Introduction
Endometrium with a thickness <8 mm in the middle
luteal phase (6–10 days following ovulation) is considered as thin
endometrium (1), which is a
potential cause of infertility. Hysteroscopy has been reported to
demonstrate no adhesion in the pelvic cavity as the endometrium is
smooth and thin in patients with thin endometrium, which may affect
the implantation of blastocysts in the endometrium (2,3). There
are a number of potential factors that may lead to thin
endometrium, with decidualization failure being one of the major
causes (4,5).
Under the effects of estrogen and progestogen,
endometrial stromal cells (ESCs) are able to undergo a typical
transformation, including morphological, biochemical and vascular
system changes, which are termed as endometrial decidualization
(6). Endometrial decidualization is
one of the essential preconditions for blastocyst implantation,
placentation and pregnancy maintenance (5). Furthermore, the morphology of human
ESCs in decidualization is altered from spindle to polygon shape,
the cell becomes larger and round, the nucleus becomes large and
pale, the cytoplasm is rich in glycogen and lipid droplets and the
cells reveal epithelioid changes (7). In addition, the intercellular
boundaries become unclear as prolactin (PRL), insulin-like growth
factor-binding protein 1 (IGFBP-1), tissue factors, neuropeptide,
and extracellular matrix components are secreted from the cells,
among which PRL and IGFBP-1 are considered as the markers of ESC
decidualization (8–10). Previous studies have revealed that
PRL is expressed in gestational endometrium and decidual cells
induced in vitro (10), and
that IGFBP-1 is overexpressed in decidualized endometrium stroma
and positively associated with the degree of stromal cell
decidualization (11). Additionally,
the expression of IGFBP-1 in the decidualized endometrium is
regulated by the progesterone receptor (PR) (12).
Phytoestrogen is a plant-derived non-steroidal
compound (13). The structure and
biological activity of phytoestrogen are similar to those of
endogenous estrogen (13). However,
phytoestrogen has bidirectional regulatory effects: It demonstrates
anti-estrogenic activity when the level of estrogen is high in the
body, and reveals estrogenic activity when the level of estrogen is
relatively low (14). Icaritin is
one of the major active ingredients of epimedium. Icaritin and its
derivative, desmethylicaritin, exhibit evident estrogenic effects,
and are able to increase the expression of PR (15). However, only a small number of
studies to date have investigated the effects of Icaritin on
endometrial cells and its role in decidualization of the cells.
Therefore, the effect of Icaritin on primary ESCs was investigated
in the present study.
Materials and methods
Human endometrium
In total, 20 endometrial tissues were obtained
during hysterectomy at the Shenzhen Nanshan People's Hospital
(Shenzhen, China) between August 2014 and December 2015, with
written informed consent obtained from all patients (aged 30–39),
following the approved procedures of the Institutional Review Board
of Shenzhen Nanshan People's Hospital. Subsequently, the collected
endometrium samples were used for the separation and culture of
primary ESCs. The T HESC human ESC cell line was provided by
Professor Haibin Wang of the Institute of Zoology, Chinese Academy
of Sciences (Beijing, China) and used for the induction of the
decidualization model. The study was approved by the Ethics
Committee of Shenzhen Institutes of Advanced Technology, Chinese
Academy of Sciences (Shenzhen, China).
Isolation, culture and identification
of primary ESCs (pESCs)
Fresh endometrial tissues were obtained during
hysterectomy, placed in ice-cold sterilized PBS and transferred to
the laboratory for the separation and culture of pESCs within 1 h.
Tissues were minced and digested with high concentrations of
collagenase (1 mg/ml) and DNase (0.2 µg/ml) for 1 h at 37°C, and
then filtered through a mesh. The glandular epithelial and stromal
cells were separated by the adhesion purification method, as
described previously (16).
The stromal cells reached confluence after 3 days of
culture in the complete culture medium (cDMEM, high glucose
Dulbecco's modified Eagle's medium with 10% fetal bovine serum;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C with 5% CO2. Next, the culture medium was
discarded, cells were washed with PBS, and 0.25% trypsin and 0.53
mM EDTA solution (Invitrogen; Thermo Fisher Scientific, Inc.) was
added to disassociate the cells for 2 min at room temperature, and
the cells were gently resuspended. The cell suspension was
transferred to a centrifuge tube and centrifuged at 230 × g for 5
min at room temperature. The supernatant was then discarded and the
fresh culture medium was added to resuspend the cell pellet gently.
The cells were subsequently split at a ratio of 1:3.
For the purity identification of the primary ESCs,
they were passaged three times, and were then seeded into a 24-well
culture plate with a density of 1×104 cells/ml. Once the
cells had reached 80% confluence, the culture medium was discarded,
and the cells were washed with PBS twice. Next, 1 ml ice-cold 4%
paraformaldehyde (PFA) was added into each well to fix the cells
for 20 min on ice. Finally, the fixed cells were washed with PBS
and stored at 4°C in PBS for immunoflurescence identification with
vimentin and cytokeratin 7 (CK7) as described below.
In vitro decidualization model of
human ESCs
Induction of the decidualization of the stromal
cells (105 cells) was performed following with estradiol
(E2, 10 nM) + progesterone (P4; 1 µM) (both from Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and/or cAMP (0.5 mM) using an
induction method, as previously reported (14). Briefly, the confluence ESCs from the
third passage after thaw were used to induce decidualization with
different treatment groups: PE (P4+E2), PEC (P4+E2+cAMP), PC
(P4+cAMP) and control (complete culture medium) groups. The
concentrations of E2, P4 and cAMP were selected according to a
previous study (17).
Following the successful induction of the
decidualization model, Icaritin (10 µM; Beijing Shenogen Pharma
Group, Beijing, China) was added to the induction cocktail to
investigate its effect with the following groups: PI (P4+Icaritin),
PE (P4+E2), PEI (P4+E2+Icaritin), PEC (P4+E2+cAMP), PECI
(P4+E2+cAMP+Icaritin), PC (P4+cAMP), PCI (P4+cAMP+Icaritin) and the
control (complete culture medium) groups. Then, the induced
decidualization was performed for all groups as described
above.
Each group contained triplicates, and each condition
had been performed three times. Cells from different treatment
groups were all observed and photographed with an inverted
microscope during the treatment period. In all treatments,
following 96 h of induction, the culture media from different
treatment groups were collected and stored at −80°C for IGFBP-1
ELISA analysis later. Furthermore, a number of cells from different
groups were harvested for total RNA extraction, and a number of the
cells were fixed with 4% PFA at room temperature for 15 min, then
washed with PBS and stored at 4°C in PBS for immunofluorescence
staining.
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression of PRL and IGFBP-1 mRNA in
decidualized ESC cells was measured by RT-qPCR according to the
previously described methods (18).
Primer sequences were as follows: PRL sense, ATGGAAGTCCCGACCAGAC
and antisense, ATT GAG GAG CAA ACC AAACG; IGFBP-1 sense, CTG CGT
GCA GGA GTC TGA and antisense, CCC AAA GGA TGG AAT GATCC.
ELISA
IGFBP-1 protein expression in the supernatant of
decidualized ESCs was measured with IGFBP-1 ELISA kits (OKDA00085;
Alpha Diagnostics, San Antonio, TX, USA), according to the
manufacturer's protocol. The optical density, measured using a
microplate reader, was put into the software to calculate the
protein concentration, from which a curve was plotted using
GraphPad Prism 6 software (Premier Biosoft International, Inc.,
Palo Alto, CA, USA).
Immunofluorescence
Fixed pESCs were incubated with anti-vimentin
(1:200, ab92547) and anti-CK7 (1:200, ab181598) (both from Abcam,
Cambridge, MA, USA), and fixed ESCs from different treatments were
incubated with anti-PRL (1:150, ab64377; Abcam) primary antibody
overnight at 4°C separately, washed with PBS and incubated with
FITC-conjugated secondary antibody (1:200, ab6717; Abcam). After
washing with PBS, cells were mounted with anti-fading mounting
medium with DAPI and observed with a fluorescence microscope
(magnification, ×40). Images were captured with a charge coupled
device camera.
Statistical analysis
SPSS for Windows 17.0 software (SPSS Inc., Chicago,
IL, USA) was used for statistical analysis. Quantitative data are
presented as the mean ± standard deviation. One-way analysis of
variance was used for the analysis of single factors amongst
multiple groups. The Student-Newman-Keuls and Fisher's least
significant difference t-tests were used for pair-wise comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Morphology and identification of
primary ESCs
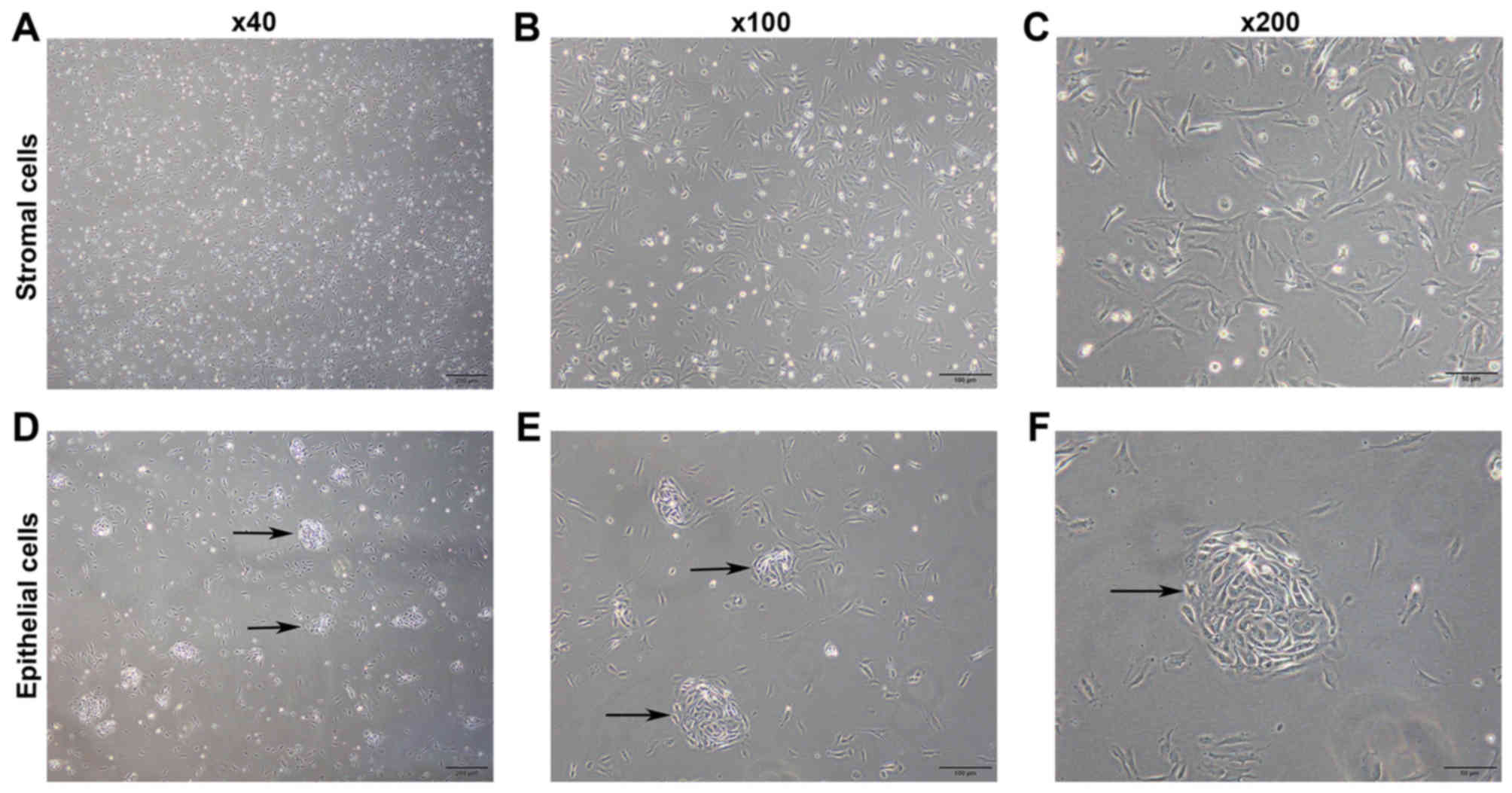
Images of pESCs are presented in Fig. 1. Stromal cells began to adhere within
several min following culture, and the adhesion was generally
completed at 1–2 h of culture. Cells were triangular at an early
stage following adhesion (Fig.
1A-C). The glandular epithelial cells predominantly aggregated
and began to adhere at 2 h of culture. Furthermore, the cells
exhibited a tadpole-like appearance at an early stage following
adhesion, and subsequently grew into a nest shape (Fig. 1D-F).
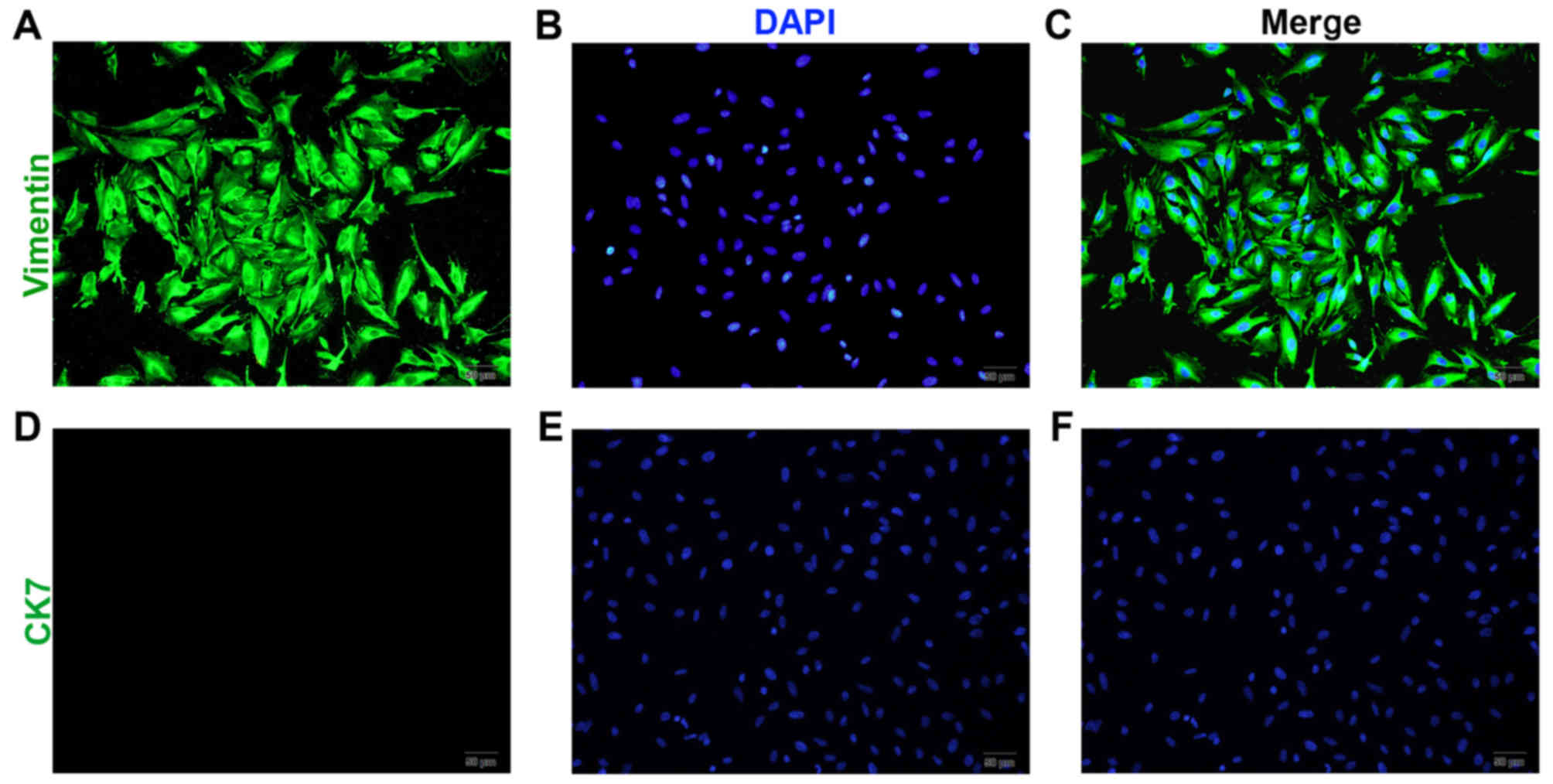
Following 4–6 days of culture, the pESCs reached
confluence and became spindle-shaped (Fig. 2A-C). Among the stromal cells, <5%
were identified as glandular epithelial cells. Following passage of
the cells three times, relatively pure stromal cells were obtained,
with a purity of 95%, and positive vimentin (Fig. 2A-C) and negtive CK7 expression
(Fig. 2D-F).
Morphological characteristics of the
ESCs following decidualization
An inverted microscope was used to observe the in
vitro cultured decidualized stromal cells (Fig. 3). The results revealed that the shape
of the cells was irregular. The cells were polygon-shaped and
revealed epithelioid changes. Multinucleation was observed for the
cell nuclei, and the cell bodies were large and round in the PEC
and PC groups (Fig. 3E and G)
compared with the control, PI and PE groups (Fig. 3A-C), with moderate changes observed
in the PEI, PECI and PCI groups (Fig.
3D, F and H).
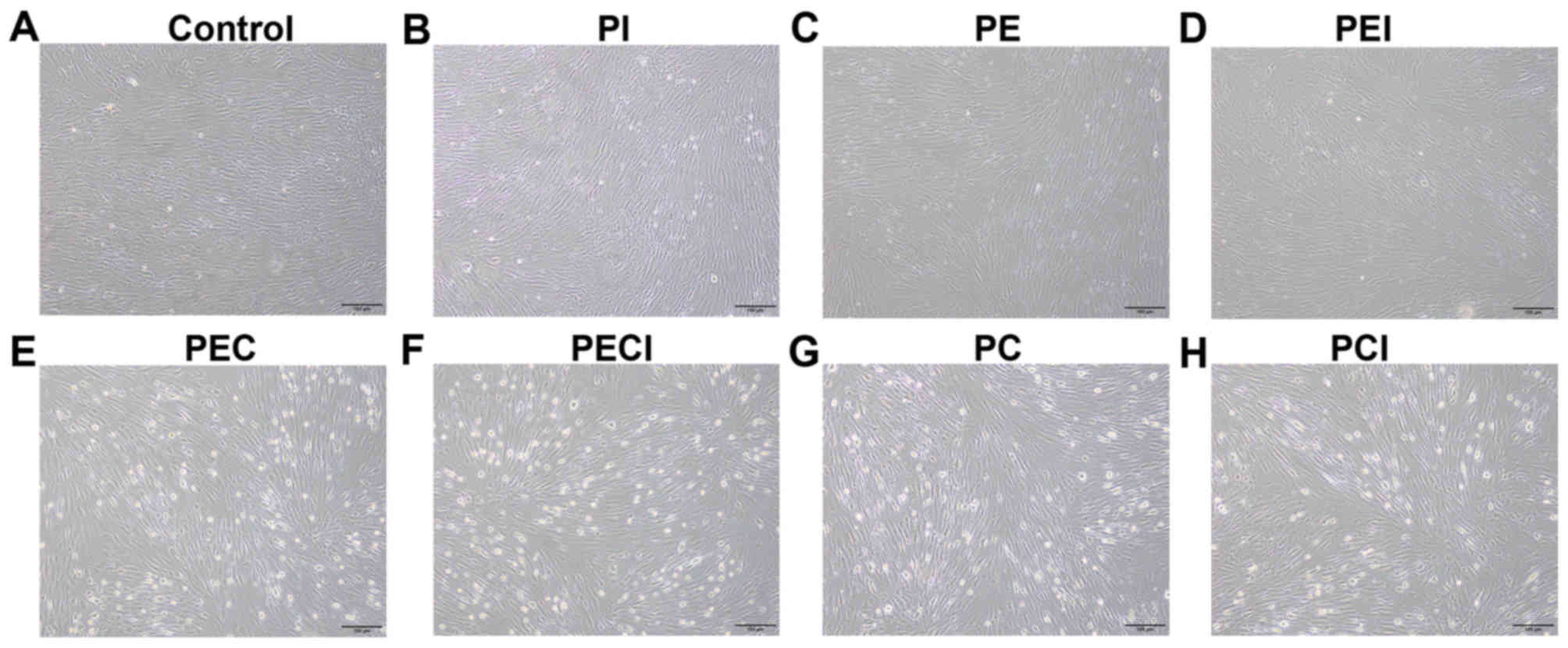 | Figure 3.Morphology of decidualized stromal
cells. Morphology of the stromal cells was observed under the
following treatments: (A) Control, (B) PI, (C) PE, (D) PEI, (E)
PEC, (F) PECI, (G) PC and (H) PCI. Magnification, ×100. P4,
progesterone; E2, estradiol; PI, P4+Icaritin; PE, P4+E2; PEI,
P4+E2+Icaritin; PEC, P4+E2+cAMP; PECI, P4+E2+cAMP+Icaritin; PC,
P4+cAMP; PCI, P4+cAMP+Icaritin; cAMP, cyclic adenosine
monophosphate. |
Icaritin inhibits the expression of
PRL and IGFBP-1 mRNA in the decidualized cells
The results revealed that PEC and PC were able to
induce the decidualization of ESCs, as the expression of PRL
(Table I) and IGFBP-1 mRNA (Table II) was significantly higher in the
two groups compared with the control group, and the expression in
the PEC group was also significantly higher compared with the PC
group. However, the expression levels of PRL and IGFBP-1 was
significantly decreased in the PCI group compared with the PC group
and in the PECI group compared with the PEC group following
addition of Icaritin.
 | Table I.PRL mRNA expression following Icaritin
treatment in decidualized cells (n=10 per group). |
Table I.
PRL mRNA expression following Icaritin
treatment in decidualized cells (n=10 per group).
| Group | Relative
expression |
|---|
| Control |
8.722×10−6±1.08866×10−5 |
| PI |
3.96×10−6±2.21×10−6 |
| PE |
5.82×10−6±2.10297×10−6 |
| PEI |
4.86×10−6±1.31×10−6 |
| PEC |
0.0041±0.000446 |
| PECI |
0.002995±0.00038 |
| PC |
0.00348±0.000416 |
| PCI |
0.002929±0.000704 |
 | Table II.IGFBP-1 mRNA expression following
Icaritin treatment in decidualized cells (n=10 per group). |
Table II.
IGFBP-1 mRNA expression following
Icaritin treatment in decidualized cells (n=10 per group).
| Group | Relative
expression |
|---|
| Control |
0.000076362±3.45985×10−5 |
| PI |
9.31374×10−6±4.64574×10−6 |
| PE |
1.03501×10−5±2.74771×10−6 |
| PEI |
1.68732×10−5±3.79461×10−6 |
| PEC |
0.001787477±0.000487733 |
| PECI |
0.001435567±0.000317134 |
| PC |
0.001470706±0.000377389 |
| PCI |
0.000961847±0.000164622 |
Icaritin inhibits the expression of
decidualization proteins
Cell immunofluorescence of PRL revealed that the PRL
was negative in the cell groups that were not decidualized in the
control, PI, PE, and PEI groups (Fig.
4A-D) but positively expressed in the decidualized cells of the
PEC, PECI, PC and PCI groups (Fig.
4E-H). By evaluating the decidualized marker IGFBP-1 secreted
into the culture media, the IGFBP-1 protein level was demonstrated
to be significantly higher in the PEC group compared with the PC
group. However, the expression of IGFBP-1 protein was significantly
decreased following addition of Icaritin (Table III).
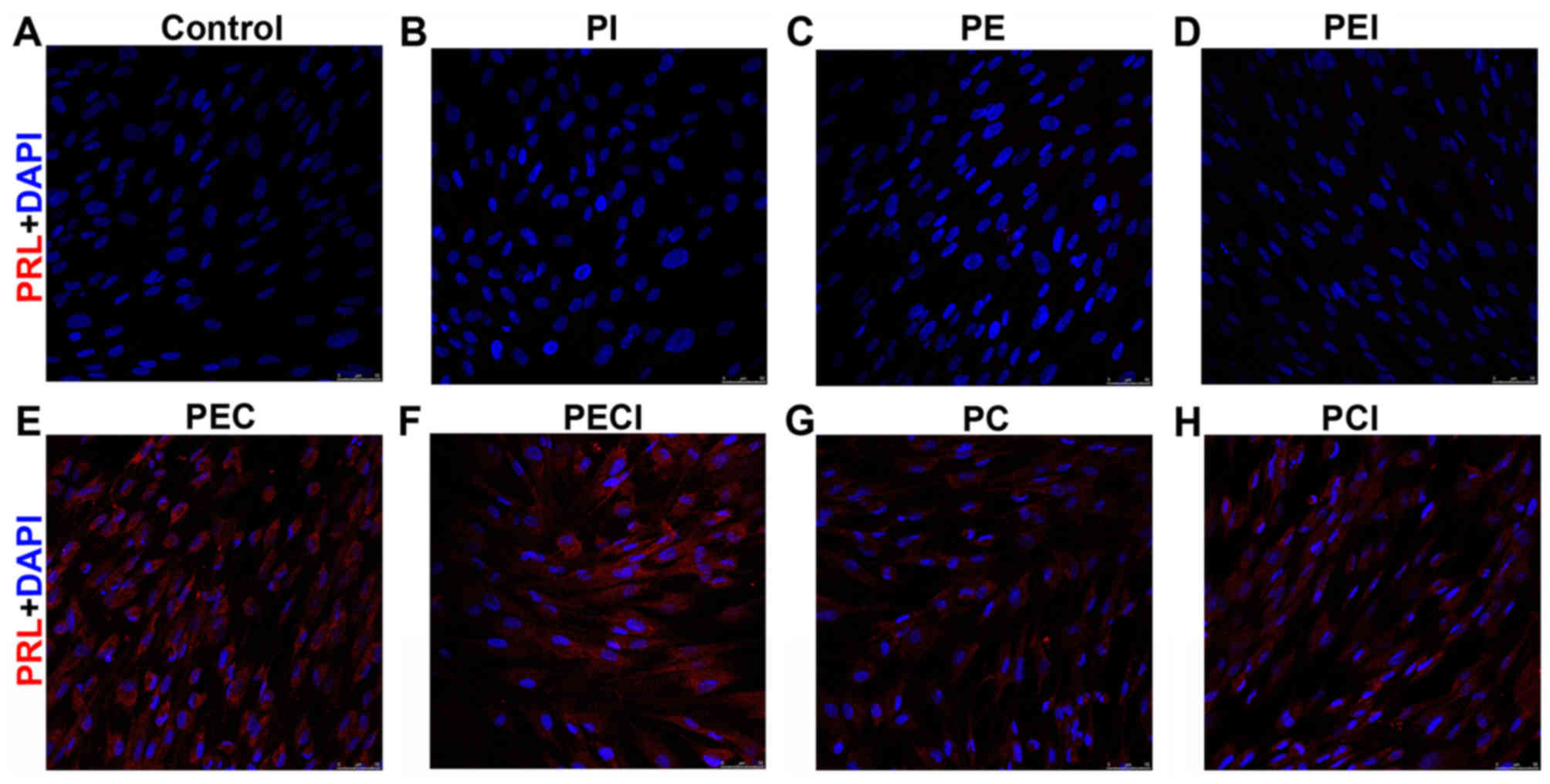 | Figure 4.PRL immunofluorescent staining of the
stromal cells was observed under the following treatments: (A)
Control, (B) PI, (C) PE, (D) PEI, (E) PEC, (F) PECI, (G) PC and (H)
PCI. Magnification, ×200. PRL, prolactin; PI, P4+Icaritin; PE,
P4+E2; PEI, P4+E2+Icaritin; PEC, P4+E2+cAMP; PECI,
P4+E2+cAMP+Icaritin; PC, P4+cAMP; PCI, P4+cAMP+Icaritin; cAMP,
cyclic adenosine monophosphate; E2, estradiol; P4,
progesterone. |
 | Table III.IGFBP-1 protein expression following
Icaritin treatment in decidualized cells (n=6 per group). |
Table III.
IGFBP-1 protein expression following
Icaritin treatment in decidualized cells (n=6 per group).
| Group | Relative
expression |
|---|
| PEC |
10.438125±0.781819935 |
| PECI |
9.0145±0.753969874 |
| PC |
8.7955±1.028489461 |
| PCI |
6.4853±0.056983423 |
Discussion
The acceptability of the growing environment of
endometrium is critical to the successful implantation of embryos,
and an endometrium that is too thin may reduce the implantation
rate of embryos (3). Fatemi and
Popovic-Todorovic (19) previously
demonstrated that insufficient endometrium growth may result in a
decreased rate of embryo implantation. The endometrium consists of
ESCs and endometrial glandular epithelial cells. Usually, specimens
of thin endometrium are difficult to obtain due to the low
incidence of thin endometrium. In the present study, such specimens
were obtained during hysterectomy for uterine fibroid or
adenomyosis. High concentrations of collagenase and DNase were used
in combination to digest the cells, after which the cells were
filtered through a mesh and the adhesion purification method was
used to separate the glandular epithelial and stromal cells. As
glandular epithelial cells are difficult to passage and purify,
only ESCs with relatively high purity were obtained in the present
study. As the response of pESCs to hormone treatments various among
pESCs derived from different patients, the T HESC cell (ESCs) line
was used to investigate the effect of Icaritin on the
decidualization to minimize the variation from different pESCs. Li
et al (20) recently
demonstrated that the use of BerEP4-coated magnetic beads was able
to assist in obtaining high-purity ESCs. Chan et al
(21) previously indicated that a
BerEP4 antibody exhibited specificity for luminal and glandular
epithelia in the endometrium. In the present study, a 95% purity of
pESCs was obtained via the adhesion culture method, which is
comparable to other methods used for ESC isolation (20,21).
Physiological levels of estrogen and progestogen are
required for the regulation of menstruation and pregnancy
maintenance (6). From the late stage
of secretion, endometrial cells begin to proliferate and
differentiate under the effects of estrogen and progestogen, and
then undergo decidualization to form decidual cells (22). Such cells are irregular in shape;
appear polygon shaped and the cell bodies become large and round,
with a rich cytoplasm (6). Cell
nuclei separate to demonstrate multinuclear changes (6). In humans and mice, the degree of
decidualization is consistent with the degree of trophoblast
invasion (6,23,24).
Such decidualization may then exert regulatory effects on
blastocyst implantation, placenta formation and maintenance of
normal pregnancy (23). If defects
in decidualization exist, trophoblast invasion may be blocked and
thus lead to infertility and recurrent spontaneous abortion
(24). Patients with a thin
endometrium exhibit a low menstrual blood volume, which maybe
associated with insufficient decidualization of the endometrium
(4).
Decidualization is predominantly regulated by
estrogen and progestogen (6).
Therefore, adding estrogen and progestogen to human or murine ESCs
is able to induce a model of decidualization in vitro.
Previous studies have revealed that progesterone, estrogen and cAMP
co-regulate the decidualization of human ESCs in a time-dependent
manner (25–27). Carden et al (28) previously used E2+P4 to treat ESCs for
14 days and successfully induce decidualization. The present study
confirmed that using E2+P4 and/or cAMP to treat ESCs for 96 h was
able to induce decidualization. Additionally, the expression of
decidualization-related proteins was significantly higher in the
PEC group compared with the PC group. It was speculated that this
may be associated with the evidence that P4 serves a dominant role
in the process of decidualization (29). However, the regulation by trace
estrogen and its receptors is also required. Furthermore, the
regulatory effects of estrogen are required for in vitro
cultured ESCs to regulate decidualization, whereas a lower
expression level of decidualization-related proteins in the PC
group (compared with the PEC group) may be associated with the
insufficiency of estrogen.
Previous studies have revealed that the regulatory
effects of estrogen are more precise than the effects of
progestogen (30–32). Icaritin has estrogenic effects, and
thus maypromote the proliferation of the endometrium and improve
the expression of progestogen receptors (15). Human ESCs carry progestogen and
estrogen receptors, and thus can mimic the in vivo
morphological and functional changes in decidualization in
vitro (33). It was hypothesized
that the estrogenic effects of Icaritin were able to affect the
decidualization of stromal cells, and thus affect the proliferation
and secretion of the endometrium. In order to avoid the individual
difference in primary ESCs, E2+P4 and/or cAMP were used to treat T
HESC cells in the present study in order to induce the
decidualization model, after which Icaritin was added for
induction. Immunostaining revealed that PRL was positively
expressed, and the expression of IGFBP-1 was positively associated
with the degree of decidualization. Following addition of Icaritin
into the PEC and PC groups, in which decidualization was induced,
the expression of PRL and IGFBP-1 mRNA and the level of IGFBP-1
protein was decreased. These observations indicated that Icaritin
was able to inhibit the expression of decidualization-related genes
in ESCs in vitro. Icaritin is a plant-derived estrogen that
reveals anti-estrogenic effects when the estrogen level in the body
is relatively high (34). Therefore,
additional Icaritin in E2-induced decidualization may exert
anti-estrogenic activities. Wu et al (35) recently indicated that providing
excess estrogen at an early stage of pregnancy was able to inhibit
the decidualization of the endometrium and lead to decidualization
dysfunction. However, additional studies are required to
investigate the mechanisms associated with the inhibitory effects
of Icaritin on decidualization further.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of Guangdong (grant no.
2016A030313033).
References
|
1
|
Riad ON and Hak AA: Assessment of
endometrial receptivity using Doppler ultrasonography in infertile
women undergoing intrauterine insemination. Gynecol Endocrinol.
30:70–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gleicher N, Vidali A and Barad DH:
Successful treatment of unresponsive thin endometrium. Fertil
Steril. 95:2123.e13–e17. 2011. View Article : Google Scholar
|
|
3
|
Aydin T, Kara M and Nurettin T:
Relationship between endometrial thickness and in vitro
Fertilization-intracytoplasmic sperm injection outcome. Int J
Fertil Steril. 7:29–34. 2013.PubMed/NCBI
|
|
4
|
Gargett CE and Healy DL: Generating
receptive endometrium in Asherman's syndrome. J Hum Reprod Sci.
4:49–52. 2011.PubMed/NCBI
|
|
5
|
Deligdisch L: Hormonal pathology of the
endometrium. Mod Pathol. 13:285–294. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramathal CY, Bagchi IC, Taylor RN and
Bagchi MK: Endometrial decidualization: Of mice and men. Semin
Reprod Med. 28:17–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwab KE, Chan RW and Gargett CE:
Putative stem cell activity of human endometrial epithelial and
stromal cells during the menstrual cycle. Fertil Steril. 84 Suppl
2:S1124–S1130. 2005. View Article : Google Scholar
|
|
8
|
Dunn CL, Kelly RW and Critchley HO:
Decidualization of the human endometrial stromal cell: An enigmatic
transformation. Reprod Biomed Online. 7:151–161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kane NM, Jones M, Brosens JJ, Kelly RW,
Saunders PT and Critchley HO: TGFβ1 attenuates expression of
prolactin and IGFBP-1 in decidualized endometrial stromal cells by
both SMAD-dependent and SMAD-independent pathways. PLoS One.
5:e129702010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guzel E, Buchwalder L, Basar M, Kayisli U,
Ocak N, Bozkurt I, Lockwood CJ and Schatz F: Effects of tibolone
and its metabolites on prolactin and insulin-like growth factor
binding protein-1 expression in human endometrial stromal cells.
Gynecol Endocrinol. 31:414–418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang DK, Qi HB, Luo X, Xiao XQ and Jia
XY: Comparative study of placental α-microglobulin-1, insulin-like
growth factor binding protein-1 and nitrazine test to diagnose
premature rupture of membranes: A randomized controlled trial. J
Obstet Gynaecol Res. 40:1555–1560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ammoun S, Schmid MC, Zhou L, Ristic N,
Ercolano E, Hilton DA, Perks CM and Hanemann CO: Insulin-like
growth factor-binding protein-1 (IGFBP-1) regulates human
schwannoma proliferation, adhesion and survival. Oncogene.
31:1710–1722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lóránd T, Vigh E and Garai J: Hormonal
action of plant derived and anthropogenic non-steroidal estrogenic
compounds: Phytoestrogens and xenoestrogens. Curr Med Chem.
17:3542–3574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma HP, Ming LG, Ge BF, Zhai YK, Song P,
Xian CJ and Chen KM: Icariin is more potent than genistein in
promoting osteoblast differentiation and mineralization in vitro. J
Cell Biochem. 112:916–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye HY and Lou YJ: Estrogenic effects of
two derivatives of icariin on human breast cancer MCF-7 cells.
Phytomedicine. 12:735–741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pierro E, Minici F, Alesiani O, Miceli F,
Proto C, Screpanti I, Mancuso S and Lanzone A: Stromal-epithelial
interactions modulate estrogen responsiveness in normal human
endometrium. Biol Reprod. 64:831–838. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kusama K, Yoshie M, Tamura K, Kodaka Y,
Hirata A, Sakurai T, Bai H, Imakawa K, Nishi H, Isaka K, et al:
Regulation of decidualization in human endometrial stromal cells
through exchange protein directly activated by cyclic AMP (Epac).
Placenta. 34:212–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang
Y and Zhang S: Endometrial stem cells repair injured endometrium
and induce angiogenesis via AKT and ERK pathways. Reproduction.
152:389–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fatemi HM and Popovic-Todorovic B:
Implantation in assisted reproduction: A look at endometrial
receptivity. Reprod Biomed Online. 27:530–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li T, He H, Liu R, Wang SX and Pu DM:
Isolation and identification of epithelial and stromal stem cells
from eutopic endometrium of women with endometriosis. Eur J Obstet
Gynecol Reprod Biol. 178:89–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan RW, Schwab KE and Gargett CE:
Clonogenicity of human endometrial epithelial and stromal cells.
Biol Reprod. 70:1738–1750. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Franco HL, Dai D, Lee KY, Rubel CA, Roop
D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK and DeMayo
FJ: WNT4 is a key regulator of normal postnatal uterine development
and progesterone signaling during embryo implantation and
decidualization in the mouse. FASEB J. 25:1176–1187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gellersen B, Brosens IA and Brosens JJ:
Decidualization of the human endometrium: Mechanisms, functions,
and clinical perspectives. Semin Reprod Med. 25:445–453. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cha J, Sun X and Dey SK: Mechanisms of
implantation: Strategies for successful pregnancy. Nat Med.
18:1754–1767. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Logan PC, Ponnampalam AP, Steiner M and
Mitchell MD: Effect of cyclic AMP and estrogen/progesterone on the
transcription of DNA methyltransferases during the decidualization
of human endometrial stromal cells. Mol Hum Reprod. 19:302–312.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Latil A, Bièche I, Vidaud D, Lidereau R,
Berthon P, Cussenot O and Vidaud M: Evaluation of androgen,
estrogen (ER alpha and ER beta), and progesterone receptor
expression in human prostate cancer by real-time quantitative
reverse transcription-polymerase chain reaction assays. Cancer Res.
61:1919–1926. 2001.PubMed/NCBI
|
|
27
|
Gellersen B and Brosens JJ: Cyclic
decidualization of the human endometrium in reproductive health and
failure. Endocr Rev. 35:851–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carden D, Xiao F, Moak C, Willis BH,
Robinson-Jackson S and Alexander S: Neutrophil elastase promotes
lung microvascular injury and proteolysis of endothelial cadherins.
Am J Physiol. 275:H385–H392. 1998.PubMed/NCBI
|
|
29
|
Lee K, Jeong J, Tsai MJ, Tsai S, Lydon JP
and DeMayo FJ: Molecular mechanisms involved in progesterone
receptor regulation of uterine function. J Steroid Biochem Mol
Biol. 102:41–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stricker R, Eberhart R, Chevailler MC,
Quinn FA, Bischof P and Stricker R: Establishment of detailed
reference values for luteinizing hormone, follicle, stimulating
hormone, estradiol, and progesterone during different phases of the
menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab
Med. 44:883–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tan J, Paria BC, Dey SK and Das SK:
Differential uterine expression of estrogen and progesterone
receptors correlates with uterine preparation for implantation and
decidualization in the mouse. Endocrinology. 140:5310–5321. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Curtis Hewitt S, Goulding EH, Eddy EM and
Korach KS: Studies using the estrogen receptor alpha knockout
uterus demonstrate that implantation but not
decidualization-associated signaling is estrogen dependent. Biol
Reprod. 67:1268–1277. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hatayama H, Kanzaki H, Iwai M, Kariya M,
Fujimoto M, Higuchi T, Kojima K, Nakayama H, Mori T and Fujita J:
Progesterone enhances macrophage colony-stimulating factor
production in human endometrial stromal cells in vitro.
Endocrinology. 135:1921–1927. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang HK, Lee SB, Kwon H, Sung CK, Park YI
and Dong MS: Peripubertal administration of icariin and icaritin
advances pubertal development in female rats. Biomol Ther (Seoul).
20:189–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bin Wu: Preliminary study on the effect of
estrogen on the spontaneous pregnancy and the process of pregnancy
(unpublished PhD thesis). Peking Union Medical College. 2014.
|