug-containing poultice) for lower back pain during the
intervention, which may have affected the efficacy of test
supplements. Thus, 55 subjects (mean age, 52.6±1.1 years; n=26 in
the placebo group, n=29 in the proteoglycan group) were judged to
be eligible for assessment of the efficacy of the test supplement
(Fig. 1 and Table I).
 | Table I.Baseline characteristics of subjects
in the placebo and PG groups. |
Table I.
Baseline characteristics of subjects
in the placebo and PG groups.
| Variable | Placebo (n=26) | PG (n=29) | P-value |
|---|
| Age (years) |
53.9±1.9 |
51.3±1.2 | 0.3 |
| Male/female
(n) | 10/16 | 13/16 | 0.8 |
| Height (cm) | 163.1±1.8 | 164.1±1.3 | 0.6 |
| Weight (kg) |
57.0±2.1 |
58.2±2.0 | 0.7 |
| Body mass index
(kg/m2) |
21.3±0.5 |
21.5±0.5 | 0.7 |
| Systolic blood
pressure (mmHg) | 112.5±2.1 |
111.6±2.2 | 0.8 |
| Diastolic blood
pressure (mmHg) |
72.1±1.6 |
71.0±1.4 | 0.6 |
| Pulse rate
(beats/min) |
71.5±1.6 |
69.7±1.9 | 0.5 |
| Kellgren and
Lawrence grade, 0:1 |
|
|
|
| Right
knee (n) | 16:10 | 14:15 | 0.4 |
| Left
knee (n) | 14:12 | 14:15 | 0.8 |
| JKOM (total
score) |
46.3±1.6 |
46.5±1.7 | 0.8 |
| VAS (mm) |
|
|
|
| Pain at
rest |
16.6±5.4 |
23.8±4.6 | 0.3 |
| Pain at
walking |
49.2±4.0 |
53.2±3.2 | 0.4 |
| Pain at
going up and down stairs |
61.3±3.3 |
62.4±3.2 | 0.8 |
| C1,2C (µg/ml
×10−1) |
7.7±0.4 |
7.9±0.3 | 0.7 |
| PIICP (ng/ml) |
47.2±2.0 |
47.7±2.2 | 0.9 |
| C1,2C/PIICP ratio
(×10−2) |
1.7±0.1 |
1.8±0.1 | 0.8 |
| CS846 (ng/ml) |
154.8±13.8 |
160.7±16.0 | 0.8 |
| COMP (ng/ml) |
154.1±10.2 |
174.9±32.9 | 0.6 |
Study design
A prospective randomized double-blind
placebo-controlled, parallel-group comparative study was designed
to compare the actions of salmon nasal cartilage proteoglycan and a
placebo on cartilage metabolism. Markers for type II collagen
synthesis (PIICP) and degradation (C1,2C), aggrecan (CS846) and
COMP in individuals with knee joint discomfort were measured. In
addition, the safety of the test was evaluated throughout. Subjects
were enrolled and screened from February 2015 to April 2015, and
the test supplements were administered from May 2015 to August 2015
in the Nihonbashi Sakura Clinic (Tokyo, Japan). The study protocol
was registered at the UMIN Clinical Trials Registry (trial no.
UMIN000016470), and involved the Nihonbashi Sakura Clinic. The
study protocol was approved by the Aisei Hospital Ueno Clinic
Research Ethics Committee (Tokyo, Japan) and was conducted in
accordance with the principles of the amended Declaration of
Helsinki and Ethical Guidelines for Epidemiological Research
(established by the Japanese Government in 2008). Written informed
consent was obtained from all participants prior to their
enrollment in the study. The study consisted of a 4-week run-in
(screening) period and a 16-week intervention period. Subjects were
screened at a baseline visit by physical examination, knee
radiograph according to a standardized method, a symptom
questionnaire and routine laboratory tests. In addition, medical
examinations were performed at weeks 4, 8, 12 and 16 during the
intervention, and laboratory tests were performed at weeks 12 and
16 during the intervention for the enrolled subjects.
Intervention and subject
assignment
The test supplement was manufactured in the form of
a hard capsule (220 mg in a capsule) by Ichimaru Pharcos, Co., Ltd.
(Gifu, Japan) and consisted of 12.5 mg salmon nasal cartilage
extract (containing 10 mg proteoglycan) and 207.5 mg dextrin (as a
vehicle). The placebo capsule contained only dextrin powder
(Ichimaru Pharcos, Co., Ltd.). Salmon proteoglycan was extracted
from salmon (Oncorhynchus keta) nasal cartilage, as
previously reported (35). Briefly,
frozen (−20°C) salmon nasal cartilage was dissolved in a solution
of 4–5% acetic acid and salmon proteoglycan was extracted.
High-performance liquid chromatography analysis was performed and
detected a salmon proteoglycan peak as 450,000 molecular size, as
previously reported (36,37).
Subjects were randomly assigned to receive a 10-mg
salmon nasal cartilage proteoglycan-containing capsule
(proteoglycan group) or a placebo capsule containing only vehicle
(placebo group). All subjects were instructed to take the test
supplement or placebo with water once a day between breakfast and
lunch for 16 weeks. The daily dose of salmon nasal cartilage
proteoglycan (10 mg/day) was determined based on the results of a
previous study (29). Adherence to
the intervention was evaluated on the basis of a consumption record
in the study diary and <80% adherence was considered a protocol
violation.
Serum and second void of morning urine were
collected from the subjects in a fasting state at baseline, and at
weeks 12 and 16 during the intervention. Blood samples were
collected by venipuncture, and incubated for 20–30 min at 25°C,
followed by centrifugation at 1,000 × g for 10 min at 4°C to
isolate sera. Serum and urine samples were immediately used for
routine laboratory tests; sera were also aliquoted and stored at
−80°C until the assays for C1,2C, PIICP, CS846 and COMP were
conducted.
Evaluation of cartilage
metabolism
To evaluate the effect of the test supplement on
cartilage metabolism, the levels of type II collagen degradation
(C1,2C) and synthesis (PIICP) markers, CS846 and COMP were
evaluated in serum samples. Serum C1,2C and PIICP were measured
using a Collagen Type I and II Cleavage ELISA kit (60-1002-001;
IBEX Technologies Inc., Montreal, QC, Canada) and ELISA kit for
Procollagen II C-Terminal Propeptide (SEA964Hu; Wuhan USCN Business
Co., Ltd., Wuhan, China), respectively. The C1,2C/PIICP ratio was
then calculated. Serum CS846 and COMP were measured using an
Aggrecan Chondroitin Sulfate 846 Epitope ELISA kit (60–1004; IBEX
Technologies, Inc.) and Human COMP Quantikine ELISA kit (DCMP0;
R&D Systems, Inc., Minneapolis, MN, USA), respectively.
Evaluation of knee pain
The efficacy of the test supplement was also
evaluated based on the changes in subscale scores of JKOM (33). This was measured at baseline, and at
weeks 4, 8, 12 and 16 during the intervention. JKOM is a
self-answered evaluation questionnaire that includes five
subcategories: i) Degree of knee pain as determined by VAS; ii)
Pain and stiffness in knees (8 questions); iii) Condition in daily
life (10 questions); iv) General activities (5 questions); and v)
Health conditions (2 questions). The responses to each question
(ii-v subcategories) are scored from 1 to 5 points, with 1 point
indicating the best functional status and 5 points indicating the
worst functional status. The JKOM score is higher in subjects with
increased pain and physical dysfunction, and is reliable and valid
for studying the clinical outcomes of knee osteoarthritis (38). The outcome of JKOM is closely
correlated with that of other arthritis-related scales, including
the Western Ontario and McMaster Universities Arthritis Index
(38).
Safety evaluation
Safety and tolerability were assessed throughout the
study. The incidence and severity of intervention-related adverse
events (side effects) were recorded, as well as abnormal changes in
physical parameters, such as blood pressure and pulse rate. The
results of laboratory tests, including hematology, biochemical
profile and urinalysis, were also recorded. Any changes in physical
conditions and the use of pharmaceutical products were recorded in
a diary by the enrolled subjects.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean, unless otherwise noted. Regarding the baseline
characteristics of subjects, the distributions of males and
females, and Kellgren and Lawrence grades were analyzed using
Pearson's χ2 test, and other parameters were analyzed using the
Student's t-test. Subcategory I of JKOM and VAS subscales were
compared using the Student's t-test. Subcategories II–V of JKOM
were compared between different time points using the Wilcoxon rank
sum test, and between different groups using the Mann Whitney-U
test. Safety data, C1,2C, PIICP, CS846 and COMP levels, and
C1,2C/PIICP ratios were compared using the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of study groups
Table I presents the
baseline characteristics of the 55 subjects (n=26 in the placebo
group; n=29 in the proteoglycan group) who completed the study and
fulfilled the eligibility criteria. The baseline characteristics
included demographic characteristics (age, and distribution of male
and female subjects), physiological measurement parameters (body
height, body weight and body mass index), physiological
examinations (systolic blood pressure, diastolic blood pressure and
pulse rate), distribution of Kellgren and Lawrence grades, JKOM
(total score), VAS subscales (pain at rest, pain at walking, and
pain at going up and down stairs), and levels of biomarkers for
type II collagen metabolism (C1,2C, PIICP and C1,2C/PIICP ratio),
CS846 and COMP. There were no significant differences in these
parameters between the placebo and proteoglycan groups at the
baseline. Adherence to the allotted supplement was 96–100% among
the 58 subjects who completed the study.
Assessment of cartilage metabolism
using markers for type II collagen degradation and synthesis, CS846
and COMP
In addition to the markers for type II collagen
degradation (C1,2C) and synthesis (PIICP), aggrecan (CS846) and
COMP, the ratio of type II collagen degradation to synthesis may be
used to predict the progression of joint damage in patients with
knee osteoarthritis (39,40). The effect of salmon cartilage
proteoglycan on cartilage metabolism was evaluated by measuring the
C1,2C/PIICP ratio as well as levels of C1,2C, PIICP, CS846 and COMP
using sera collected at baseline, and at weeks 12 and 16 of the
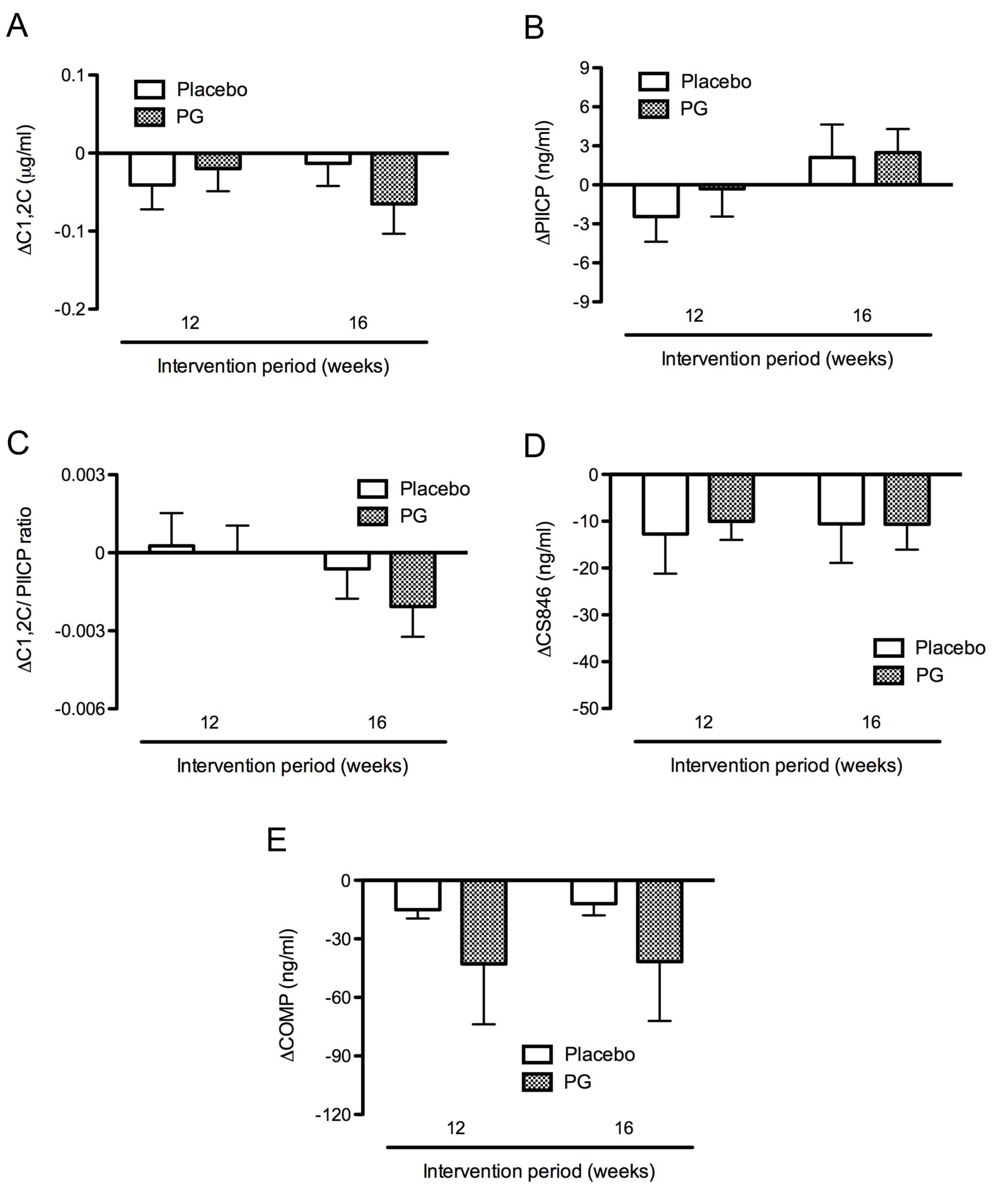
intervention. The levels of C1,2C and PIICP did not significantly
change in the placebo and proteoglycan groups after 16 weeks
intervention. There were also no significant differences in C1,2C
and PIICP levels between the placebo and proteoglycan groups.
However, C1,2C levels slightly decreased from baseline levels of
0.77±0.04 and 0.79±0.03 µg/ml to 0.76±0.03 and 0.73±0.03 µg/ml
after the intervention for 16 weeks in the placebo and proteoglycan
groups, respectively. Thus, C1,2C levels decreased slightly more in
the proteoglycan group (−0.06±0.04 µg/ml) compared with the placebo
group (−0.01±0.03 µg/ml) after the intervention for 16 weeks
(Fig. 2A). By contrast, PIICP levels
were marginally increased from the baseline levels of 47.20±2.00
and 47.66±2.15 ng/ml to 49.31±2.57 and 50.14±2.58 ng/ml after the
intervention for 16 weeks in the placebo and proteoglycan groups,
respectively. PIICP levels increased slightly more in the
proteoglycan group (2.49±1.80 ng/ml) compared with the placebo
group (2.11±2.52 ng/ml) after 16 weeks intervention, although this
difference was not significant (Fig.
2B). Furthermore, the C1,2C/PIICP ratios decreased from the
baseline after 16 weeks intervention in both groups. The
C1,2C/PIICP ratios decreased slightly more in the proteoglycan
group (−0.002±0.001) compared with the placebo group (−0.001±0.001)
after the intervention for 16 weeks (Fig. 2C), although there was no significant
difference between the two groups.
Levels of CS846 were not markedly altered during the
intervention period in the placebo and proteoglycan groups
(Fig. 2D), while levels of COMP
decreased somewhat more in the proteoglycan group than in the
placebo group (Fig. 2E). However,
there were no significant differences in COMP levels between the
placebo and proteoglycan groups at weeks 12 and 16.
Assessment of cartilage metabolism in
subjects with severe knee pain
In order to improve clarity regarding the effect of
the test supplement, further assessments focused on subjects with a
high level of pain and physical dysfunction, as determined by their
JKOM score (33). Subjects with a
total JKOM score of <41 (25% of the 55 subjects) were excluded.
Thus, 41 subjects (mean age, 52.3±1.2 years; n=19 in placebo group,
n=22 in proteoglycan group) with a total score of JKOM ≥41
(increased pain and physical dysfunction) were evaluated. Table II presents the baseline
characteristics of these subjects, including demographic
characteristics, physiological characteristics, distribution of
Kellgren and Lawrence grades, JKOM (total score), VAS subscales and
levels of biomarkers. There were no significant differences between
these parameters in the placebo and proteoglycan groups.
 | Table II.Baseline characteristics of the
subjects with a total JKOM score ≥41 points in the placebo and PG
groups. |
Table II.
Baseline characteristics of the
subjects with a total JKOM score ≥41 points in the placebo and PG
groups.
| Variable | Placebo (n=19) | PG (n=22) | P-value |
|---|
| Age (years) |
53.4±2.1 |
51.3±1.2 | 0.4 |
| Male/female
(n) | 8/11 | 13/9 | 0.4 |
| Height (cm) | 163.1±2.1 | 165.5±1.5 | 0.3 |
| Weight (kg) |
56.9±2.5 |
60.6±2.4 | 0.3 |
| Body mass index
(kg/m2) |
21.2±0.6 |
22.0±0.6 | 0.4 |
| Systolic blood
pressure (mmHg) | 114.7±2.2 | 111.6±2.2 | 0.3 |
| Diastolic blood
pressure (mmHg) |
73.4±1.7 |
71.0±1.6 | 0.3 |
| Pulse rate
(beats/min) |
71.8±2.0 |
69.3±2.0 | 0.4 |
| Kellgren and
Lawrence grade, 0:1 |
|
|
|
| Right
knee (n) | 13:06 | 11:11 | 0.3 |
| Left
knee (n) | 10:09 | 11:11 | >0.9 |
| JKOM (total
score) |
49.4±1.7 |
50.1±1.6 | 0.7 |
| VAS (mm) |
|
|
|
| Pain at
rest |
13.6±5.1 |
24.7±5.2 | 0.1 |
| Pain at
walking |
48.5±4.4 |
51.5±4.1 | 0.6 |
| Pain at
going up and down stairs |
63.9±3.5 |
61.7±4.0 | 0.7 |
| C1,2C (µg/ml
×10−1) |
7.4±0.4 |
8.0±0.4 | 0.3 |
| PIICP (ng/ml) |
47.3±2.3 |
48.0±2.6 | 0.8 |
| C1,2C/PIICP ratio
(×10−2) |
1.7±0.1 |
1.8±0.2 | 0.5 |
| CS846 (ng/ml) |
165.4±18.1 |
158.4±18.9 | 0.8 |
| COMP (ng/ml) |
160.5±13.5 |
190.2±42.7 | 0.5 |
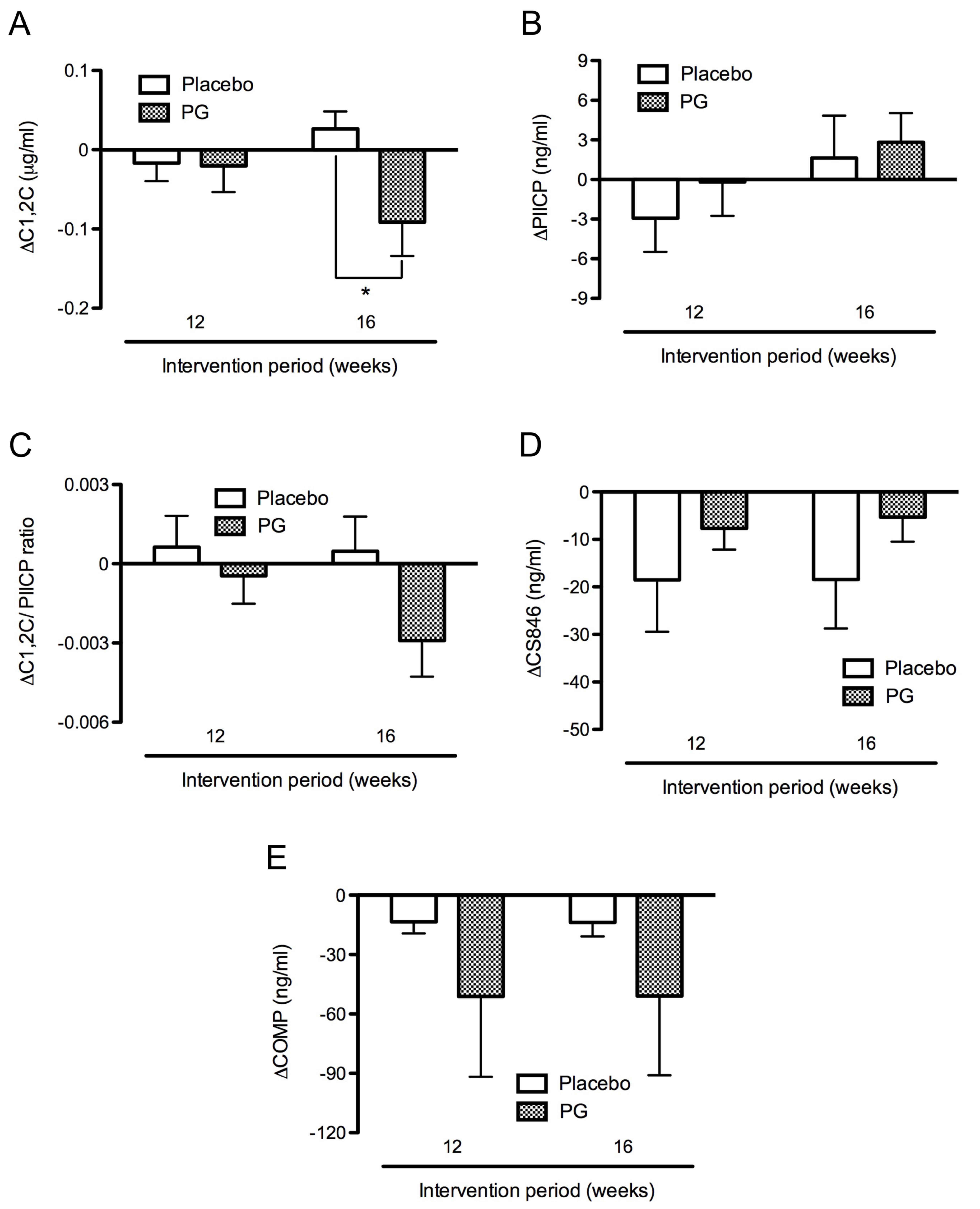
In the proteoglycan group, C1,2C levels decreased
from the baseline levels of 0.80±0.04 to 0.71±0.03 µg/ml after 16
weeks intervention. In the placebo group, C1,2C levels increased
from the baseline levels of 0.74±0.04 to 0.77±0.04 µg/ml after 16
weeks intervention in the placebo group. Thus, the C1,2C levels
were significantly decreased in the proteoglycan group (−0.09±0.04
µg/ml) compared with the placebo group (0.03±0.02 µg/ml) after 16
weeks intervention (P<0.05; Fig.
3A). By contrast, PIICP levels were slightly increased from the
baseline levels of 47.34±2.30 and 48.03±2.55 ng/ml to 48.96±3.14
and 50.86±3.12 ng/ml in the placebo and proteoglycan groups,
respectively following 16 weeks intervention. PIICP levels
increased slightly more in the proteoglycan group (2.83±2.20 ng/ml)
compared with the placebo group (1.63±3.21 ng/ml) after 16 weeks
intervention (Fig. 3B). Furthermore,
C1,2C/PIICP ratios were markedly decreased in the proteoglycan
group (−0.003±0.001) compared with the placebo group (0.001±0.001)
after 16 weeks intervention, though this difference was not
significant (Fig. 3C).
Levels of CS846 were markedly decreased during the
intervention period in the placebo group relative to the
proteoglycan group (Fig. 3D), while
levels of COMP were reduced to a greater extent in the proteoglycan
group than in the placebo group (Fig.
3E). However, levels of CS846 and COMP did not differ
significantly between the placebo and proteoglycan groups at weeks
12 and 16.
Assessment of cartilage metabolism in
subjects with constant knee pain
The effect of the test supplement was also evaluated
in subjects with constant knee joint discomfort, based on the
subscale of VAS (pain at rest). Thus, 25 subjects without pain at
rest (VAS subscale of 0) were excluded. A total of 30 subjects
(mean age, 50.1±1.4 years n=12 in the placebo group and n=18 in the
proteoglycan group) with a subscale of VAS (pain at rest) ≥1 mm
were evaluated. Table III presents
the baseline characteristics of these subjects. No significant
differences in these parameters were found between the placebo and
proteoglycan groups.
 | Table III.Baseline characteristics of subjects
with knee pain at rest in the placebo and PG groups. |
Table III.
Baseline characteristics of subjects
with knee pain at rest in the placebo and PG groups.
| Variable | Placebo (n=12) | PG (n=18) | P-value |
|---|
| Age (years) | 49.6±2.9 | 50.5±1.4 | 0.9 |
| Male/female
(n) | 5/7 | 8/10 | >0.9 |
| Height (cm) | 164.9±3.2 | 163.4±1.4 | 0.9 |
| Weight (kg) | 56.3±3.2 | 57.2±2.2 | 0.8 |
| Body mass index
(kg/m2) | 20.7±0.6 | 21.3±0.7 | 0.5 |
| Systolic blood
pressure (mmHg) | 113.4±3.6 | 110.0±2.5 | 0.4 |
| Diastolic blood
pressure (mmHg) | 72.3±2.8 | 70.1±1.4 | 0.4 |
| Pulse rate
(beats/min) | 70.0±2.5 | 69.4±2.1 | 0.9 |
| Kellgren and
Lawrence grade, 0:1 |
|
|
|
| Right
knee (number of knees) | 06:06 | 09:09 | >0.9 |
| Left
knee (number of knees) | 05:07 | 08:10 | >0.9 |
| JKOM (total
score) | 48.8±3.1 | 47.7±2.4 | 0.9 |
| VAS (mm) |
|
|
|
| Pain at
rest | 35.9±9.0 | 38.3±4.9 | 0.8 |
| Pain at
walking | 53.5±6.8 | 54.6±3.9 | 0.9 |
| Pain at
going up and down stairs | 62.1±4.7 | 61.2±3.9 | 0.9 |
| C1,2C (µg/ml
×10−1) | 7.9±0.4 | 8.0±0.4 | >0.9 |
| PIICP (ng/ml) | 48.4±2.8 | 47.5±2.6 | 0.8 |
| C1,2C/PIICP ratio
(×10−2) | 1.7±0.2 | 1.8 ±0.1 | 0.8 |
| CS846 (ng/ml) | 173.5±26.8 | 162.8±24.4 | 0.8 |
| COMP (ng/ml) | 142.6±16.2 | 189.4±52.5 | 0.5 |
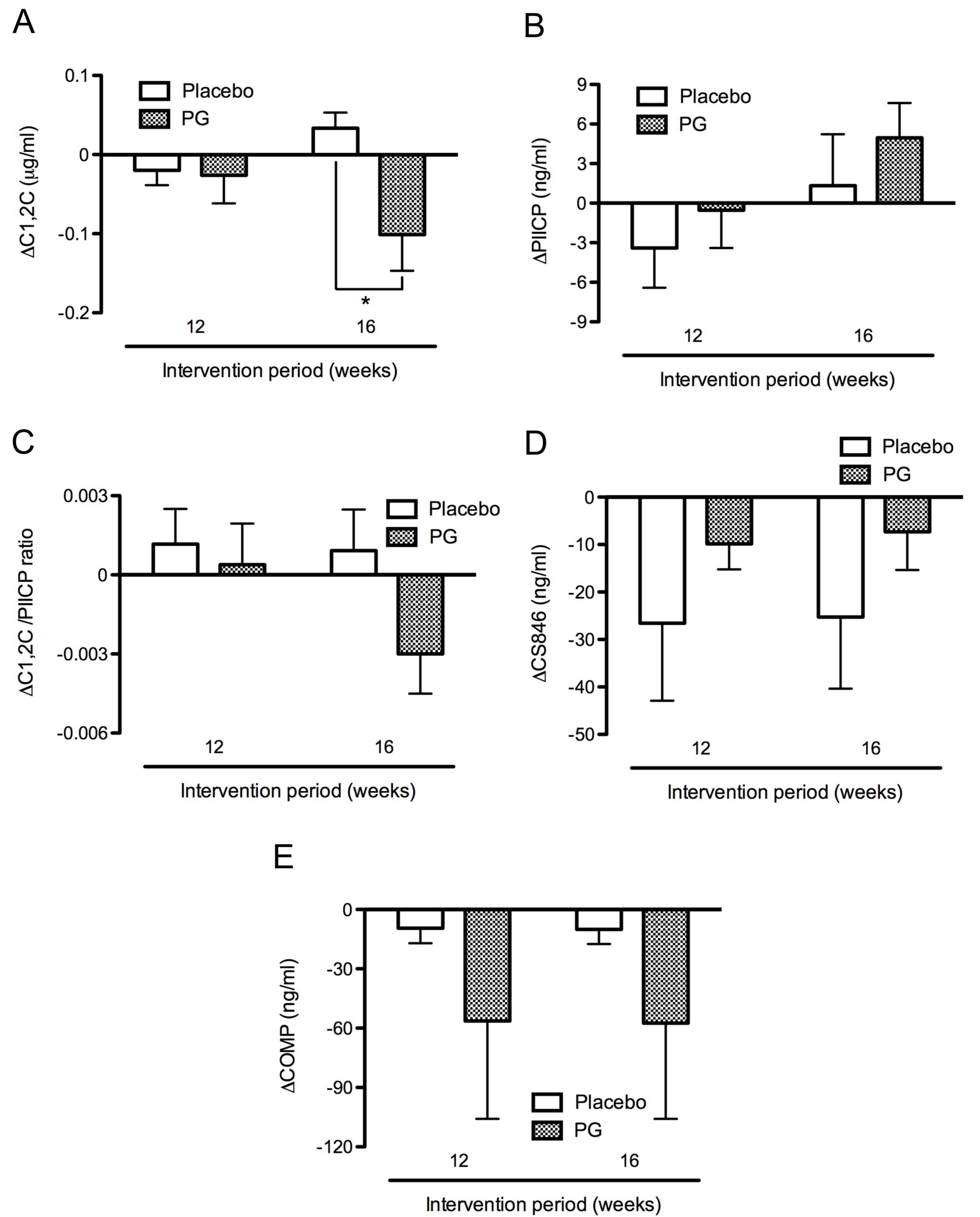
In the proteoglycan group, C1,2C levels decreased
from the baseline level of 0.80±0.04 to 0.70±0.03 µg/ml after 16
weeks intervention. In the placebo group, C1,2C levels increased
from the baseline levels of 0.79±0.04 to 0.83±0.05 µg/ml after 16
weeks intervention. Thus, C1,2C levels were significantly decreased
in the proteoglycan group (−0.10±0.05 µg/ml) compared with the
placebo group (0.03±0.02 µg/ml) after 16 weeks intervention
(P<0.05; Fig. 4A). By contrast,
PIICP levels were slightly increased from the baseline levels of
48.37±2.77 and 47.46±2.59 ng/ml to 49.70±3.89 and 52.42±3.69 ng/ml
in the placebo and proteoglycan groups respectively, after 16 weeks
intervention. Thus, PIICP levels from the baseline were slightly
increased in the proteoglycan group (4.96±2.64 ng/ml) compared with
the placebo group (1.33±3.90 ng/ml) after 16 weeks intervention
(Fig. 4B). Furthermore, the
C1,2C/PIICP ratio decreased in the proteoglycan group
(−0.003±0.001) whereas it increased in the placebo group
(0.001±0.002) after 16 weeks intervention, although this difference
was not significant (Fig. 4C).
By contrast, levels of CS846 were markedly decreased
during the intervention period in the placebo group relative to the
proteoglycan group (Fig. 4D), while
levels of COMP were reduced to a greater extent in the proteoglycan
group than in the placebo group (Fig.
4E). However, levels of CS846 and COMP did not differ
significantly between the placebo and proteoglycan groups at weeks
12 and 16 (Fig. 4D and E).
Assessment of JKOM and VAS scores
The test supplement was evaluated based on changes
in the subscale scores of JKOM (33)
and VAS. However, there were no significant differences between the
placebo and proteoglycan groups during the intervention at weeks 4,
8, 12 or 16 in the subscale scores of JKOM and VAS subscales, among
the subjects of the initial analysis (Table I), the subjects with a total score of
JKOM ≥41 (Table II) and the
subjects with pain at rest (Table
III) (data not shown).
Together these observations suggest that oral
administration of test supplement containing salmon nasal cartilage
proteoglycan may have a protective effect on cartilage metabolism
in subjects with severe and constant knee pain, by improving the
C1,2C/PIICP ratio (lowering type II collagen degradation and
increasing type II collagen synthesis).
Assessment of safety and
tolerability
Out of all 60 enrolled subjects, 6 (26.7%) in the
placebo group and 11 (36.7%) in the proteoglycan group experienced
one or more adverse events during the intervention period. The
total number of adverse events reported was 11 and 21 in the
placebo and proteoglycan groups, respectively, and there was no
significant difference in the frequency of adverse events occurring
between the two groups (P=0.580). Major adverse events reported
from the subjects of the placebo and proteoglycan groups were
symptoms of a common cold (sore throat, cough and/or bronchitis),
headache, back pain and neck/shoulder pain. All adverse events were
of mild or moderate intensity and judged by the medical
investigator to be unrelated to the intervention.
Furthermore, changes in physical measurement
parameters (body weight and body mass index), physiological
examinations (systolic and diastolic blood pressures, and pulse
rate) and laboratory tests (urinalysis, hematology and blood
chemistry) were minimal and within the reference values during the
intervention in both groups.
Discussion
The biomarkers for cartilage metabolism,
particularly type II collagen metabolism, are used to screen for
the risk of progressive destruction of joint cartilage and also to
monitor the effects of structure-modifying agents or dietary
supplements on joint diseases, such as osteoarthritis (8,9). For
example, the actions of chondroprotective agents, (such as
glucosamine and chondroitin sulfate), have been evaluated using
type II collagen degradation biomarkers including CTX-II and C2C or
C1,2C) (21,41–43).
Type II collagen synthesis biomarkers, such as PIICP, have also
been used in combination with type II collagen degradation
biomarkers, including CTX-II and C2C or C1,2C, to monitor the
disease state and progression of osteoarthritis. The combination of
type II collagen degradation and synthesis biomarkers has been
demonstrated to be more effective at predicting the progression of
osteoarthritis or monitoring the action of chondroprotective agents
on cartilage metabolism in osteoarthritis (39,40,44).
The present study aimed to evaluate the action of
salmon nasal cartilage proteoglycan on human joint health. A
randomized double-blind placebo-controlled clinical trial was
conducted to evaluate the effect of oral administration of
proteoglycan (10 mg/day) on cartilage metabolism in subjects with
knee joint discomfort by measuring the serum levels of type II
collagen degradation (C1,2C) and synthesis (PIICP) markers, as well
as aggrecan (CS846) and COMP.
The C1,2C antibody used to measure C1,2C can
recognize the cleaved fragment (neoepitope) of type I collagen as
well as that of type II collagen in the cartilage, on the basis of
sequence homology (8,9). In this regard, the C1,2C antibody has
been used to detect the increased degradation of type I collagen in
atheromatous plaques (45) and the
modulation of type I collagen degradation in acute respiratory
distress syndrome (46). However, in
the present study, subjects with hypertension (atherosclerosis) and
lung disorders were excluded. Thus, in the present study, serum
levels of C1,2C should reflect alterations in type II collagen
metabolism in the cartilage.
The mechanism by which salmon nasal cartilage
proteoglycan exerts protective action on cartilage metabolism
remains unknown. Previous studies have reported that salmon nasal
proteoglycan induces the proliferation of chondrocytes and the
production of proteoglycans by chondrocytes in vitro
(29,30). The administration of salmon
proteoglycan attenuated not only collagen-induced arthritis in
mice, but also mouse experimental colitis in vivo by
suppressing the expression of inflammatory cytokines, including
interleukin (IL)-1 and IL-6 (30,47). It
has been determined that salmon nasal proteoglycan can be absorbed
in the jejunum of the small intestine by clathrin-mediated
endocytosis (48). Furthermore, type
II collagen is degraded by collagenases, including MMP-1, MMP-8 and
MMP-13, and the expression of these enzymes is upregulated by
inflammatory cytokines produced in the articular cartilage
(49,50). Based on these findings, it is
reasonable to speculate that orally administered salmon nasal
cartilage proteoglycan is absorbed in the small intestine.
Proteoglycan or its metabolites may exert anti-inflammatory actions
via the suppression of inflammatory cytokine production, as well as
a chondroprotective effect at the joint tissue via the
proliferation of chondrocytes and the production of proteoglycans.
This may inhibit the expression of MMPs and the degradation of type
II collagen in the articular cartilage, as demonstrated by a
decrease in C1,2C levels. However, the detailed mechanism for the
chondroprotective action of salmon nasal cartilage proteoglycan in
humans remains to be elucidated.
In the initial analysis of the results (Fig. 2), a statistically significant effect
of salmon nasal cartilage proteoglycan on cartilage metabolism
could not be detected. Thus, in the second and third analyses, only
subjects with increased pain and physical dysfunction (total JKOM
score ≥41), and pain at rest were analyzed, and consequently salmon
nasal cartilage proteoglycan was proved to be significantly
effective at improving the cartilage metabolism. These observations
suggested that additional inclusion criteria (such as subscales of
JKOM and VAS), should be introduced when screening subjects in
randomized double-blind placebo-controlled clinical trials to
identify the potential action of dietary supplements on the
cartilage metabolism in individuals with knee joint discomfort but
without a diagnosis of osteoarthritis. In this context, it is
interesting to note that a previous study by the current authors
demonstrated that administration of salmon nasal cartilage
proteoglycan (10 mg/day for 12 weeks) relieved the symptoms of
osteoarthritis, including the subscale scores of JKOM and VAS, in
subjects with knee osteoarthritis (29). By contrast, in the present study, the
administration of salmon nasal proteoglycan (10 mg/day for 16
weeks) did not improve the symptoms of knee discomfort (evaluated
by the subscale scores of JKOM and VAS) in subjects without the
diagnosis of knee osteoarthritis. Notably, the total JKOM score
(53.7±3.7), VAS score of pain at rest (33.7±5.7 mm) and Kellgren
and Lawrence grade (mainly 1 and 2) were higher in the subjects
(n=12) in the previous study (29),
compared with subjects (n=55) in the present study (total JKOM
score, 46.4±1.2; VAS score of pain at rest, 20.4±3.5 mm; Kellgren
and Lawrence grade, mainly 0 and 1). Thus, salmon nasal cartilage
proteoglycan is expected to more potently exert chondroprotective
action and improve the knee joint discomfort in subjects
experiencing greater levels of pain and dysfunction.
A previous open-label study demonstrated that the
administration of salmon nasal cartilage proteoglycan relatively
reduces the degradation and increases the synthesis of type II
collagen, thereby improving the symptoms of osteoarthritis in
individuals with knee osteoarthritis (30). However, the effect of the
administration of salmon nasal proteoglycan on cartilage metabolism
has not yet been confirmed in a blind study. Thus, to the best of
our knowledge, this is the first randomized double-blind
placebo-controlled clinical trial to demonstrate the effect of oral
administration of salmon nasal proteoglycan on cartilage
metabolism. The efficacy and safety of proteoglycan observed in the
present study suggest that a dietary supplement containing salmon
nasal proteoglycan is safe to administer and may exert a
chondroprotective action in individuals with knee joint discomfort
by improving type II collagen metabolism in cartilage. Thus, salmon
nasal proteoglycan-containing supplement is a potential candidate
for maintaining or improving joint health.
Acknowledgements
The authors would like to thank Mr. Takashi
Nakagawa, Ms. Kaori Yoshimura and Professor Tetsuro Yamamoto (Total
Technological Consultant Co., Ltd., Tokyo, Japan) for their helpful
discussion and statistical expertise in the preparation of this
manuscript.
The present study was funded by Ichimaru Pharcos
Co., Ltd., which produces salmon nasal proteoglycan; however, the
company had no input on the design and conduct of the study,
subject recruitment, collection, management or analysis of the
data. Dr Tatsuji Takahashi, Dr Yuka Tsuda Tanaka and Dr Makoto
Tsuboi participated in the analyses of biomarkers as members of our
research group and are employees of Ichimaru Pharcos Co., Ltd. Dr
Akihito Tomonaga and Dr Kumie Ito performed the clinical study, and
Professor Isao Nagaoka had control over the design of the study and
preparation of the manuscript.
References
|
1
|
Ravenda V, Manette C, Lemmens R, Mariani
AM, Struvay N and Reginster JY: Prevalence and impact of
osteoarthritis and osteoporosis on health-related quality of life
among active subjects. Aging Clin Exp Res. 19:55–60. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jinks C, Jordan K and Croft P:
Osteoarthritis as a public health problem: The impact of developing
knee pain on physical function in adults living in the community:
(KNEST 3). Rheumatology (Oxford). 46:877–881. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshimura N, Muraki S, Oka H, Mabuchi A,
En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, et
al: Prevalence of knee osteoarthritis, lumbar spondylosis, and
osteoporosis in Japanese men and women: The research on
osteoarthritis/osteoporosis against disability study. J Bone Miner
Metab. 27:620–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi C and Changlin H: Effects of moving
training on histology and biomarkers levels of articular cartilage.
J Surg Res. 135:352–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garnero P, Rousseau JC and Delmas PD:
Molecular basis and clinical use of biochemical markers of bone,
cartilage, and synovium in joint diseases. Arthritis Rheum.
43:953–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garnero P, Piperno M, Gineyts E, Christgau
S, Delmas PD and Vignon E: Cross sectional evaluation of
biochemical markers of bone, cartilage and synovial tissue
metabolism in patients with knee osteoarthritis: Relations with
disease activity and joint damage. Ann Rheum Dis. 60:619–626. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poole AR: Biochemical/immunochemical
biomarkers of osteoarthritis: Utility for prediction of incident or
progressive osteoarthritis. Rheum Dis Clin North Am. 29:803–818.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elsaid KA and Chichester CO: Review:
Collagen markers in early arthritic diseases. Clin Chim Acta.
365:68–77. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rousseau JC and Delmas PD: Biological
markers in osteoarthritis. Nat Clin Pract Rheumatol. 3:346–356.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garnero P and Delmas PD: Biomarkers in
osteoarthritis. Curr Opin Rheumatol. 15:641–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christgau S, Garnero P, Fledelius C, Moniz
C, Ensig M, Gineyts E, Rosenquist C and Qvist P: Collagen type II
C-telopeptide fragments as an index of cartilage degradation. Bone.
29:209–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
Development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Billinghurst RC, Dahlberg L, Ionescu M,
Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O,
Tschesche H, et al: Enhanced cleavage of type II collagen by
collagenases in osteoarthritic articular cartilage. J Clin Invest.
99:1534–1545. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shinmei M, Ito K, Matsuyama S, Yoshihara Y
and Matsuzawa K: Joint fluid carboxy-terminal type II procollagen
peptide as a marker of cartilage collagen biosynthesis.
Osteoarthritis Cartilage. 1:121–128. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rizkalla G, Reiner A, Bogoch E and Poole
AR: Studies of the articular cartilage proteoglycan aggrecan in
health and osteoarthritis. Evidence for molecular heterogeneity and
extensive molecular changes in disease. J Clin Invest.
90:2268–2277. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lohmander LS, Ionescu M, Jugessur H and
Poole AR: Changes in joint cartilage aggrecan after knee injury and
in osteoarthritis. Arthritis Rheum. 42:534–544. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vilím V, Vytásek R, Olejárová M, Machácek
S, Gatterová J, Procházka B, Kraus VB and Pavelka K: Serum
cartilage oligomeric matrix protein reflects the presence of
clinically diagnosed synovitis in patients with knee
osteoarthritis. Osteoarthritis Cartilage. 9:612–618. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hunter DJ, Li J, LaValley M, Bauer DC,
Nevitt M, DeGroot J, Poole R, Eyre D, Guermazi A, Gale D and Felson
DT: Cartilage markers and their association with cartilage loss on
magnetic resonance imaging in knee osteoarthritis: The Boston
Osteoarthritis Knee Study. Arthritis Res Ther. 9:R1082007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jansen NW, Roosendaal G, Lundin B, Heijnen
L, Mauser-Bunschoten E, Bijlsma JW, Theobald M and Lafeber FP: The
combination of the biomarkers urinary C-terminal telopeptide of
type II collagen, serum cartilage oligomeric matrix protein, and
serum chondroitin sulfate 846 reflects cartilage damage in
hemophilic arthropathy. Arthritis Rheum. 60:290–298. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clayton JJ: Nutraceuticals in the
management of osteoarthritis. Orthopedics. 30:624–631.
2007.PubMed/NCBI
|
|
21
|
Nagaoka I: Recent aspects of the
chondroprotective and anti-Inflammatory actions of glucosamine, a
functional food. Juntendo Med J. 60:580–587. 2014. View Article : Google Scholar
|
|
22
|
Fenton JI, Chlebek-Brown KA, Peters TL,
Caron JP and Orth MW: Glucosamine HCl reduces equine articular
cartilage degradation in explant culture. Osteoarthritis Cartilage.
8:258–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gouze JN, Bordji K, Gulberti S, Terlain B,
Netter P, Magdalou J, Fournel-Gigleux S and Ouzzine M:
Interleukin-1beta downregulates the expression of
glucuronosyltransferase I, a key enzyme priming glycosaminoglycan
biosynthesis: Influence of glucosamine on
interleukin-1beta-mediated effects in rat chondrocytes. Arthritis
Rheum. 44:351–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura H, Shibakawa A, Tanaka M, Kato T
and Nishioka K: Effects of glucosamine hydrochloride on the
production of prostaglandin E2, nitric oxide and metalloproteases
by chondrocytes and synoviocytes in osteoarthritis. Clin Exp
Rheumatol. 22:293–299. 2004.PubMed/NCBI
|
|
25
|
Derfoul A, Miyoshi AD, Freeman DE and Tuan
RS: Glucosamine promotes chondrogenic phenotype in both
chondrocytes and mesenchymal stem cells and inhibits MMP-13
expression and matrix degradation. Osteoarthritis Cartilage.
15:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McAlindon TE, Lavalley MP, Gulin JP and
Felson DT: Glucosamine and chondroitin for treatment of
osteoarthritis: A systematic quality assessment and meta-analysis.
JAMA. 283:1469–1475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: A randomized, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pavelká K, Gatterová J, Olejarová M,
Machacek S, Giacovelli G and Rovati LC: Glucosamine sulfate use and
delay of progression of knee osteoarthritis: A 3-year, randomized,
placebo-controlled, double-blind study. Arch Intern Med.
162:2113–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi T, Masutani T, Tomonaga A,
Watanabe K, Yamamoto T, Ito K, Tsuboi M, Yamaguchi H and Nagaoka I:
Influence on improvement of osteoarthritis by oral intake of
proteoglycan extracted from salmon nasal cartilage. Proceedings of
the International Conference and Exhibition on Nutraceuticals and
Functional Foods. Sapporo. Abstract P112. 2011;
|
|
30
|
Ohshika S, Ishibashi Y, Kon A, Kusumi T,
Kijima H and Toh S: Potential of exogenous cartilage proteoglycan
as a new material for cartilage regeneration. Int Orthop.
36:869–877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshimura S, Asano K and Nakane A:
Attenuation of collagen-induced arthritis in mice by salmon
proteoglycan. Biomed Res Int. 2014:4064532014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthritis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akai M, Iwaya T, Kurosawa H, Doi T, Nasu
T, Hayashi K and Fujino K: Development of new disease-specific QOL
measure for patients with knee osteoarthritis: Japanese knee
osteoarthritis measure (JKOM). J Phys Med. 16:55–62. 2005.(In
Japanese).
|
|
34
|
Okuda M, Omokawa S, Okahashi K, Akahane M
and Tanaka Y: Validity and reliability of the Japanese Orthopaedic
Association score for osteoarthritic knees. J Orthop Sci.
17:750–756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Majima M, Takagaki K, Sudo S, Yoshihara S,
Kudo Y and Yamagishi S: Effect of proteoglycan on experimental
colitis. Int Cong Ser. 1223:221–224. 2001. View Article : Google Scholar
|
|
36
|
Takahashi T, Matsubara J, Wakamatsu K, Tsu
Da Tanaka Y, Masutani T, Yonezuka M, Ito K, Tsuji-Takayama K and
Tsuboi M: Ingestion of salmon nasal cartilage-derived proteoglycan
improves skin condition: A randomized, double-blind, controlled
study. Immun Endoc Metab Agents Med Chem. 15:160–167. 2015.
View Article : Google Scholar
|
|
37
|
Takahashi T, Maeda M, Matsubara J, Fujita
Y, Masutani T, Yonezuka M, Ito K, Tsuji-Takayama K and Tsuboi M:
Safety evaluation of industrially extracted, highly purified
proteoglycan from salmon nasal cartilatge. Pharmacometrics.
89:15–22. 2015.
|
|
38
|
Akai M, Doi T, Fujino K, Iwaya T, Kurosawa
H and Nasu T: An outcome measure for Japanese people with knee
osteoarthritis. J Rheumatol. 32:1524–1532. 2005.PubMed/NCBI
|
|
39
|
Cahue S, Sharma L, Dunlop D, Ionescu M,
Song J, Lobanok T, King L and Poole AR: The ratio of type II
collagen breakdown to synthesis and its relationship with the
progression of knee osteoarthritis. Osteoarthr Cartil. 15:819–823.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sharif M, Kirwan J, Charni N, Sandell LJ,
Whittles C and Garnero P: A 5-yr longitudinal study of type IIA
collagen synthesis and total type II collagen degradation in
patients with knee osteoarthritis-association with disease
progression. Rheumatology (Oxford). 46:938–943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Christgau S, Henrotin Y, Tankó LB, Rovati
LC, Collette J, Bruyere O, Deroisy R and Reginster JY:
Osteoarthritic patients with high cartilage turnover show increased
responsiveness to the cartilage protecting effects of glucosamine
sulfate. Clin Exp Rheumatol. 22:36–42. 2004.PubMed/NCBI
|
|
42
|
Cibere J, Thorne A, Kopec JA, Singer J,
Canvin J, Robinson DB, Pope J, Hong P, Grant E, Lobanok T, et al:
Glucosamine sulfate and cartilage type II collagen degradation in
patients with knee osteoarthritis. J Rheumatol. 32:896–902.
2005.PubMed/NCBI
|
|
43
|
Mazières B, Hucher M, Zaïm M and Garnero
P: Effect of chondroitin sulfate in symptomatic knee
osteoarthritis: A multicentre, randomised, double-blind,
placebo-controlled study. Ann Rheum Dis. 66:639–645. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Conrozier T, Poole AR, Ferrand F, Mathieu
P, Vincent F, Piperno M, Verret C, Ionescu M and Vignon E: Serum
concentrations of type II collagen biomarkers (C2C, C1, 2C and
CPII) suggest different pathophysiologies in patients with hip
osteoarthritis. Clin Exp Rheumatol. 26:430–435. 2008.PubMed/NCBI
|
|
45
|
Sukhova GK, Schönbeck U, Rabkin E, Schoen
FJ, Poole AR, Billinghurst RC and Libby P: Evidence for increased
collagenolysis by interstitial collagenases-1 and -3 in vulnerable
human atheromatous plaques. Circulation. 99:2503–2509. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Armstrong L, Tickett DR, Mansell JP,
Ionescu M, Hoyle E, Billinghurst RC, Poole AR and Millar AB:
Changes in collagen turnover in early acute respiratory distress
syndrome. Am J Respir Crit Care Med. 160:1910–1915. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mitsui T, Sashinami H, Sato F, Kijima H,
Ishiguro Y, Fukuda S, Yoshihara S, Hakamada K and Nakane A: Salmon
cartilage proteoglycan suppresses mouse experimental colitis
through induction of Foxp3+ regulatory T cells. Biochem Biophys Res
Commun. 402:209–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsuchiya Y, Tomita M, Tsuboi M, Takahashi
Y, Yonezuka M, Kikuchi S, Nagasawa S, Kumazawa A and Kubota J:
Absorption of proteoglycan via clathrin-mediated endocytosis in the
small intestine of rats. Biosci Biotechnol Biochem. 77:654–656.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Billinghurst RC, Wu W, Ionescu M, Reiner
A, Dahlberg L, Chen J, van Wart H and Poole AR: Comparison of the
degradation of type II collagen and proteoglycan in nasal and
articular cartilages induced by interleukin-1 and the selective
inhibition of type II collagen cleavage by collagenase. Arthritis
Rheum. 43:664–672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rousset F, Hazane-Puch F, Pinosa C, Nguyen
MV, Grange L, Soldini A, Rubens-Duval B, Dupuy C, Morel F and Lardy
B: IL-1beta mediates MMP secretion and IL-1beta neosynthesis via
upregulation of p22(phox) and NOX4 activity in human articular
chondrocytes. Osteoarthritis Cartilage. 23:1972–1980. 2015.
View Article : Google Scholar : PubMed/NCBI
|