Introduction
Exposure to chlorine gas may result in serious
adverse effects and potentially, patient mortality. Between 2000
and 2005, ~9,000 cases of chlorine gas exposure were annually
reported to poison control centers in the United States (1). Chlorine gas is a potent pulmonary
irritant known to cause acute damage in the upper and lower
respiratory tracts (2). Chlorine gas
inhalation typically occurs following accidental exposure from
chemicals used in manufacturing, or in the household and at
swimming pools (3). Short-term,
high-level exposure due to traffic accidents, chlorine spills or
other disasters may result in symptoms of acute airway obstruction
occurring, including wheezing, cough, chest tightness and dyspnea.
More severely affected individuals may suffer from acute lung
injury and acute respiratory distress syndrome (2–4) and ~1%
of exposed individuals succumb (4).
Mortality occurs primarily due to pulmonary edema with respiratory
failure and circulatory collapse (5). Inhaling a large amount of gas may lead
to the development of respiratory and circulatory disorders, or
even cardiopulmonary arrest (6).
Myocardial infarction, acute ischemic stroke and hyperglycemia may
be triggered by acute chlorine gas inhalation (5). By contrast, workplace and public
exposures are usually long-term, low-level exposures, which may
result in increased airway reactivity. It is very difficult to
provide a range for asymptomatic exposure as it is related to the
materials that generate chlorine gas, the exposure time and the
difference in an individual's sensitivity to chlorine gas. An
acceptable chlorine concentration is considered to be <0.5 parts
per million (ppm) and symptoms may appear following exposure to 2–5
ppm chlorine (7). Serious symptoms,
including dyspnea, unconsciousness and mortality, usually occur 30
min to 1 h following exposure to 30 to 60 ppm chlorine gas. The
current study presents the case of a patient exposed to chlorine
gas, resulting in bronchial damage and diffuse alveolar hemorrhage,
confirmed by fiberoptic bronchoscopy (FB).
Case report
A 55-year-old male patient brought to the emergency
clinic at the Mito Medical Center (Mito, Japan) presented with
severe dyspnea. The patient became lethargic and found it
impossible to talk due to severe shortness of breath. In addition,
impaired consciousness and hypoxia were observed in the patient,
who was admitted with a primary diagnosis of acute respiratory
failure due to an unknown cause. The vital signs of the patient
were measured and recorded, and were as follows: Pulse rate, 190
beats/min (normal range: ≤100 beats/min); blood pressure, 111/86
mmHg (within the normal range); respiratory rate, 34 breaths/min
(normal range: ≤20 breaths/min); temperature, 37.8°C (normal range:
≤37.0°C) and oxygen saturation measured by pulse oximetry
(SpO2), 82% (normal range: ≥90%). Physical examination
revealed rigorous inspiratory retraction and coarse crackles. Chest
radiograph and computed tomography (CT) scan indicated diffuse
ground glass opacity in the left and right lung (Fig. 1). The patient was monitored and
catheterized. Due to a decrease in SpO2 to 70% with 10
l/min of oxygen inhalation using a reservoir mask, noninvasive
positive pressure ventilation (NPPV) was applied using a
Respironics V60 ventilator (Philips Japan, Tokyo, Japan) 14 h
following hospital admittance, as SpO2 could not be
spontaneously maintained by the patient in this condition. The
initial setting of NPPV was continuous positive airway pressure
(CPAP) mode with 10 mmHg inhaling 100% oxygen. Following
application of NPPV, SpO2 increased to 90%. CPAP
treatment gradually weakened the positive pressure in the airway to
atmospheric pressure whilst simultaneously improving
SpO2 levels and NPPV was discontinued following 24 h
treatment, and 38 h after the patient was admitted to hospital. At
this point, physicians were made aware that the patient had treated
chloric acid and inhaled chlorine gas twice for a few sec 13 h
prior to the arrival at hospital and that dyspnea and bloody sputa
began 11 h prior to patient admittance. FB was performed when the
patient was in a conscious state and under local anesthesia (4 ml
4% lidocaine delivered by bronchoscopic injection), after the
termination of NPPV to identify tracheobronchial appearance and
perform bronchoalveolar lavage (BAL) using FB (Olympus BF-260;
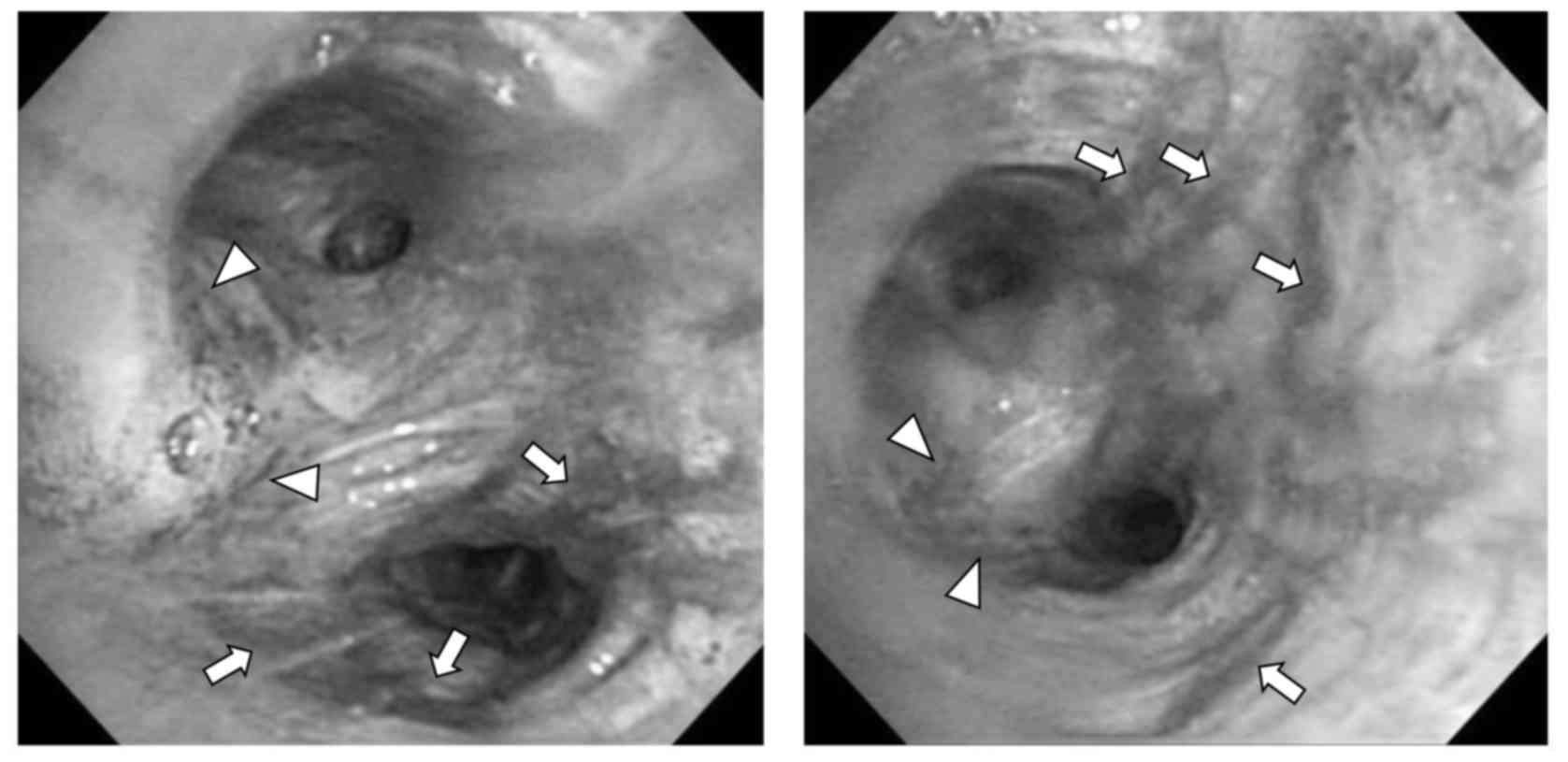
Olympus Corporation, Tokyo, Japan). Following FB, hyperemic and
edematous change was observed in the trachea and bronchus,
specifically at interlober spurs between the upper and lower
bronchus in the left lung (Fig. 2).
BAL fluid from the left lower lobe of the lung had reddish brown
appearance with hemosiderin-laden macrophages, suggesting pulmonary
hemorrhage. Respiratory condition was maintained using NPPV and
oxygen therapy; no corticosteroids or antibiotics were administered
to the patient. Diffuse ground glass opacity in the left and right
lungs had disappeared on the fifth day of treatment, as indicated
by a CT scan. The patient completely recovered and was discharged
home five days following hospital admittance with no medication.
Once a month for six months, the patient was followed up, but there
was no deterioration of respiratory condition.
Discussion
The mechanism of respiratory tract injury by
chlorine gas inhalation is associated with its ability to form
hypochlorous and hydrochloric acids in the respiratory tract
(8). Due to the relatively low
solubility of chlorine gas in water, chlorine is able to reach the
periphery of the lungs and cause extensive damage, unlike ammonia
and other highly soluble gasses, which are removed from the
proximal airway by mucocilliary clearance (3,8).
Chlorine gas has a highly irritant effect on the airway epithelium,
however; its effect depends on the concentration of inhaled gas as
well as the duration of exposure (6,9). A large
amount and long duration of chlorine gas inhalation may lead to the
development of respiratory and circulatory disorders or even
cardiopulmonary arrest, however a small amount and short duration
of exposure may be asymptomatic (6).
Therefore, chest radiographs and CT scans may not detect any
unusual signs, such as pulmonary edema, patchy consolidation,
diffuse nodular opacity or vascular congestion (8–10).
Previous case reports have indicated that chlorine
gas inhalation may affect various systemic systems other than the
respiratory system (5,6). Kose et al (5) documented a case of acute ischemic
stroke, myocardial infarction and hyperglycemia triggered by acute
chlorine gas inhalation, and emphasized that physicians should keep
in mind all intoxications that may affect various systems and
thereby trigger various diseases. However, the patient observed in
the present study had a 10-year history of diabetes mellitus and
4-year history of arrhythmia, but no deterioration of these
diseases during the clinical course was observed. Li et al
(6) reported two cases of
pneumomediastinum following acute inhalation of chlorine gas; this
complication was not observed in the patient in the present study.
Additionally, later complications, such as bronchial asthma,
decrease residual volume and increase airway responsiveness have
been reported following chlorine gas inhalation (11–14).
Therefore, patients require careful follow up observations to
detect and prevent the development of such late complications. In
the present study, the patient was followed up once a month for six
months, however there was no deterioration of respiratory
condition. The importance of FB in patients with toxic gas
inhalation has been previously reported (9,15,16),
however performing FB is complicated, due to the severity of the
respiratory condition in the majority of patients presenting with
symptoms of chlorine gas inhalation. Yarkin et al (9) reported tracheobronchial mucosal injury
in a patient treated for chlorine gas inhalation and this was the
first reported case to provide FB images. In this patient,
hemorrhagic inflammation associated with white necrotic lines on
the mucosal surface and disappearance of the cartilage rings of
trachea was observed (9). The major
bronchial lumens were obstructed with yellowish hard materials
thus; FB could not be advanced distally through the main bronchus.
Therefore, BAL could not be performed and the patient succumbed to
severe acidosis, hypoxemia and high fever on the third day of
admission (9). The present study
presents a case with FB images as well as BAL findings in a patient
with respiratory tract injury due to chlorine gas inhalation. As
mentioned previously, there was a difference in the concentration
and exposure time of inhaled chlorine gas. Therefore, it seems that
there may be a difference in the severity of symptoms experienced
by the patient in the study by Yarkin et al (9) and the patient in the current study. To
the best of our knowledge, the current case report presents the
first successfully treated patient with chlorine gas inhalation,
with FB images as well as BAL findings of chlorine gas
inhalation.
Yamamoto et al (17) reported two cases of delayed-onset
acute lung injury following chlorine gas exposure. Based on the
results of Yamamoto et al (17) and other previous reports (18–20), it
has been suggested that there may be a latent period lasting up to
10 h and that symptoms may worsen 48 h following exposure to
chlorine gas. In the current study, the patient developed
respiratory failure after 4 h and symptoms worsened 13–14 h
following initial exposure. The application and discontinuation of
NPPV occurred 14 and 38 h following exposure, respectively and FB
was performed 40 h following exposure to chlorine gas. This
clinical course suggests that the patient in the present study had
‘delayed-onset’ lung injury and not ‘immediate-onset’, as the
development and deterioration of symptoms in the patient did not
begin rapidly following the inhalation. The amount of chlorine gas
inhaled appeared to be small and occurred for a short duration as
the patient demonstrated a rapid improvement of respiratory
condition without any residual signs or symptoms, despite the
diffuse alveolar hemorrhage and endobronchial injury that
occurred.
Obtaining a precise medical history is important in
order to correctly diagnose patients with toxic gas inhalation. In
addition, timely and proper evaluation using chest imaging and FB
may provide useful clinical information. Therefore, clinicians
should consider performing FB if the circumstances permit. It may
be difficult to provide detailed criteria of the appropriate
general and respiratory conditions to perform FB safely, as
patients may have been exposed to chlorine gas for different
durations, inhaled different concentrations of chlorine and respond
very differently to chlorine gas inhalation. However, it is
important to clarify indications and contraindications of FB as
this may provide clinicopathological information regarding central
and peripheral airways in patients that have experienced chlorine
gas inhalation.
References
|
1
|
Becker M and Forrester M: Pattern of
chlorine gas exposures reported to Texas poison control centers,
2000 through 2005. Tex Med. 104:51–57. 2008.
|
|
2
|
Sexton JD and Pronchik DJ: Chlorine
inhalation: The big picture. J Toxicol Clin Toxicol. 36:87–93.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winder C: The toxicology of chlorine.
Environ Res. 85:105–114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White CW and Martin JG: Chlorine gas
inhalation: Human clinical evidence of toxicity and experience in
animal models. Proc Am Thorac Soc. 7:pp. 257–263. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kose A, Kose B, Açikalin A, Gunay N and
Yildirim C: Myocardial infarction, acute ischemic stroke and
hyperglycemia triggered by acute chlorine gas inhalation. Am J
Emerg Med. 27:1022.e1–4. 2009. View Article : Google Scholar
|
|
6
|
Li B, Jia L, Shao D, Liu H, Nie S, Tang W,
Xu B, Hu Z and Sun H: Pneumomediastinum from acute inhalation of
chlorine gas in 2 young patients. Am J Emerg Med. 29:357.e1–4.
2011. View Article : Google Scholar
|
|
7
|
National Research Council, . Emergency and
continuous exposure limits for selected airborne contaminants. 2.
Washington, DC: National Academy Press. Committee on Toxicology,
Board on Toxicology and Environmental Health Hazards, Commission on
Life Sciences, National Research Council; pp. 5–110. 1984
|
|
8
|
Kanne JP, Thoongsuwan N, Parimon T and
Stern EJ: Trauma cases from Harborview Medical Center. Airway
injury after acute chlorine exposure. AJR Am J Roentgenol.
186:232–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yarkın T, Adıgüzel N, Karakurt Z, Güngör
G, Aksoy F and Baran R: Chlorine-induced extensive tracheobronchial
necrosis concomitantly benzene-induced pancytopenia presented with
severe pneumonia. Tuberk Toraks. 58:439–443. 2010.PubMed/NCBI
|
|
10
|
Parimon T, Kanne JP and Pierson DJ: Acute
inhalation injury with evidence of diffuse bronchiolitis following
chlorine gas exposure at a swimming pool. Respir Care. 49:291–294.
2004.PubMed/NCBI
|
|
11
|
Kilburn KH: Chlorine-induced damage
documented by neurophysiological, neuropsychological, and pulmonary
testing. Arch Environ Health. 55:31–37. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gautrin D, Leroyer C, Infante-Rivard C,
Ghezzo H, Dufour JG, Girard D and Malo JL: Longitudinal assessment
of airway caliber and responsiveness in workers exposed to
chlorine. Am J Respir Crit Care Med. 160:1232–1237. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwartz DA, Smith DD and Lakshminarayan
S: The pulmonary sequelae associated with accidental inhalation of
chlorine gas. Chest. 97:820–825. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donnelly SC and FitzGerald MX: Reactive
airways dysfunction syndrome (RADS) due to chlorine gas exposure.
Ir J Med Sci. 159:275–277. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akhavan A, Ajalloueyan M, Ghanei M and
Moharamzad Y: Late laryngeal findings in sulfur mustard poisoning.
Clin Toxicol (Phila). 47:142–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen MA and Guzzardi LJ: Inhalation of
products of combustion. Ann Emerg Med. 12:6281983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto R, Fujishima S and Ueno K: Two
cases of delayed-onsetacute lung injury after chlorine gas
exposure. JJAAM. 20:390–396. 2009.(In Japanese).
|
|
18
|
Tian X, Tao H, Brisolara J, Chen J, Rando
RJ and Hoyle GW: Acute lung injury induced by chlorine inhalation
in C57BL/6 and FVB/N mice. Inhal Toxicol. 20:783–793. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Babu RV, Cardenas V and Sharma G: Acute
respiratory distress syndrome from chlorine inhalation during a
swimming pool accident: A case report and review of the literature.
J Intensive Care Med. 23:275–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Batchinsky AI, Martini DK, Jordan BS, Dick
EJ, Fudge J, Baird CA, Hardin DE and Cancio LC: Acute respiratory
distress syndrome secondary to inhalation of chlorine gas in sheep.
J Trauma. 60:944–957. 2006. View Article : Google Scholar : PubMed/NCBI
|