Introduction
Obesity-induced systemic inflammation originates in
adipose tissue prior to hepatic tissue (1,2). The
human body contains various fat deposits, which can be divided into
white and brown fat. White adipose tissue (WAT) is a
multifunctional organ that stores nutrients in the form of fat
droplets. In addition, WAT secretes cytokines that affect the
body's metabolic state, thus it is sometimes regarded as the
largest endocrine organ in the body (3). Excessive energy intake induces
adipocyte hypertrophy and hyperplasia, which may lead to the
development of high fat diet-induced obesity. Hypertrophic
adipocytes release chemokines and proinflammatory cytokines to
activate and attract inflammatory cells into WAT. This contributes
to systemic insulin resistance and ultimately, a state of chronic
low-grade adipose tissue inflammation (4). Polyphenol intake is positively
associated with a decrease in the incidence of metabolic and
obesity-associated disorders. Quercetin is a polyphenolic flavonoid
compound present in a variety of fruits and vegetables, including
onions, broccoli, tomatoes, apples and berries. It has a wide range
of biological activities and health-promoting effects, including
anti-carcinogenic (5), antiviral
(6), antioxidant (7), antidiabetic (8), anti-inflammatory (9), anti-aging (10) and angioprotective properties
(11). Furthermore, it has recently
been suggested that quercetin exerts anti-obesity activity via the
mitogen-activated protein kinase (MAPK) and 5′-adenine
monophosphate-activated protein kinase α1 (AMPKα1) signaling
pathways (12). Resveratrol, a
phytoalexin found in the skin and seeds of grapes and in red wine,
may also protect against diet-induced obesity and metabolic
diseases including hepatic steatosis and insulin resistance
(13). In the present study, the
effect of combination treatment with quercetin and resveratrol
(CQR) was investigated in rats fed a HFD. The impact on CQR on
HFD-induced fat accumulation, insulin resistance, proinflammatory
cytokine levels, mast infiltration and AMPKα1/sirtuin 1 (SIRT1)
signaling in adipose tissues was assessed.
Materials and methods
Animals
The present study was approved by the Institutional
Animal Care and Use Committee of Shanghai University of Traditional
Chinese Medicine (Shanghai, China), and all procedures were
performed in accordance with the National Institute of Health's
guidelines (14). A total of 46 male
8-week-old Wistar rats with a mean weight of 200±10 g were provided
by the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China).
Rats were raised in an environment of 22±0.5°C and 40–70% relative
humidity under a 12 h light/dark cycle, with food and water freely
available. Following 1-week habituation, the rats were randomly
divided into 2 groups. The normal diet (ND) group, (n=10) were fed
a regular chow diet containing 5.4% fat and the HFD group, (n=36)
were fed a HFD containing 45% fat. After 3 weeks, rats in the HFD
group were ranked according to weight gain, and rats in the lower
third (n=12) were excluded from the study as they were deemed to be
obesity resistant. The remaining 24 rats in the HFD group were
randomly divided into 2 sub-groups: i) A HFD group (n=12); and ii)
a HFD+CQR (n=12) group. CQR treatment consisted of 120 mg/kg/day
resveratrol (purity, ≥98%; Hangzhou Great Forest Biomedical Co.,
Ltd., Hangzhou, China) and 240 mg/kg/day quercetin (purity ≥98;
Nanjing Zelang Medical Technology Co., Ltd., Nanjing, China) The
body weight and food intake of the rats was recorded each week.
After 11 weeks, rats were anesthetized with isoflurane and
sacrificed following a 12 h fast. Blood was extracted from the
abdominal aorta and centrifuged at 5,000 × g for 15 min at 4°C. The
serum was separated and stored at −80°C prior to analysis.
Following blood collection, subcutaneous adipose tissues (SATs),
epididymis adipose tissues (EATs) and perinephric adipose tissues
were promptly removed, rinsed, weighed on ice and then snap frozen
in liquid nitrogen and stored at −80°C.
Biochemical parameters
Total cholesterol (C), triglycerides (TG),
high-density lipoprotein-C (HDL-C) and low-density lipoprotein-C
(LDL-C) in the serum were measured and quantified using a Hitachi
7600 automatic biochemical analyzer (Hitachi, Ltd., Tokyo, Japan)
with the corresponding kits as follows: Quick Auto Neo T-CHOLII
assay reagent and Quick Auto Neo TGII assay reagent, manufactured
by Shino-Test Corporation (Tokyo, Japan); L-type HDL-C reagent 1
(cat no. 998-09011), reagent 2 (cat no. 994-09111); and L-type
LDL-C reagent 1 (cat no. 997-39893) and reagent 2, (cat no.
993-39993), manufactured by Wako Pure Chemical Industries, Ltd.
(Osaka, Japan).
Cytokine quantification
Levels of leptin, adiponectin, insulin,
interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and monocyte
chemotactic protein-1 (MCP-1) in the serum were quantified using
the corresponding commercial rat ELISA kits as follows: Rat leptin,
LEP ELISA kit (cat no. CSB-E07433r); rat adiponectin, ADP ELISA
kit, (cat no. CSB-E07271r); rat insulin, INS ELISA kit
(CSB-E05070r); rat IL-6, IL-6 ELISA kit (cat no. CSB-E04640r); rat
TNF-α ELISA kit (cat no. CSB-E11987r); and rat MCP-1/monocyte
chemotactic and activating factor, MCP-1/MCAF ELISA kit (cat no.
CSB-E07429r; Cusabio Biotech Co., Ltd., Wuhan, China) according to
the manufacturer's protocols.
Histopathology
Adipose tissues were collected at 11 weeks and fixed
in 4% formalin at room temperature for 24 h, embedded in paraffin
and sliced serially into sections 5-µm thick. To determine
adipocyte size, hematoxylin and eosin staining was conducted on
EATs at room temperature for 30 min. Five visual fields were
randomly selected from each section with an Olympus BX51 light
microscope (Olympus Corporation, Tokyo, Japan) and examined using
Image-Pro Plus version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA) to determine the average adipocyte diameter. Toluidine blue
staining was also performed on EATs for 1 h by briefly submerging
tissue sections in 0.1% aqueous toluidine blue (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at room temperature, the
histological images were used to quantify the number of mast cells
present, as previously described (15). Mast cell numbers were counted using a
light microscope and were presented as cell
numbers/mm2.
Protein extraction and western blot
analysis
Plasma membrane proteins were extracted from adipose
tissues using a radioimmunoprecipitation assay lysis buffer (cat
no. 89900, Pierce; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), total protease inhibitor and phosphatase inhibitor, as
described previously (16). Protein
concentration was measured using a commercial bicinchoninic acid
assay kit and a microplate reader at 570 nm. Subsequently protein
(40 µg/lane) was separated by 10% SDS-PAGE and transferred to a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were blocked with 2% bovine serum
albumin (cat no. K720; Ameresco, Inc., Framingham, MA, USA) at room
temperature and TBS with Tween-20 for 1 h, then incubated overnight
at 4°C with AMPKα1 antibody (cat no. 2795), phosphorylated
(p)-AMPKα1 (Thr172; cat no. 2535), or SIRT1 rabbit monoclonal
antibodies (cat no 3931). Membranes were also incubated with
monoclonal mouse anti-human β-actin antibody (cat no. 4967; all
1:1,000 dilution; all from Cell Signalling Technology, Inc.,
Danvers, MA, USA) as the loading control. Following extensive
washing in Tween-PBS, membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody
(1:4,000; cat no. sc-2030; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for 1 h. Bands were visualized using LightShift™
Chemiluminescent electrophoretic mobility shift assay kit (cat no.
20148; Thermo Fisher Scientific, Inc.) and the ImageQuant™ LAS 4000
Mini, quantified using ImageQuant TL 7.0 software (both from GE
Healthcare, Chicago, IL, USA) and expressed as the ratio of pAMPKα1
to AMPKα1 or SIRT1 to β-actin.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. For multiple comparisons, differences were analyzed
using one-way analysis of variance followed by Tukey's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference. All statistics were analyzed
using Graphpad Prism version 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
Treatment with CQR results in a lower
body and visceral adipose tissue weight in an HFD-rat model
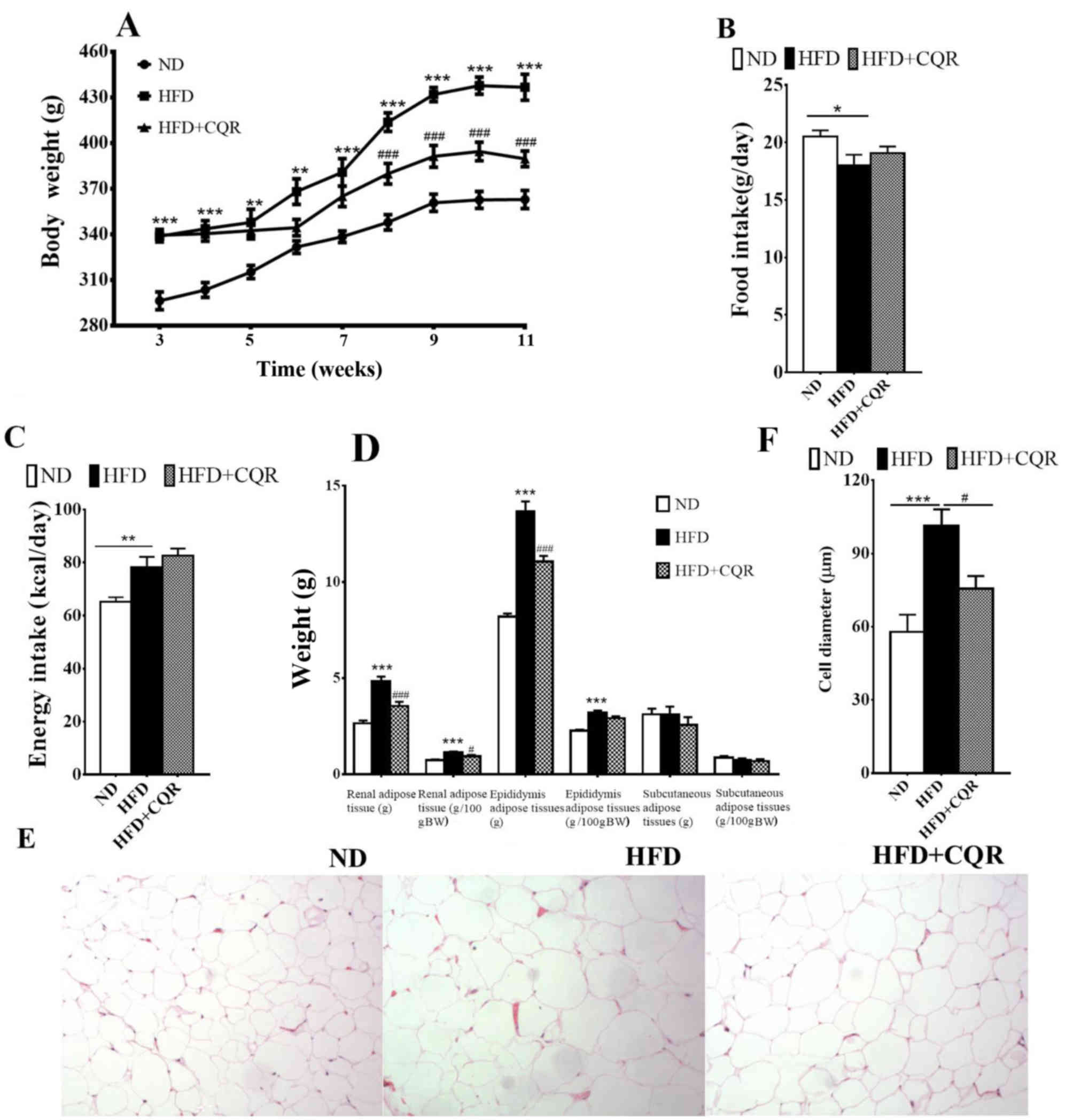
After 3 weeks, at the point of CQR intervention, the
body weight of rats in the HFD group was significantly higher than
those in the ND group (Fig. 1A). The
body weight increase was alleviated by CQR between weeks 8 and 11
and following 11 weeks intervention; HFD+CQR rats had a
significantly lower body weight than HFD rats (Fig. 1A). Food intake was significantly
lower in HFD rats compared with ND rats (Fig. 1B), however energy intake was
significantly higher (Fig. 1C). CQR
did not exert a marked effect on food and energy intake. At the end
of week 11, HFD+CQR rats had a notably lower visceral (epididymal
and perirenal) adipose tissue weight (Fig. 1D) and a significantly smaller adipose
cell diameter compared with the HFD group (Fig. 1E and F). Subcutaneous adipose tissue
weight did not differ significantly across all groups (Fig. 1D). These results suggest that CQR
treatment may inhibit HFD-induced obesity.
CQR treatment affects lipid levels in
the serum
The HFD group exhibited significantly higher levels
of total C, TG and LDL-C in the serum compared with the ND group
(Table I). CQR significantly
attenuated these lipid levels compared with the HFD. However, CQR
did not reverse the decrease in serum HDL-C induced by HFD
(Table I).
 | Table I.CQR normalizes the concentrations of
serum constituents in HFD fed rats. |
Table I.
CQR normalizes the concentrations of
serum constituents in HFD fed rats.
| Parameter | ND | HFD | HFD+CQR |
|---|
| Serum total C
(µmol/l) |
1536±101.5a |
1987±100.6 |
1587±70.36a |
| Serum TG
(µmol/l) |
685.8±89.93c |
1366±129.3 |
746.4±80.98c |
| Serum HDL-C
(µmol/l) |
1010±24.72b |
778.3±30.79 |
901.7±23.09 |
| Serum LDL-C
(µmol/l) |
275.0±13.76b |
462.5±29.39 |
305.0±31.66b |
| Serum insulin
(IU/ml) |
66.66±4.422a |
84.65±4.917 |
68.16±3.454a |
| Serum leptin
(pg/ml) |
542.5±27.07c |
904.2±47.88 |
667.7±36.90c |
| Serum adiponectin
(pg/ml) |
68.78±4.889a |
38.74±3.740 |
60.70±3.934 |
| Serum TNF-α
(pg/ml) |
41.50±6.000b |
71.28±3.545 |
50.34±5.403a |
| Serum IL-6
(pg/ml) |
1.141±0.0871b |
2.063±0.2744 |
1.131±0.0842b |
| Serum MCP-1
(pg/ml) |
47.56±5.594a |
72.47±3.848 |
49.01±4.671a |
CQR treatment reduces insulin and
leptin levels in serum
HFD significantly elevated serum insulin and leptin
levels but lowered the serum adipinectin levels compared with the
ND (Table I). CQR significantly
decreased serum insulin and leptin levels but exhibited no
significant effect on serum adipinectin compared with the HFD
group.
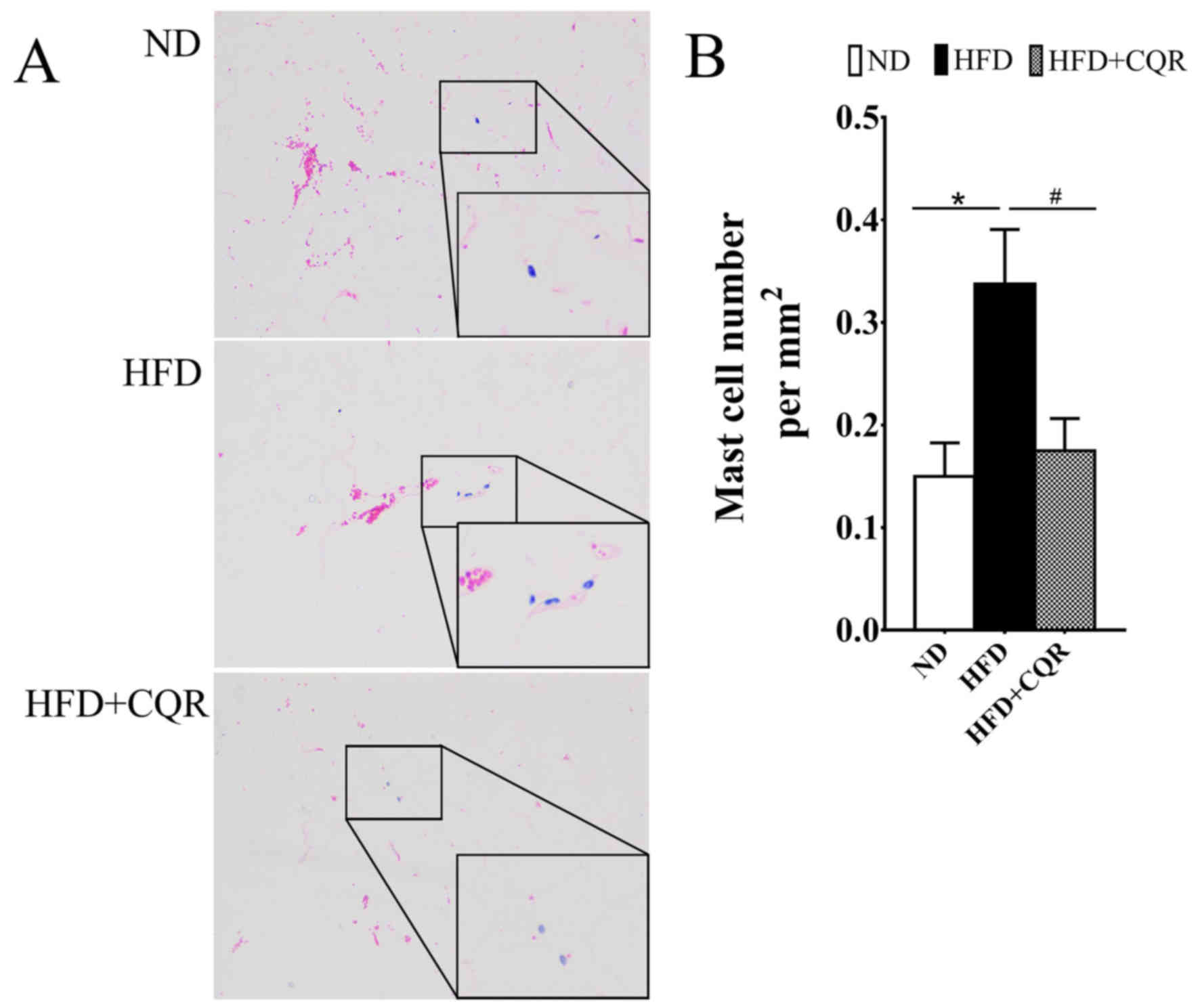
CQR suppresses the clustering of mast
cells in adipose tissue
During the formation of HFD induced-obesity and
exacerbation of adipose tissue inflammation or insulin resistance,
a large amount of mast cells infiltrate the adipose tissue
(17). It was reported that HFD
induced mast cell clustering in WAT and promoted obesity and
insulin resistance (18), while
quercetin suppresses the release of cytokines from mast cells in
vitro and obesity mice (16,19). The
number of mast cells in EAT was calculated to evaluate the effects
of CQR on mast cell in adipose tissue of HFD-induced rats (Fig. 2). The results demonstrated that HFD
significantly promoted the transition of mast cells into EATs,
while CQR significantly reversed this effect (both P<0.05).
CQR suppresses proinflammatory
cytokines
A variety of proinflammatory cytokines are secreted
by hypertrophic adipocytes and cause inflammatory cell infiltration
during the development and progression of obesity (20). Several important proinflammatory
cytokines involved in insulin resistance were detected in this
study; results from ELISA determined that levels of the
proinflammatory cytokines TNF-α, IL-6 and MCP-1, which increased in
rats on a HFD, were significantly suppressed by CQR (Table I). These results suggest that CQR may
relieve systematic inflammation induced by obesity.
CQR upregulates the AMPKα1/SIRT1
signalling pathway
AMPKα1 and SIRT1 are two key nutrient sensors
linking nutrient metabolism and inflammation (21–22).
AMPKα1 negatively regulates lipid-induced inflammation, which acts
through SIRT1 to protect against obesity, inflammation and insulin
resistance (23). It has been
demonstrated that quercetin alleviates obesity-associated adipose
tissue macrophage infiltration and inflammation in mice via the
AMPKα1/SIRT1 signaling pathway (16). Resveratrol also induces beneficial
effects on obesity and metabolic disturbances by activating the
AMPKα1/SIRT1 signaling pathway (24). Consistent with previous studies,
AMPKα1 phosphorylation (Fig. 3A) and
SIRT1 expression (Fig. 3B) in the
EAT of rats fed a HFD were significantly suppressed. Treatment with
CQR significantly reversed the suppression of AMPKα1
phosphorylation in a rat model (Fig.
3A).
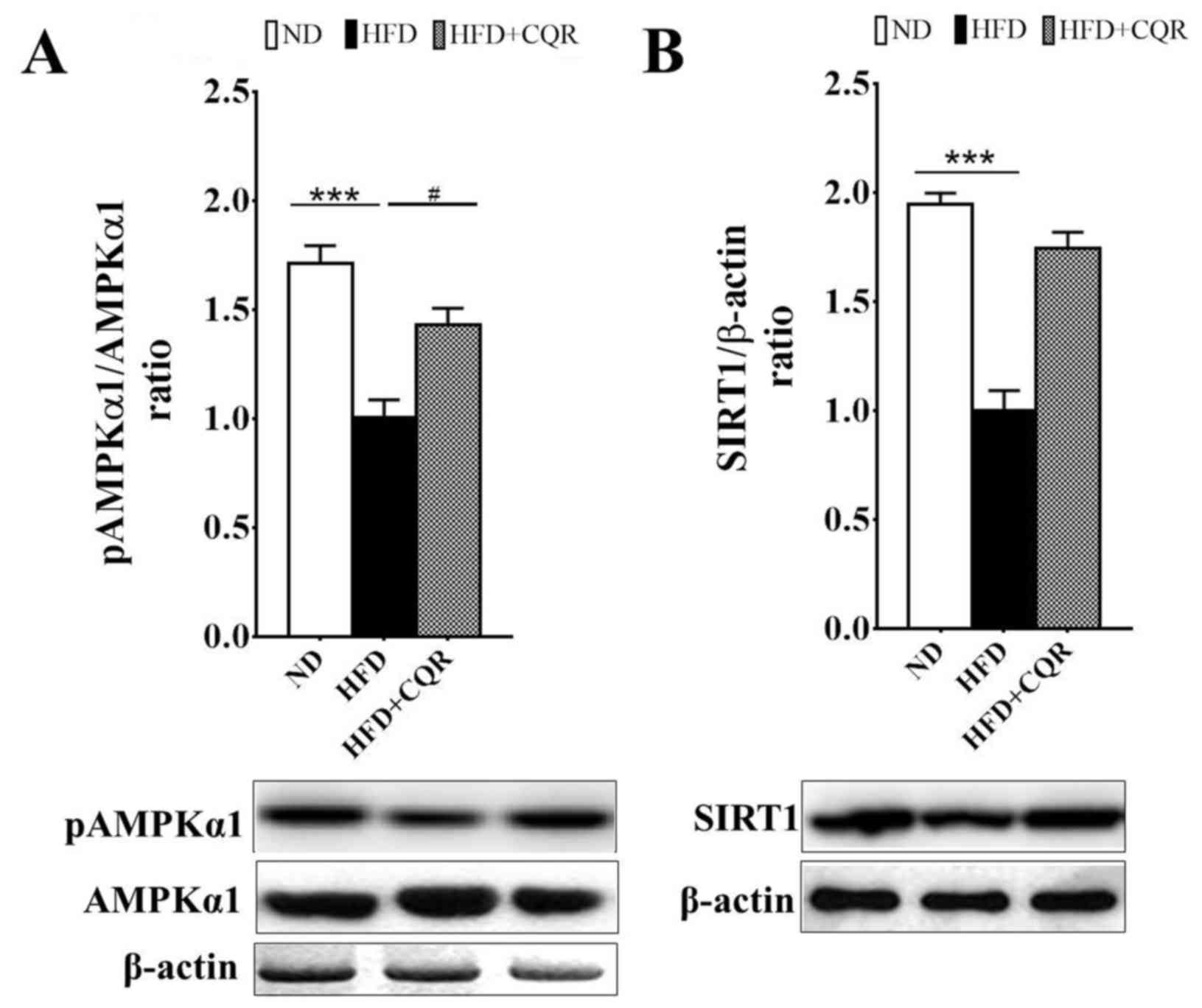 | Figure 3.Treatment with CQR increases AMPKα1
phosphorylation and SIRT1 expression in the EAT of rats fed a HFD.
After 11 weeks, tissue samples were obtained from each group and
the (A) protein and phosphorylation levels of AMPKα1 and (B) the
protein expression of SIRT1 in EATs, were measured. Quantification
of AMPKα1 activity and SIRT1 expression was presented as the ratio
of pAMPKα1 to total AMPKα1 and SIRT1 to β-actin, respectively.
Statistical differences between groups were identified using a
one-way ANOVA test followed by Tukey's multiple comparison test
(n=8 per group). All data are presented as the mean ± standard
error of mean. ***P<0.001 and #P<0.05. AMPKα1,
5′-adenosine monophosphate-activated protein kinase α1; SIRT1,
sirtuin 1; EAT, epididymal adipose tissue; HFD, high fat diet; ND,
normal diet; CQR, combination of quercetin and resveratrol; p,
phosphorylated. |
Discussion
Quercetin and resveratrol are two types of dietary
polyphenols, which may have beneficial effects on metabolic
syndrome (25–33). It has also been reported that
quercetin and resveratrol have a therapeutic effect on
triacylglycerol metabolism in WAT (25). The results of the present study
suggest that the combination of quercetin and resveratrol
ameliorate insulin resistance and adipose tissue inflammation in
obese rats. Furthermore, CQR treatment was able to reverse the
changes in AMPKα1 phosphorylation and SIRT1 expression that occur
in adipose tissues. To the best of our knowledge, the current study
is the first to demonstrate that CQR has synergic effects on body
fat accumulation and adipose inflammation by activating the
AMPKα1/SIRT1 signaling pathway.
Previous studies have demonstrated that resveratrol
and quercetin may reduce body fat accumulation in animal models
(26–31). In our previous study, CQR exhibited
synergistic effects on a HFD-induced metabolic phenotype in mice
(32). Since polyphenols are
efficient, particularly at higher doses (33), doses of 120 mg/kg/day resveratrol and
240 mg/kg/day quercetin were used in the current study. These are
similar to the doses used in other comparable studies performed in
rodents (31,34–36). The
recommended dosage of quercetin and resveratrol in obesity or
insulin-resistance studies remains controversial and it was
reported that treatment with lower doses of resveratrol may
activate SIRT1, whereas higher doses activate AMPK in a
SIRT1-independent manner (37). The
doses of quercetin and resveratrol used in some studies are 30
mg/kg/day and 15 mg/kg/day, respectively (26,38). In
addition, quercetin is considered to be safe as it does not induce
carcinogenicity and genotoxicity following oral administration at
high doses (up to 2,000 mg/kg) (39). Lagouge et al (31) demonstrated that dietary treatment
with 200 or 400 mg/kg/day resveratrol, delivered in either chow or
a HFD, significantly increased the aerobic capacity and resistance
to HFD induced-obesity in mice.
The anti-obesity effects of quercetin and
resveratrol on HFD-induced body weight gain remain controversial
and negative results have been reported by several research groups
(40–42). Similar to previous studies (24,26,29,32,43), the
results of the present study demonstrated that the body weight gain
between weeks 9 and 11 in rats fed a HFD and treated with CQR was
significantly attenuated compared with rats fed a HFD alone.
Treatment with CQR also reduced the weights of renal adipose
tissues and EATs, however it did not affect SAT weight or food and
energy intake. CQR therefore appears to have a mild weight-reducing
and visceral fat-reducing effect. Visceral adipose tissue is a
proinflammatory endocrine tissue and may be responsible for the
increased cardiometabolic risk that occurs as body mass index rises
(44). Regardless of adiposity
status, visceral adiposity is associated with an adverse
cardiometabolic profile, including inflammation, insulin resistance
and myocardial dysfunction, all of which are hallmarks of an
‘obese’ phenotype (44). In addition
the current study demonstrated that, HFD-induced dyslipidemia
caused an increase in total C, TGs and LDL-C and a decrease in
HDL-C in the blood. Chaudhari et al (45) also reported that a HFD induced
significant increases in total C, TG and LDL-C in the rat serum,
whereas it reduced HDL-C in rat serum (41). In the present study, CQR treatment
increased serum HDL-C and decreased serum TC, TG and LDL-C,
demonstrating that CQR is able to reduce the effects of a HFD in a
rat model of obesity, which is consistent with previous reports
(12,46–48).
Chronic low-grade adipose tissue inflammation serves
an important role in the development of HFD-induced obesity and
insulin resistance (4,49,50). The
proinflammatory or anti-inflammatory molecules abnormally secreted
from obese adipose tissue are called adipokines and provide
evidence that there is a direct association between obesity and
systemic inflammation (51). The
adipose tissue of obese individuals exhibits increased expression
of proinflammatory adipokines, including TNF-α, MCP-1 and IL-6 but
a reduced expression of adiponectin (52). Systemic leptin is increased in
animals fed a HFD or with inflammation and/or infection states and
directly affects cytokine production (53); thus it was hypothesized that CQR may
ameliorate the inflammation in adipose tissue induced by a HFD. The
results of the current study demonstrated that CQR attenuates
adipocyte growth in EAT and mast cell clustering into adipose
tissues. In the serum, CQR treatment reduced leptin, as well as
TNF-α, MCP-1 and IL-6 levels.
To reveal the molecular mechanisms by which CQR
attenuates obesity associated adipose tissue inflammation, two
important nutrient sensors and inflammatory regulators in EAT were
assessed in the current study; AMPKα1 and SIRT1 (54,55). CQR
significantly increased HFD-suppressed AMPKα1 phosphorylation and
markedly increased SIRT1 expression in EATs, suggesting that CQR
influences the AMPKα1/SIRT1 signaling pathway in adipose tissues.
The AMPKα1/SIRT1 signaling pathway may be a novel cellular target
due to its anti-inflammatory effects in adipocytes (24,56).
AMPKα1 activates SIRT1 and inhibits inflammation in macrophages
(57); furthermore, AMPKα1 may
inhibit the activation of the nuclear factor-κB system, a key
regulator of innate immunity and inflammation (55). Activation of AMPKα1 may suppress the
synthesis of pro-inflammatory cytokines, such as IL-6 and IL-8, in
adipocytes (58).
Aminoimidazole-4-carboxami-deriboside, a pharmacological activator
of AMPKα1, may inhibit inflammatory responses via
AMPKα1-independent pathways, and reverse lipopolysaccharide and
HFD-induced inflammation (55–59).
Reduction of EAT SIRT1 expression may induce ectopic inflammatory
gene expression and the overexpression of SIRT1 inhibits
HFD-induced increases in inflammation in adipose tissue (60). Dong et al (16) reported that dietary quercetin
suppressed adipose tissue macrophage infiltration and inflammation
via the AMPKα1/SIRT1 pathway in mice fed a HFD. Furthermore,
Bitterman and Chung (61) reported
that the AMPKα1/SIRT1 signaling pathway is the primary target for
the metabolic effects of resveratrol.
In conclusion, the results of the present study
suggest that CQR ameliorates not only excessive body weight gain
and dyslipidemia, but also adipose tissue inflammation in rats with
HFD-induced obesity. The anti-obese effect of CQR is associated
with a reduction in body weight gain, adipocyte diameter, adipose
tissue weight and an improvement of dyslipidemia in serum. Its
anti-obese effect is closely associated with its anti-inflammation
effects by which it reduces adipokine secretion and activates the
AMPKα1/SIRT1 signaling pathway. These results indicate that CQR has
the potential to reduce HFD-induced obesity and inflammation.
Acknowledgements
The present study was supported by the Education
Department of Guizhou Province 1 (grant no. 2014-31), the
Scientific and Technologic Innovated Team of Guizhou Province 2
(grant no. 2015-4025), the High-level Innovation Talents 3 (grant
no. 2015-4029), the Teacher Development Project of Shanghai
Municipal Education Commission and the Innovation Project of Amway
(China) Research and Development Center Ltd., Co.
References
|
1
|
Wensveen FM, Valentić S, Šestan M, Turk
Wensveen T and Polić B: The ‘Big Bang’ in obese fat: Events
initiating obesity-induced adipose tissue inflammation. Eur J
Immunol. 45:2446–2456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Heijden RA, Sheedfar F, Morrison
MC, Hommelberg PP, Kor D, Kloosterhuis NJ, Gruben N, Youssef SA, de
Bruin A, Hofker MH, et al: High-fat diet induced obesity primes
inflammation in adipose tissue prior to liver in C57BL/6j mice.
Aging (Albany NY). 7:256–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ronti T, Lupattelli G and Mannarino E: The
endocrine function of adipose tissue: An update. Clin Endocrinol
(Oxf). 64:355–365. 2006.PubMed/NCBI
|
|
4
|
Ouchi N, Parker JL, Lugus JJ and Walsh K:
Adipokines in inflammation and metabolic disease. Nat Rev Immunol.
11:85–97. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brito AF, Ribeiro M, Abrantes AM, Pires
AS, Teixo RJ, Tralhão JG and Botelho MF: Quercetin in cancer
treatment, alone or in combination with conventional therapeutics?
Curr Med Chem. 22:3025–3039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiow KH, Phoon MC, Putti T, Tan BK and
Chow VT: Evaluation of antiviral activities of Houttuynia cordata
Thunb. Extract, quercetin, quercetrin and cinanserin on murine
coronavirus and dengue virus infection. Asian Pac J Trop Med.
9:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: From antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eid HM and Haddad PS: The antidiabetic
potential of quercetin: Underlying mechanisms. Curr Med Chem.
24:355–364. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Yao J, Han C, Yang J, Chaudhry MT,
Wang S, Liu H and Yin Y: Quercetin, inflammation and immunity.
Nutrients. 8:1672016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Corrêa RCG, Peralta RM, Haminiuk CWI,
Maciel GM, Bracht A and Ferreira ICFR: New phytochemicals as
potential human anti-aging compounds: Reality, promise, and
challenges. Crit Rev Food Sci Nutr. 13:1–16. 2016. View Article : Google Scholar
|
|
11
|
Pashevin DA, Tumanovska LV, Dosenko VE,
Nagibin VS, Gurianova VL and Moibenko AA: Antiatherogenic effect of
quercetin is mediated by proteasome inhibition in the aorta and
circulating leukocytes. Pharmacol Rep. 63:1009–1018. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nabavi SF, Russo GL, Daglia M and Nabavi
SM: Role of quercetin as an alternative for obesity treatment: You
are what you eat! Food Chem. 179:305–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aguirre L, Fernández-Quintela A, Arias N
and Portillo M: Resveratrol: Anti-obesity mechanisms of action.
Molecules. 19:18632–18655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
15
|
Altintas MM, Azad A, Nayer B, Contreras G,
Zaias J, Faul C, Reiser J and Nayer A: Mast cells, macrophages, and
crown-like structures distinguish subcutaneous from visceral fat in
mice. J Lipid Res. 52:480–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong J, Zhang X, Zhang L, Bian HX, Xu N,
Bao B and Liu J: Quercetin reduces obesity-associated ATM
infiltration and inflammation in mice: A mechanism including
AMPKα1/SIRT1. J Lipid Res. 55:363–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun S, Ji Y, Kersten S and Qi L:
Mechanisms of inflamatory responses in obese adipose tissue. Annu
Rev Nutr. 32:261–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Divoux A, Sun J, Zhang J, Clément
K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al:
Genetic deficiency and pharmacological stabilization of mast cells
reduce diet-induced obesity and diabetes in mice. Nat Med.
15:940–945. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park HH, Lee S, Son HY, Park SB, Kim MS,
Choi EJ, Singh TS, Ha JH, Lee MG, Kim JE, et al: Flavonoids inhibit
histamine release and expression of proinfl ammatory cytokines in
mast cells. Arch Pharm Res. 31:1303–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee WH, Lin RJ, Lin SY, Chen YC, Lin HM
and Liang YC: Osthole enhances glucose uptake through activation of
AMP-activated protein kinase in skeletal muscle cells. J Agric Food
Chem. 59:12874–12881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sag D, Carling D, Stout RD and Suttles J:
Adenosine 5′-monophosphate-activated protein kinase promotes
macrophage polarization to an anti-inflammatory functional
phenotype. J Immunol. 181:8633–8641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshizaki T, Milne JC, Imamura T, Schenk
S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR and Olefsky
JM: SIRT1 exerts anti-inflammatory effects and improves insulin
sensitivity in adipocytes. Mol Cell Biol. 29:1363–1374. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Z, Kahn BB, Shi H and Xue BZ:
Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK)
antagonizes fatty acid-induced inflammation through SIRT1. J Biol
Chem. 285:19051–19059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Ligt M, Timmers S and Schrauwen P:
Resveratrol and obesity: Can resveratrol relieve metabolic
disturbances? Biochim Biophys Acta. 1852:1137–1144. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arias N, Macarulla MT, Aguirre L, Milton I
and Portillo MP: The combination of resveratrol and quercetin
enhances the individual effects of these molecules on
triacylglycerol metabolism in white adipose tissue. Eur J Nutr.
55:341–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gómez-Zorita S, Fernández-Quintela A,
Macarulla MT, Aguirre L, Hijona E, Bujanda L, Milagro F, Martínez
JA and Portillo MP: Resveratrol attenuates steatosis in obese
Zucker rats by decreasing fatty acid availability and reducing
oxidative stress. Br J Nutr. 107:202–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rivera L, Morón R, Sánchez M, Zarzuelo A
and Galisteo M: Quercetin ameliorates metabolic syndrome and
improves the inflammatory status in obese Zucker rats. Obesity
Silver (Spring). 16:2081–2087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung CH, Cho I, Ahn J, Jeon TI and Ha TY:
Quercetin reduces high-fat diet-induced fat accumulation in the
liver by regulating lipid metabolism genes. Phytother Res.
27:139–143. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobori M, Masumoto S, Akimoto Y and Oike
H: Chronic dietary intake of quercetin alleviates hepatic fat
accumulation associated with consumption of a Western-style diet in
C57/BL6J mice. Mol Nutr Food Res. 55:530–540. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alberdi G, Rodríguez VM, Miranda J,
Macarulla MT, Arias N, Andrés-Lacueva C and Portillo MP: Changes in
white adipose tissue metabolism induced by resveratrol in rats.
Nutr Metab (Lond). 8:292011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lagouge M, Argmann C, Gerhart-Hines Z,
Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P,
Elliott P, et al: Resveratrol improves mitochondrial function and
protects against metabolic disease by activating SIRT1 and
PGC-1alpha. Cell. 127:1109–1122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou M, Wang S, Zhao A, Wang K, Fan Z,
Yang H, Liao W, Bao S, Zhao L, Zhang Y, et al: Transcriptomic and
metabonomic profiling reveal synergistic effects of quercetin and
resveratrol supplementation in high fat diet fed mice. J Proteome
Res. 11:4961–4971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amiot MJ, Riva C and Vinet A: Effects of
dietary polyphenols on metabolic syndrome features in humans: A
systematic review. Obes Rev. 17:573–586. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Andrade JM, Frade AC, Guimarães JB,
Freitas KM, Lopes MT, Guimarães AL, de Paula AM, Coimbra CC and
Santos SH: Resveratrol increases brown adipose tissue thermogenesis
markers by increasing SIRT1 and energy expenditure and decreasing
fat accumulation in adipose tissue of mice fed a standard diet. Eur
J Nutr. 53:1503–1510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu Z, Zhao J, Xu H, Lyv Y, Feng X, Fang Y
and Xu Y: Maternal quercetin administration during gestation and
lactation decrease endoplasmic reticulum stress and related
inflammation in the adult offspring of obese female rats. Eur J
Nutr. 53:1669–1683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Guo X, Chu Y and Lu S: Heart
protective effects and mechanism of quercetin preconditioning on
anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene.
545:149–155. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Price NL, Gomes AP, Ling AJ, Duarte FV,
Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro
JS, et al: SIRT1 is required for AMPK activation and the beneficial
effects of resveratrol on mitochondrial function. Cell Metab.
15:675–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arias N, Macarulla MT, Aguirre L, Miranda
J and Portillo MP: Liver delipidating effect of a combination of
resveratrol and quercetin in rats fed an obesogenic diet. J Physiol
Biochem. 71:569–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harwood M, Danielewska-Nikiel B,
Borzelleca JF, Flamm GW, Williams GM and Lines TC: A critical
review of the data related to the safety of quercetin and lack of
evidence of in vivo toxicity, including lack of
genotoxic/carcinogenic properties. Food Chem Toxicol. 45:2179–2205.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Panchal SK, Poudyal H and Brown L:
Quercetin ameliorates cardiovascular, hepatic, and metabolic
changes in diet-induced metabolic syndrome in rats. J Nutr.
142:1026–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boesch-Saadatmandi C, Wagner AE, Wolffram
S and Rimbach G: Effect of quercetin on inflammatory gene
expression in mice liver in vivo-role of redox factor 1, miRNA-122
and miRNA-125b. Pharmacol Res. 65:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pan QR, Ren YL, Zhu JJ, Hu YJ, Zheng JS,
Fan H, Xu Y, Wang G and Liu WX: Resveratrol increases nephrin and
podocin expression and alleviates renal damage in rats fed a
high-fat diet. Nutrients. 6:2619–2631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peredo-Escárcega AE, Guarner-Lans V,
Pérez-Torres I, Ortega-Ocampo S, Carreón-Torres E, Castrejón-Tellez
V, Díaz-Díaz E and Rubio-Ruiz ME: The combination of resveratrol
and quercetin attenuates metabolic syndrome in rats by modifying
the serum fatty acid composition and by upregulating SIRT1 and
SIRT2 expression in white adipose tissue. Evid Based Complement
Alternat Med. 2015:4740322015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bays HE: Adiposopathy is ‘sick fat’ a
cardiovascular disease? J Am Coll Cardiol. 57:2461–2473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chaudhari HS, Bhandari U and Khanna G:
Preventive effect of embelin from embelia ribes on lipid metabolism
and oxidative stress in high-fat diet-induced obesity in rats.
Planta Med. 78:651–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Franco JG, Lisboa PC, Lima NS, Amaral TA,
Peixoto-Silva N, Resende AC, Oliveira E, Passos MC and Moura EG:
Resveratrol attenuates oxidative stress and prevents steatosis and
hypertension in obese rats programmed by early weaning. J Nutr
Biochem. 24:960–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Baek SH, Chung HJ, Lee HK, D'Souza R, Jeon
Y, Kim HJ, Kweon SJ and Hong ST: Treatment of obesity with the
resveratrol-enriched rice DJ-526. Sci Rep. 4:38792014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
D'Andrea G: Quercetin: A flavonol with
multifaceted therapeutic applications? Fitoterapia. 106:256–271.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun S, Ji Y, Kersten S and Qi L:
Mechanisms of inflammatory responses in obese adipose tissue. Annu
Rev Nutr. 32:261–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Osborn O and Olefsky JM: The cellular and
signaling networks linking the immune system and metabolism in
disease. Nat Med. 18:363–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Berg AH and Scherer PE: Adipose tissue,
inflammation, and cardiovascular disease. Circ Res. 13:939–949.
2005. View Article : Google Scholar
|
|
52
|
Weisberg SP, McCann D, Desai M, Rosenbaum
M, Leibel RL and Ferrante AW Jr: Obesity is associated with
macrophage accumulation in adipose tissue. J Clin Investig.
112:1796–1808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fantuzzi G and Faggioni R: Leptin in the
regulation of immunity, inflammation, and hematopoiesis. J Leukoc
Biol. 68:437–446. 2000.PubMed/NCBI
|
|
54
|
Weng SY and Schuppan D: AMPK regulates
macrophage polarization in adipose tissue inflammation and NASH. J
Hepatol. 58:619–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Salminen A, Hyttinen JM and Kaarniranta K:
AMP-activated protein kinase inhibits NF-κB signaling and
inflammation: Impact on healthspan and lifespan. J Mol Med (Berl).
89:667–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Eo H, Jeon YJ, Lee M and Lim Y: Brown Alga
Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis,
oxidative stress, and inflammation by activation of AMPK and SIRT1
in high-fat diet-induced obese mice. J Agric Food Chem. 63:349–359.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yang Z, Kahn BB, Shi H and Xue BZ:
Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK)
antagonizes fatty acid-induced inflammation through SIRT1. J Biol
Chem. 285:19051–19059. 2011. View Article : Google Scholar
|
|
58
|
Grisouard J, Dembinski K, Mayer D, Keller
U, Müller B and Christ-Crain M: Targeting AMP-activated protein
kinase in adipocytes to modulate obesity-related adipokine
production associated with insulin resistance and breast cancer
cell proliferation. Diabetol Metab Syndr. 3:162011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Łabuzek K, Liber S, Gabryel B and Okopień
B: AICAR (5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside)
increases the production of toxic molecules and affects the profile
of cytokines release in LPS-stimulated rat primary microglial
cultures. Neurotoxicology. 31:134–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gillum MP, Kotas ME, Erion DM, Kursawe R,
Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu
S, et al: SirT1 regulates adipose tissue inflammation. Diabetes.
60:3235–3245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bitterman JL and Chung JH: Metabolic
effects of resveratrol: Addressing the controversies. Cell Mol Life
Sci. 72:1473–1488. 2015. View Article : Google Scholar : PubMed/NCBI
|