Introduction
Pancreas is a digestive gland in mammals, playing an
important role in digestion and absorption of food and constant
blood sugar levels by secreting pancreatic juice and hormone. With
increased age, structure and function of the pancreas gradually
decline, although the underlying mechanism remains to be
determined. The theory of traditional Chinese Medicine (TCM) holds
that the bodys aging is closely related to the lack of vital Qi,
through toning Qi can delay human aging; it has a protective effect
on tissues morphology and function of human organs (1,2).
Ginsenoside Rg1 is an important medicine used in TCM
clinically, and it can strengthen healthy Qi to eliminate
pathogens, repairing damage and prolong life. The present study
showed that Ginsenoside Rg1 is an important anti-aging constituent
of ginseng, which can delay stem cell aging and antagonism in
multiple organ injury caused by the attenuation agent (3,4).
The present study used D-gal to replicate the aging
model mice, aiming to examine the protective effects of Ginsenoside
Rg1 on pancreas injury by an antagonism attenuation agent and its
mechanism of modern biology.
Materials and methods
Animals
Two-month male C57BL/6J mice (19–21 g) were supplied
by the Medical and Laboratory Animal Center of Chongqing (qualified
no. SCXK yu. 2012-0001). The animals were housed in conditions of
controlled natural lighting and temperature (20–25°C) and received
a standard free access to water and food. This study was approved
by the Animal Ethics Committee of Chongqing Medical University
Animal Center.
Reagents
Ginsenoside Rg1 (purity 98.6%) was obtained from
Jilin Hongjiu Biological Technology Co., Ltd. (Jilin, China);
D-galactose (D-gal) (purity ≥99%) was purchased from Shanghai
Shengong Biological Engineering Co., Ltd. (Shanghai, China).
Glucometer (One Touch UItra) was supplied by Johnson & Johnson
Medical Ltd. (Shanghai, China). Blood sugar test paper (steady
house type) was obtained from LifeScan Europe (Zug, Switzerland).
The advanced glycation end products (AGEs) test kit was supplied by
Abcam Trading (Shanghai) Co., Ltd. (Cambridge, UK). DBA staining
reagent was supplied by Zhongshan Golden Bridge Biotechnology Co.,
Ltd. (Beijing, China); the BAC protein concentration test kit, the
superoxide dismutase (SOD), the malonaldehyde (MDA), the total
antioxidant capacity (T-AOC) and senescence-associated
β-galactosidase (SA-β-gal) kit were supplied by Beyotime Institute
of Biotechnology (Shanghai, China).
Replication of the mouse D-gal aging
model and group administration
C57BL/6J mice were randomly divided into three
groups, of 10 animals each. The mice of D-gal model group received
hypodermic injection of D-gal (120 mg/kg/day) (5) for 6 weeks; Rg1+D-gal groups were
injected with D-gal, time and dose with D-gal model group,
intraperitoneal injection with Rg1 (40 mg/kg/day) for 27 days from
the 16th day of D-gal replication; the same dose of saline was
administered into the naive control mice at the same time. Related
indicators were measured on the second day after modeling and
administration.
General observation
In the process of modeling, each group of mice was
observed for mental state, coat color, activity and water intake
and the weight was measured daily.
Determination of fasting blood glucose
(FBG) and oral glucose tolerance test (OGTT)
Mice were fasted for 12–14 h with free access to
water. Tail venous blood samples were collected for measurement of
FBG the following morning. Thereafter, their stomachs were filled
with 20% glucose solution, and the blood glucose levels were
measured at 30, 60, 90 and 120 min after lavage. The OGTT curve was
drawn and the area under the curve (AUC) calculated.
Observation of morphology of
pancreatic tissue structure
The mice were sacrificed by cervical dislocation and
the pancreas was collected. The pancreas index was calculated as:
pancreas index = pancreas wet weight (mg)/weight (g). The
pancreatic tissues were fixed in 5% paraformaldehyde solution for 2
days, embedded in paraffin and sectioned. Serial section of one
slice at interval of 10 sections was collected for H&E
staining. The morphological change of pancreatic tissue was
observed under an Olympus BX43 microscope (Olympus Optical Co.,
Ltd., Tokyo, Japan). Randomly selected islet data of 5 views and
the number of nucleated cells in each islet under 10 times
magnification. The area of each islet, cell number within islet and
cell areas within a single islet were counted using Image-Pro Plus
6.0 (IPP) software. Frozen sections were prepared to stain
according to the kit instructions of SA-β-gal and observe the
relative optical density of stained positive cells in pancreatic
tissue. Fresh pancreatic tail tissues were taken and cut into small
sections and fixed in glutaraldehyde for 4 h and then washed with
phosphate-buffered saline 3 times, then fixed for 1 h with 1%
osmium tetroxide. Using gradient elution of ethanol and embedded
with Epon 812 to prepare the ultra-thin sections was used. Samples
were electronically double stained by uranium acetate and lead
nitrate before observation under H-600 transmission electron
microscope and the taking of images.
Measurement of pancreas oxidation and
antioxidant index
The pancreas was extracted for creating 0.1 g of
pancreas tissue homogenate, the supernatant was collected after
centrifugation as 2,500 × g for 10 min. The supernatant protein
concentration was detected using BCA Protein Assay kit for each
group. MDA content, SOD and T-AOC were measured following the kit
manufacturers instructions.
AGEs were determined
Dewaxing the paraffin section, 3% catalase was added
to remove endogenous enzyme and the tissue was sealed with 5% goat
serum. The first and second antibodies were added, following by the
developing DAB developing agent and hematoxylin for analysis of
pancreatic tissue AGEs by immumohistochemical staining. The
distribution of AGE-stained positive cell were observed at ×20
magnification, the integral optical density (IOD) of positive cells
stained for AGEs. Each section was measured using cell image
analysis system IPP.
Statistical analysis
The data were processed by using statistical
software SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) and expressed as
mean ± standard deviation. Comparison between groups was done using
one-way ANOVA test followed by post hoc test (LSD). P<0.05 was
considered to indicate a statistically significant difference.
Results
General condition of mice
After the D-gal model mice were dosed continuously
with D-gal for 6 weeks, the coat color gradually withered, and had
dim luster, and the hair was falling off. Their spirits were down,
skin elasticity was reduced, lethargic, food intake significantly
reduced and body mass increased slowly, presented the obvious signs
of aging, while the control group and Rg1+D-gal group showed no
obvious related signs.
Effect of Ginsenoside Rg1 on aging
mouse pancreas weight and viscera index
Results showed that pancreatic wet weight of D-gal
aging model group increased obviously; pancreatic wet weight of
Rg1+D-gal group were not statistically significant compared with
normal control group (Table I).
 | Table I.Effect of Ginsenoside Rg1 on pancreas
wet weight and index of D-gal model in mice (mean ± standard
deviation, n=10). |
Table I.
Effect of Ginsenoside Rg1 on pancreas
wet weight and index of D-gal model in mice (mean ± standard
deviation, n=10).
| Groups | No. | Pancreas wet weight
(g) | Body weight (g) | Pancreas index |
|---|
| Normal control | 10 |
0.2367±0.0306b | 25.7900±1.1692 |
9.1561±0.7960b |
| D-gal model | 10 | 0.4567±0.0058 | 28.0833±1.2907 | 16.2782±0.5578 |
| Rg1+D-gal model | 10 |
0.2133±0.0058b |
24.3767±0.1750a |
8.7516±0.2435b |
Effect of Rg1 on FBG in D-gal-induced
mice
The FBG in D-gal aging model was significantly
increased (C57/6J mouse blood sugar normal is 7.5 mmol/l), the FBG
were at normal level in Rg1+D-gal group and the normal control
group (P<0.01) (Fig. 1).
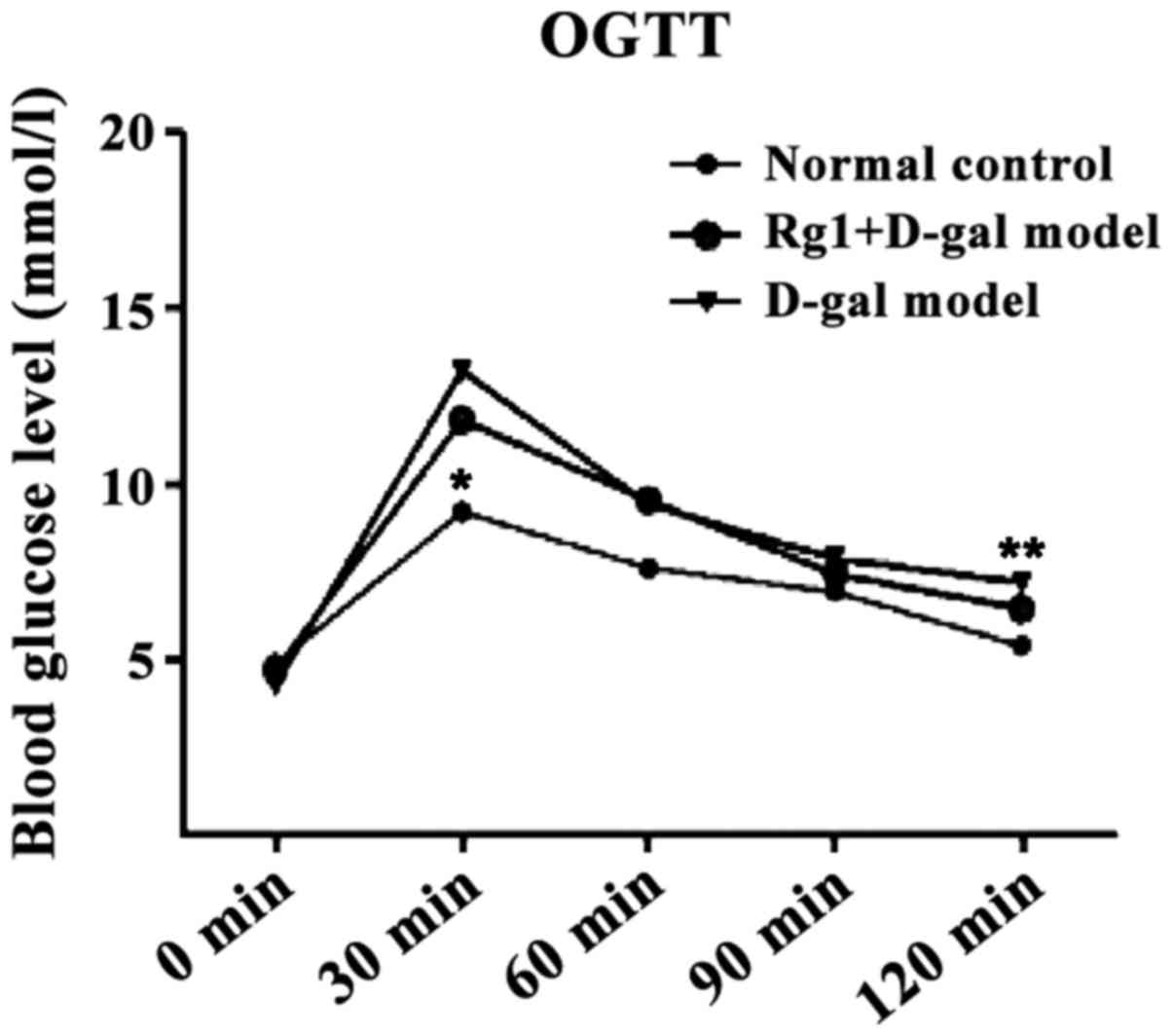
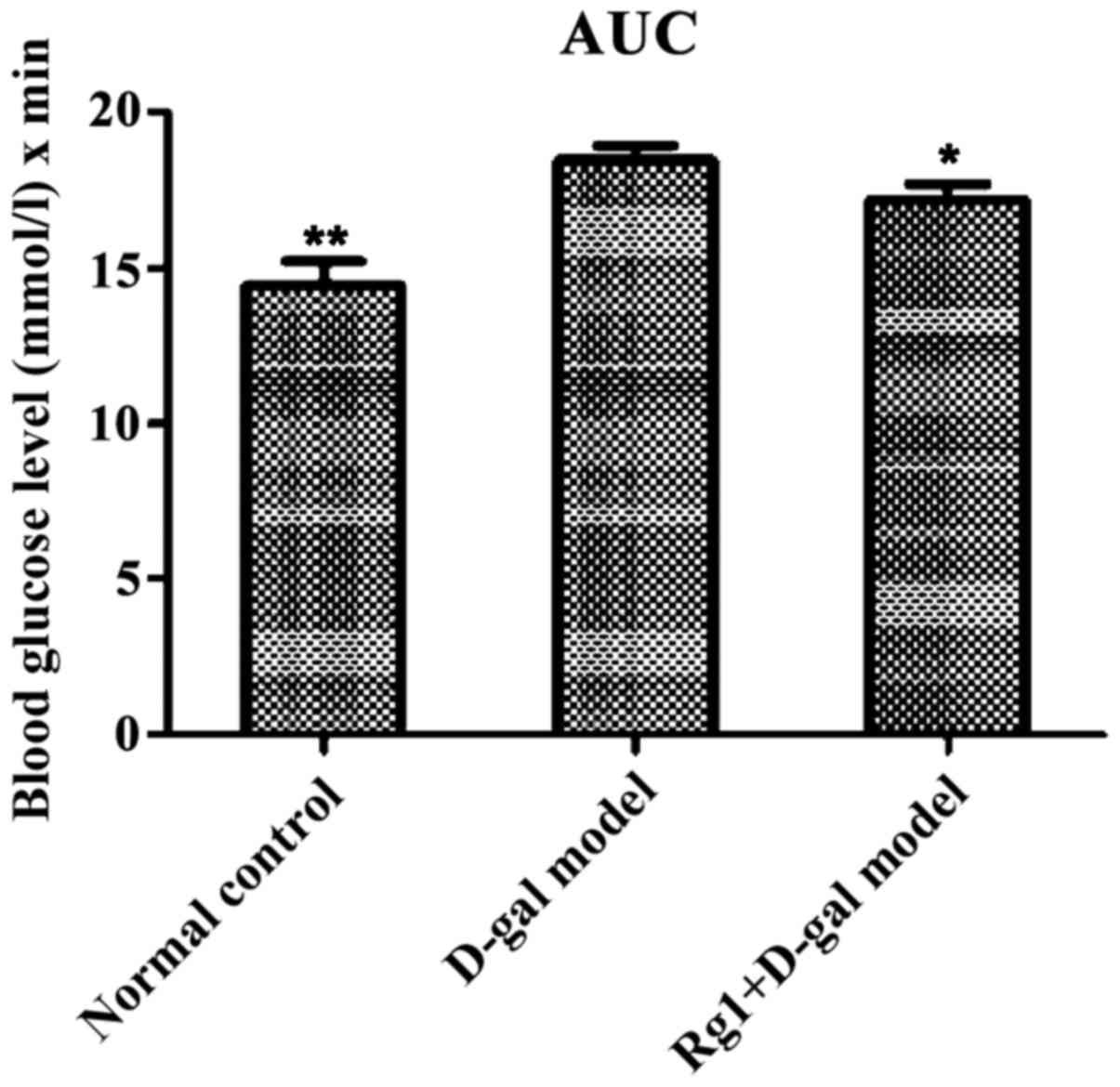
Effect of Rg1 on OGTT in mice
The blood glucose of each group reached the peak in
30 min and thereafter the level of blood glucose in all groups
showed a downward trend. D-gal aging model group was still at the
maximum during this process (Fig.
2). The AUC calculated were all less in the normal control
group (P<0.01) and Rg1+D-gal group (P<0.05)than in the D-gal
aging model group (Fig. 3).
Rg1 on D-gal aging model group effect
of pancreatic tissue
Pancreas islet morphological structures were
completed in three groups (Fig. 4).
For D-gal model group, the islet cell volume increased; the results
showed that the areas of each cell in the islets were significantly
increased compared with that of the normal control and the
Rg1+D-gal model (Fig. 5); the
percentage of total nucleated cells was increased in D-gal model
groups than that in normal control (Fig.
6).
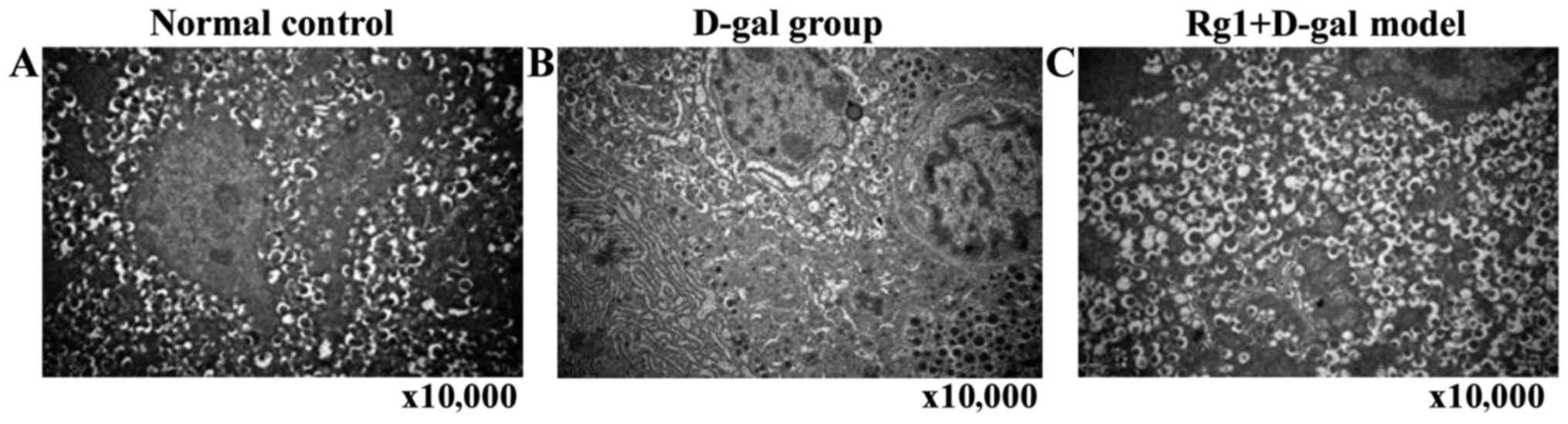
Rg1 on D-gal model group of
ultrastructure on the pancreas cells
Endocrine (islets) and exocrine pancreas cells were
observed by electron microscopy. D-gal aging model group of
external secretion cell ultrastructure showed: Swelling
mitochondria, vesicles and medullary structure formation;
endoplasmic reticulum and expanded Golgi body; individual cell
nuclei appeared with heterochromatin cohesion and perinuclear space
broadening. In Rg1+D-gal model group, mitochondria swelled
slightly, crista expanded; endoplasmic reticulum and Golgi body
expansion reduced obviously. The endocrine β-cell morphology and
number of secretory granules in each group had no significant
abnormality with normal control, aging characteristic were not
obvious (Figs. 7 and 8).
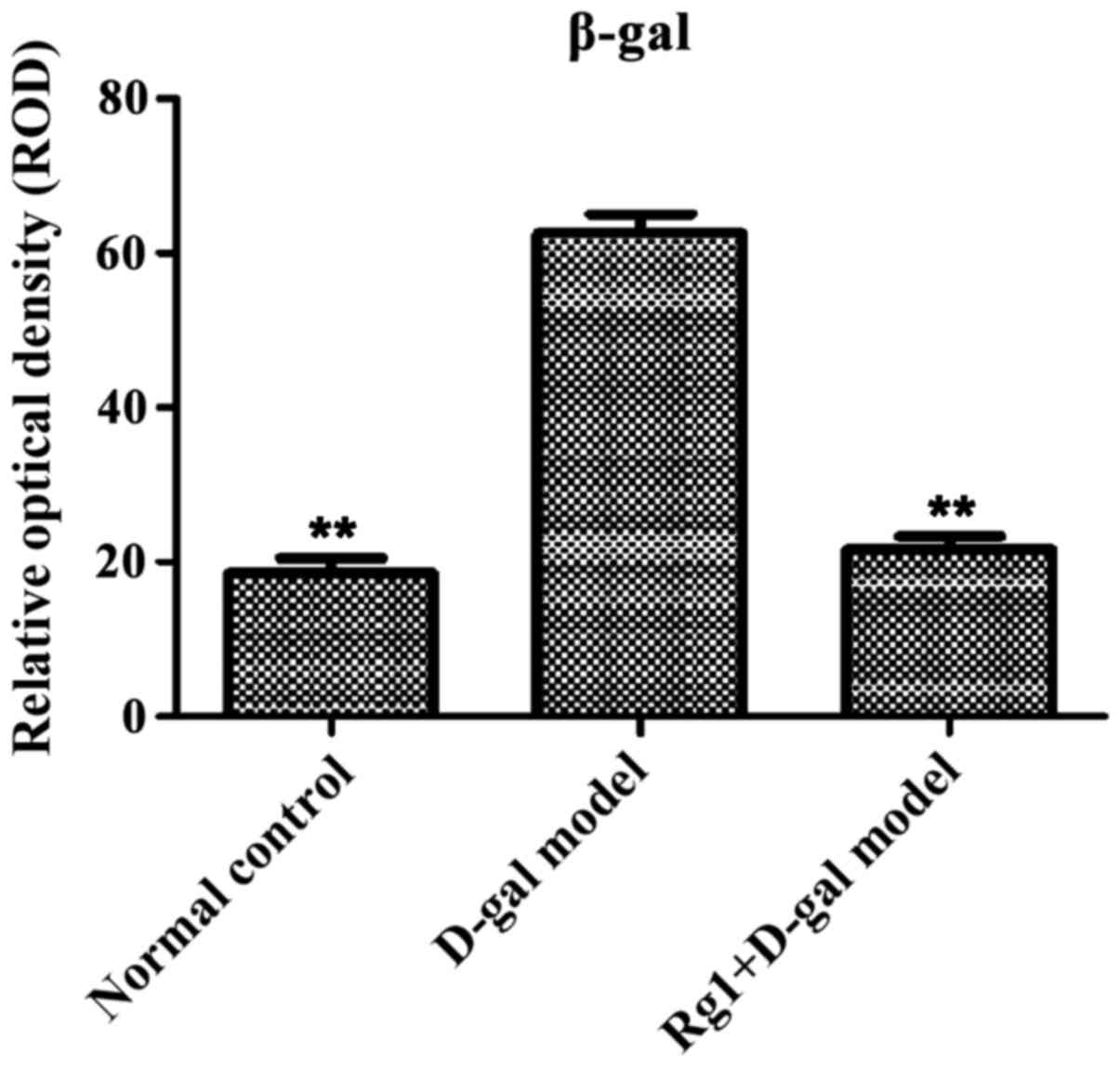
Rg1 on D-gal model group of pancreatic
tissue β-gal staining
β-galactose glucoside enzyme staining showed aging
cells in the cytoplasm with blue particles (Fig. 9). Compared with normal control and
Rg1+D-gal model group, pancreas tissue ROD was increased
(P<0.01) in D-gal aging model group (Fig. 10).
Rg1 on D-gal model group mouse
pancreas tissue oxidation and oxidative damage resistance
SOD and T-AOC were obviously decreased and MDA was
increased markedly in the D-gal aging model group. In the normal
control and Rg1+D-gal model group, the quantity of expression of
SOD and T-AOC were higher than D-gal aging model group, and the
quantity of expression of MDA was significantly decreased (Table II).
 | Table II.Effect of Rg1 on the level of SOD, MDA
and T-AOC in the pancreas of D-gal model mice (mean ± SD,
n=10). |
Table II.
Effect of Rg1 on the level of SOD, MDA
and T-AOC in the pancreas of D-gal model mice (mean ± SD,
n=10).
| Group | No. | SOD (U/mg prot) | MDA (nmol/mg) | T-AOC (U/ml) |
|---|
| Normal control | 10 |
14.669±0.365b |
3.498±0.470b |
0.266±0.011b |
| D-gal model | 10 | 11.848±0.050 | 7.421±0.293 | 0.117±0.018 |
| Rg1+D-gal model | 10 |
13.751±0.417a |
4.903±0.138b |
0.194±0.015b |
Rg1 on D-gal model group of pancreatic
AGEs
Brown areas represent AGE accumulated positive
region. D-gal model group was visible in the cytoplasm of acinar
cells and islet positive regional expression increased (Fig. 11) and IOD of pancreas AGEs was
obviously higher, with clear difference compared with normal
control (P<0.01) and Rg1+D-gal group (P<0.05) (Fig. 12).
Discussion
Pancreas is an important digestive gland to
mammalian, particularly in the human body. Pancreas secretes juice
that contains a variety of digestive enzymes. Pancreatic juice
plays an important role in digestion and absorption of food as well
as in the regulation of the constant levels of blood sugar in the
body through the secretion of insulin and glucagon and other
hormones. Indeed, pancreas is susceptible to the disease factors
damage organs, how to prevent and treat injury of the pancreas is
worth highly regarding. D-gal is now recognized as an inducer of
aging reagents that can be recapitulated in animal models of aging
model (6,7). The mechanism by which it induces damage
or aging may be related to the attacks of the mitochondrial inner
membrane lipids, protein and mitochondrial RNA that may affect the
function of mitochondria (8). The
aging model of mice made with D-gal have similarities with natural
aging mice in the level of oxidative stress-free radical injury,
non-enzymatic glycation. It may damage a number of organs including
the pancreas (9,10).
The determination of organ weight and viscera
indexes and histopathological observation can reflect the change of
organ structure to a certain degree. The results of the study
showed that the pancreas swelling was significant, wet weight and
their visceral index markedly increased in D-gal aging model group,
although pancreatic parenchyma cells of obvious damage had not seen
in the tissue slice. However, the tissue edema was obvious,
inflammatory cell infiltration, showing islet compensatory
hypertrophy on the morphology, number of islet cells and unit area
of islet cells were increased. Ultrastructure showed that
organelles of exocrine portion swelling degeneration and there was
lipofuscin deposition. Morphological structure and secretory
granule of islet cells especially the β-cell, did not change
obviously. These results indicated that the pancreas structure of
aging model mice made with D-gal was damaged. Ginsenoside Rg1 was
injected to D-gal aging model mice, but there was no evident
difference compared with normal control group in pancreatic edema,
weight, organ index or histopathology. The curative effects of
Tiped Ginsenoside Rg1 suggest antagonism effects on islet
morphology structure in aging mice damaged by D-gal.
Blood glucose level is the index to evaluate body
glucose metabolism and endurance ability, it is also reflecting
pancreatic function, especially the important index of islet
function. We observed that the value of D-gal model group FBG
exceed C57BL/6J mice maximum normal blood glucose levels, in a
state of impaired fasting glucose (IFG). OGTT is an important
indicator to determine the bodys glucose tolerance damage degree.
The blood glucose values at 30 min can reflect the insulin
resistance and pancreatic β-cell function to some extent. Blood
sugar level was not significantly different in D-gal aging model
group, and 120 min blood sugar of D-gal aging model mice did not
return to normal levels. D-gal may cause damage to glucose
tolerance in mice. While D-gal aging model mice were injected
Ginsenoside Rg1, FBG can be maintained in the normal levels. 0–120
min AUC as well as 0 and 120 min blood glucose difference value
displayed that Ginsenoside Rg1 obviously effect the reducing levels
of FBG and postprandial glucose of mice. The result indicated that
although Ginsenoside Rg1 cannot improve D-gal damage of glucose
tolerance in mice, it can, however, effectively lower the level of
blood glucose in mice (9).
SA-β-gal is an important marker of cell senescence,
which can reflect the cell lysosome function (11). AGEs by increasing ROS generation,
damaging the antioxidant system repair and inducing the formation
of their own can accelerate cellular aging process (12). Both are important indicators to
evaluate cell aging. The early stage of the research found
Ginsenoside Rg1 as anti-ageing active ingredient in ginseng
inhibited oxidative damage effect. Our data proved that D-gal aging
model group in the pancreatic tissue β-gal dyed relative optical
density of positive cells increased significantly and AGE positive
staining region of IOD increased, thus showing that D-gal can lead
to pancreas cell aging. While aging model mice were injected
Ginsenoside Rg1, the pancreas significantly reduced aging, the
degree of pancreatic aging significantly reduced, suggesting that
Ginsenoside Rg1 may counteract aging effects induced by D-gal on
mouse pancreas (13).
Oxidative damage is the mainstream theory of
cellular senescence. SOD is an important scavenging enzyme of
oxygen free radicals and has the function of protecting cellular
DNA, proteins and cell membranes (14). Oxidative damage produced more
reactive oxygen species (ROS) and disturbed cellular redox signal
(15), eventually causing cell DNA,
protein, and lipid damage (16,17). MDA
is used in the evaluation of biomarkers of oxidative stress being
the final products of ROS induced peroxidation. Our data show the
reduction of pancreatic tissue SOD, T-AOC expression and the
enhancement of MDA expression. Injection of Ginsenoside Rg1 in
aging model mice induced SOD, T-AOC expression was enhanced and MDA
expression reduced, hinting that Ginsenoside Rg1 can effectively
raise the content of antioxidant enzymes in the pancreas,
scavenging histiocytic free radicals improving its oxidative
stress.
In conclusion, Ginsenosides Rg1 is an effective
antagonist of mouse pancreas damage induced with D-gal; reducing
the FBG level. The mechanism may be through two aspects of
anti-aging and improve blood sugar levels. A tentative inference on
this result is that the mechanism may be related to reduced
oxidative damage.
Acknowledgements
The authors are grateful to grants from the National
Natural Science Foundation of China (no. 30973818).
References
|
1
|
Li XT, Wei HH, Zhang HL, Sun HB and Su YR:
Mitochondrial reactive oxygen species scavenging investigations of
Astragalus membranaceus/Codonopsispilosula Qi-invigorating herbal
tea. J Dalian Nationalities University. 5:453–457. 2015.
|
|
2
|
Enhu Zhang, Qi Zhang and Rui HU: Review on
anti-oxidant effect of Chinese Tonic-Qi herbs. Glob Tradit Chin
Med. 6:490–493. 2011.
|
|
3
|
Cheng Y, Shen LH and Zhang JT:
Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and
its mechanism of action. Acta Pharmacol Sin. 26:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan Y, Xia J, Jia D, Zhang M, Zhang Y,
Huang G and Wang Y: Mechanism of ginsenoside Rg1 renal protection
in a mouse model of d-galactose-induced subacute damage. Pharm
Biol. 54:1815–1821. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CP, Zhang MS, Liu J, Geng S, Li J, Zhu
JH, Zhang YY, Jia YY, Wang L, Wang SH, et al: Research of
anti-aging mechanism of ginsenoside Rg1 on brain. Zhongguo Zhong
Yao Za Zhi. 39:4442–4447. 2014.(In Chinese). PubMed/NCBI
|
|
6
|
Ho SC, Liu JH and Wu RY: Establishment of
the mimetic aging effect in mice caused by D-galactose.
Biogerontology. 4:152003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB,
Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, et al: Selective
beta-cell loss and alpha-cell expansion in patients with type 2
diabetes mellitus in Korea. J Clin Endocrinol Metab. 88:2300–2308.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
St-Pierre J, Buckingham JA, Roebuck SJ and
Brand MD: Topology of superoxide production from different sites in
the mitochondrial electron transport chain. J Biol Chem.
277:44784–44790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song X, Bao M, Li D and Li YM: Advanced
glycation in D-galactose induced mouse aging model. Mech Ageing
Dev. 108:239–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brownlow BS, Petro A, Feinglos MN and
Surwit RS: The role of motor activity in diet-induced obesity in
C57BL/6J mice. Physiol Behav. 60:37–41. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I and Pereira-Smith
O: A biomarker that identifies senescent human cells in culture and
in aging skin in vivo. Proc Natl Acad Sci USA. 92:pp. 9363–9367.
1995; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nowotny K, Jung T, Höhn A, Weber D and
Grune T: Advanced glycation end products and oxidative stress in
type 2 diabetes mellitus. Biomolecules. 5:194–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thorpe SR and Baynes JW: Maillard reaction
products in tissue proteins: New products and new perspectives.
Amino Acids. 25:275–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Warner HR: Superoxide dismutase, aging,
and degenerative disease. Free Radic Biol Med. 17:249–258. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sies H: Oxidative stress: A concept in
redox biology and medicine. Redox Biol. 4:180–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Das SK and Vasudevan DM: Alcohol-induced
oxidative stress. Life Sci. 81:177–187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ashok BT, Ahmad J and Ali R:
Immunochemical detection of oxidative DNA damage in cancer and
aging using anti-reactive oxygen species modified DNA monoclonal
antibody. Int J Biochem Cell Biol. 30:1367–1377. 1998. View Article : Google Scholar : PubMed/NCBI
|