Introduction
As a progressive heart muscle disease, dilated
cardiomyopathy (DCM) is characterized by a dilated ventricular
chamber, including impaired contraction of the left or both
ventricles (1,2). DCM typically leads to congestive heart
failure, thereby enhancing morbidity and mortality among patients
(3,4). Several clinical studies have indicated
that reactive oxygen species (ROS) are important in the
pathogenesis of cardiovascular diseases, including DCM (5–7).
Furthermore, it has been suggested that an imbalance between ROS
production and antioxidant defenses may result in oxidative
stress-related disorders, including DCM (8,9).
Epidemiological studies show that patients with cardiovascular
diseases can be prevented from ROS injury through antioxidant
reagents (10). Additionally,
several potential sources of ROS have been proposed, including
mitochondrial respiratory chain enzymes, xanthine oxidase,
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and
nitric oxide synthase (11).
Abnormal ROS production may further result in aberrant
morphological or functional changes, which are demonstrated as
cardiac dysfunction (12).
Omega-3 polyunsaturated fatty acid (n-3 PUFA), a
free radical scavenger, has long been suggested to be effective for
combating oxidative stress (13–15).
However, the specific mechanism through which n-3 PUFA protects the
heart from DCM remains to be elucidated. Therefore, the present
study aimed to determine the potential role of n-3 PUFA on DCM.
It has previously been indicated that the typical
pathology of DCM is exhibited by mice lacking manganese-superoxide
dismutase (Mn-SOD) activity (16,17).
Therefore, the present study used such mice as a DCM model to
explore the effect of n-3 PUFA on the parameters of heart function
and oxidative stress biomarkers in DCM mice.
Materials and methods
In vivo study protocols
A total of 8 male heart/muscle-specific
Mn-SOD-deficient (H/M-Sod2−/−) mice (age, 8 weeks;
weight, 22.5±3.1 g) were purchased from The Fourth Affiliated
Hospital of Harbin Medical University (Harbin, China). The mice
were kept in a temperature-(20–24°C) and humidity-controlled
(45–55%) environment, under a 12-h light/dark cycle, with free
access to food and water. H/M-Sod2−/− mice were fed with
3 mg/kg/day n-3 PUFA (n=4) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) or vehicle (n=4) (lauric acid, Sigma-Aldrich; Merck KGaA)
(18) for 10 weeks, and cardiac
function was evaluated by echocardiography. Mice were subsequently
sacrificed, hearts were harvested and the proteins extracted. The
present study was approved by the Zhejiang Province Hospital of
Integrated Traditional Chinese and Western Medicine (Hangzhou,
China).
Echocardiography
Two-dimensional (2D) echocardiography was performed
with certain modifications according to previously reported methods
(19). On the day of evaluation,
sodium pentobarbital (50 mg/kg; P3761, Sigma-Aldrich; Merck KGaA)
was administered by intraperitoneal injection to anesthetize the
mice. After shaving the chest, 2D echocardiography was conducted
with an echocardiographic system (model SSD-900; Hitachi-Aloka
Medical, Ltd., Tokyo, Japan) and 7.5 MHz probe (UST-987-7.5;
Hitachi-Aloka Medical, Ltd.). Furthermore, the fraction shortening
(FS), ejection fraction (EF), left ventricular internal diameter
(LVID) during systole and diastole, and endsystolic and
end-diastolic volumes were calculated using Vevo Vasc Analysis
software (version 2.2.3; Fujifilm VisualSonics, Inc., Toronto,
Canada) as previously described (20).
Primary cardiomyocyte culture
Primary cardiomyocytes from mouse neonatal hearts
were isolated as previously described (21). In brief, hearts were isolated and
digested with collagenase type II solution (Worthington Biochemical
Corporation, Lakewood, NJ, USA). Following digestion, the cells
were plated in a 25-cm2 culture flask for 2 h at 37°C in
Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to
collect cardiomyocytes. Attached cells were considered as
non-myocytes and discarded, and the unattached cells were primarily
cardiomyocytes.
Histological studies
For the histological analysis, heart tissues were
fixed at room temperature for 10 min in 10% buffered formalin.
Fixed tissues were subsequently dehydrated, embedded in paraffin,
sectioned into 4-µm slices and stained with hematoxylin and eosin
(H&E). Furthermore, Masson's trichrome was used to stain
myocardial sections in order to evaluate the cardiomyocyte diameter
and degree of fibrosis. Images were captured with a Pixera Pro600EX
camera and a VANOX-S microscope (Olympus Corporation, Tokyo,
Japan). Additionally, the fibrotic area and cardiomyocyte diameter
(>30 cells) were quantified with Qwin Plus V3 (Leica
Microsystems, Inc., Buffalo Grove, IL, USA), and the collagen
volume percentage was calculated as the mean of 5 fields for each
animal.
Hoechst 33258 staining
Primary cardiomyocytes were cultured in 6-well
tissue culture plates (1×105 cells per well). The cells
were incubated at 37°C in serum-free DMEM for 16 h at 70–80%
confluence. The cells were then washed three times with cold PBS
and fixed with 4% formaldehyde (Zhongshan Technology Co., Ltd.,
Zhongshan, China) in PBS for 20 min at room temperature. Next, the
cells were washed three times with cold PBS and stained with
Hoechst 33258 (10 µg/ml; 50 µl/slide; Sigma-Aldrich; Merck KGaA) at
room temperature for 5 min. Following staining, cold PBS was used
to further rinse the cells, and were then examined under a
fluorescence microscope.
Apoptosis assay
In order to detect the effects of n-3 PUFA on cell
apoptosis, primary cardiomyocytes isolated from mice were washed
with cold PBS three times. Next, flow cytometry was used to
determine cell apoptosis with an Annexin-V fluorescein
isothiocyanate-propidium iodide (FITC-PI) apoptosis kit
(Invitrogen, Carlsbad, CA, USA). In summary, the cells
(1×106) were washed with 1X PBS three times and
suspended at 2–3×106 cells/ml in X1 Annexin-V binding
buffer [10 mM HEPES/NaOH, (pH 7.4), 140 mM NaCl, 2.5 mM
CaCl2]. Annexin-V FITC and PI buffer were then added to
the cells, which were then incubated at room temperature for 15 min
in the dark. The cells that did not undergo any treatment were used
as an internal control. Following incubation, the cells were
filtered using a filter screen and analyzed using a flow cytometer
within 1 h of staining. Cell apoptosis was analyzed using BD
CellQuest Pro™ Analysis Software (BD Biosciences, San
Jose, CA, USA).
Determination and quantification of
ROS
Primary cardiomyocytes isolated from the mice were
cultured on slides in a 6-well chamber at 60% confluence at 37°C.
Two days later, the slides were washed with cold PBS three times.
Additionally, the slides were treated with 5 µM dihydroethidium
(DHE; Vigorous Biotechnology Beijing Co., Ltd., Beijing, China) in
serum-free DMEM F-12 medium (GE Healthcare Life Sciences) for 30
min at 37°C in darkness. Furthermore, the cells were fixed in 4%
paraformaldehyde for 30 min at room temperature. The slides were
then washed with cold PBS three times and mounted. Finally,
immunofluorescence images were captured by fluorescence microscopy,
and in order to quantify the intracellular ROS, the relative
fluorescence intensities were analyzed using flow cytometry in the
primary cardiomyocytes. Briefly, 1×106 cells were
centrifuged (200 × g) for 10 min at room temperature and the
supernatant was discarded. The cell pellet was resuspended in 1 ml
of PBS at room temperature. A 2 mM solution of H2DCFDA
(Invitrogen; Thermo Fisher Scientific, Inc.) was freshly prepared
in ethanol and 5 µl was added to the cell suspension (final
concentration 10 µM) and incubated at 37°C for 20 min. Cells were
centrifuged at 200 × g for 5 min at room temperature, the
supernatant was discarded, and the cell pellet was resuspended in
500 µl of PBS. Flow cytometric analysis was performed in duplicate
using a flow cytometer and acquired data were analyzed using WinMDI
v2.8 software (BD Biosciences).
Protein extraction, western blotting
and antibodies
Cellular proteins were extracted using RIPA buffer
[50 mM Tris/HCl, (pH 7.4), 150 mM NaCl 1% (v/v) NP-40, 0.1% (w/v)
SDS; Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China] with 0.3% (v/v) protease inhibitor (Sigma-Aldrich; Merck
KGaA), 1% (v/v) phenylmethane sulfonyl fluoride (Beijing Solarbio
Science & Technology Co., Ltd.) and 0.1% (v/v) phosphorylated
proteinase inhibitor (Sigma-Aldrich, Inc.; Merck KGaA) for 15 min
at room temperature. The lysates were collected for the total
protein following centrifugation at 10,000 × g at 4°C for 15 min.
Furthermore, the bicinchoninic acid protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.) was used to determine the protein
concentration. A total of 15 µg protein per lane was separated by
10% SDS-PAGE and transferred onto a polyvinylidene difluoride
membrane. After blocking with 8% (w/v) milk in PBST for 2 h at room
temperature, the membranes were then incubated with primary
antibodies against GAPDH (cat. no. 2118; 1:5,000), cleaved-caspase
3 (cat. no. 9664; 1:1,000), p-JNK (cat. no. 4668; 1:1,000), JNK
(cat. no. 9252; 1:1,000), p-NF-κB (cat. no. 3033; 1:1,000), NF-κB
(cat. no. 8801; 1:1,000) (all Cell Signaling Technology, Inc.,
Danvers, MA, USA) and NOX4 (cat. no. ab109225; 1:1,000; Abcam,
Cambridge, UK) overnight at 4°C. Next, the membranes were washed
with TBST three times. Additionally, they were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit or
HRP-conjugated mouse anti-goat IgG (both 1:5,000; ZB-2301,
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
for 2 h at room temperature and then washed three times with TBST.
Enhanced chemiluminescence (EMD Millipore, Billerica, MA, USA) was
used to determine the protein concentrations according to the
manufacturer's recommendations. Finally, the relative contents of
protein were normalized against GAPDH. All experiments were
repeated three times. ImageJ 1.43b software (National Institutes of
Health, Bethesda, MD, USA) was used for densitometry analysis.
Determination of protein carbonylation
and ATP content
The nuclear and mitochondrial fractions of the heart
were isolated in order to quantify cardiac protein carbonylation.
In brief, the crude nuclear fractions were isolated from tissue
homogenates at 1,000 × g for 5 min at 4°C and washed with PBS at
4°C. The mitochondrial fractions were separated as previously
described (22). The carbonylation
of mitochondrial and nuclear protein was evaluated using an Oxyblot
protein oxidation detection kit (EMD Millipore) according to the
manufacturer's instructions. Immunoreactive spots were visualized
with enhanced chemiluminescence (GE Healthcare, Buckinghamshire,
UK) and quantified with ImageJ 1.43b (National Institutes of
Health, Bethesda, MD, USA). Furthermore, the ATP content was
determined using an ATP assay kit (colorimetric/fluorometric) (cat.
no. ab83355; Abcam), according to the manufacturer's
instructions.
Statistical analysis
Data are presented as the mean ± standard error from
three independent experiments. Statistical analysis was performed
with Student's-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
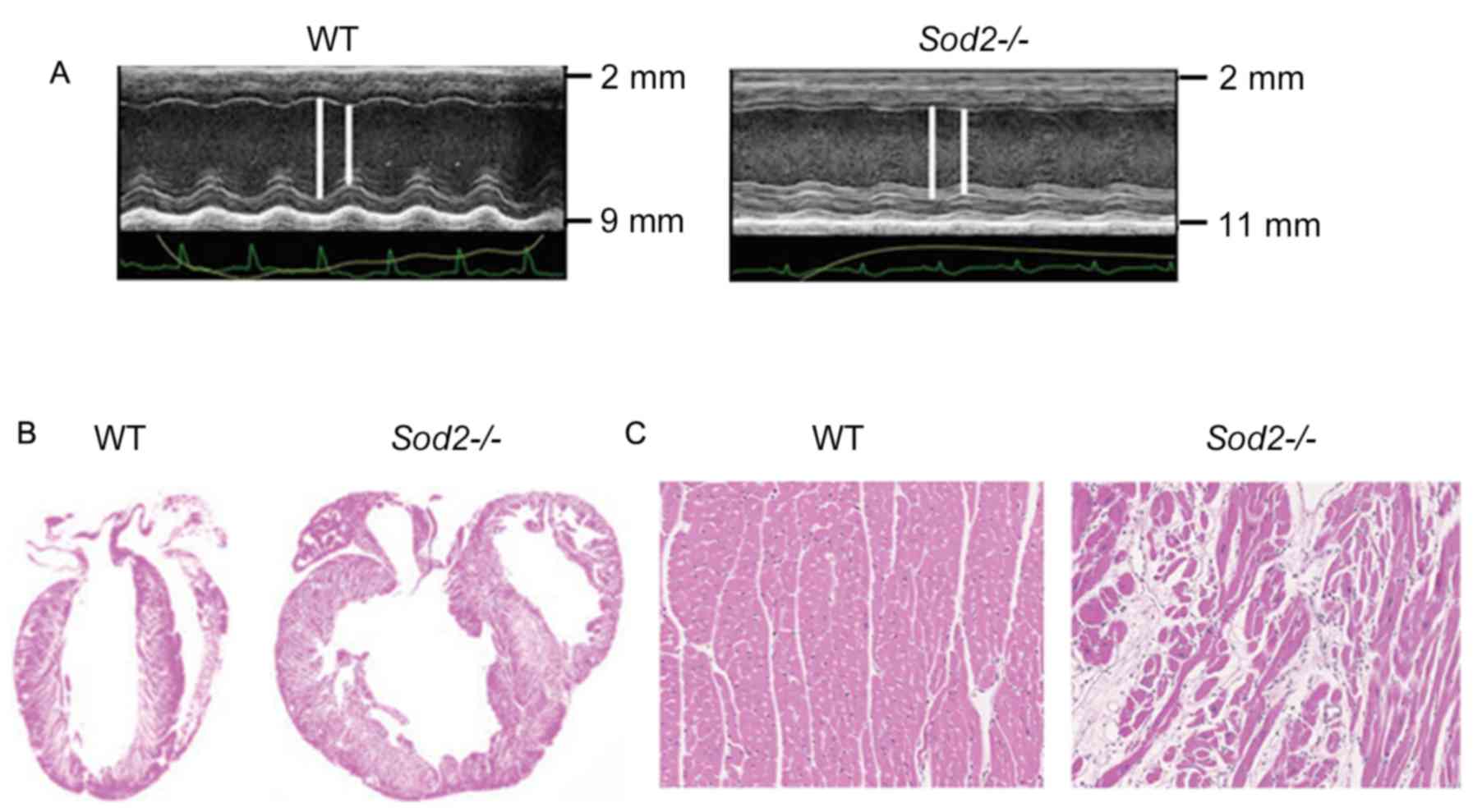
Cardiac dilation in hearts from
H/M-Sod2−/− mice
Compared with wild type (WT) mice, hearts from
H/M-Sod2−/− mice yielded increased parasternal long-axis
M-mode echocardiographic images (Fig.
1A). As shown in Fig. 1B,
H&E staining revealed an evident dilation of DCM hearts from
H/M-Sod2−/− mice compared with those from WT mice.
Furthermore, the myocardial sections from H/M-Sod2−/−
mice indicated eccentric hypertrophy and myocardial disarray
compared with hearts from WT mice (Fig.
1C). These data showed the cardiac dilation phenotype in hearts
from H/M-Sod2−/− mice.
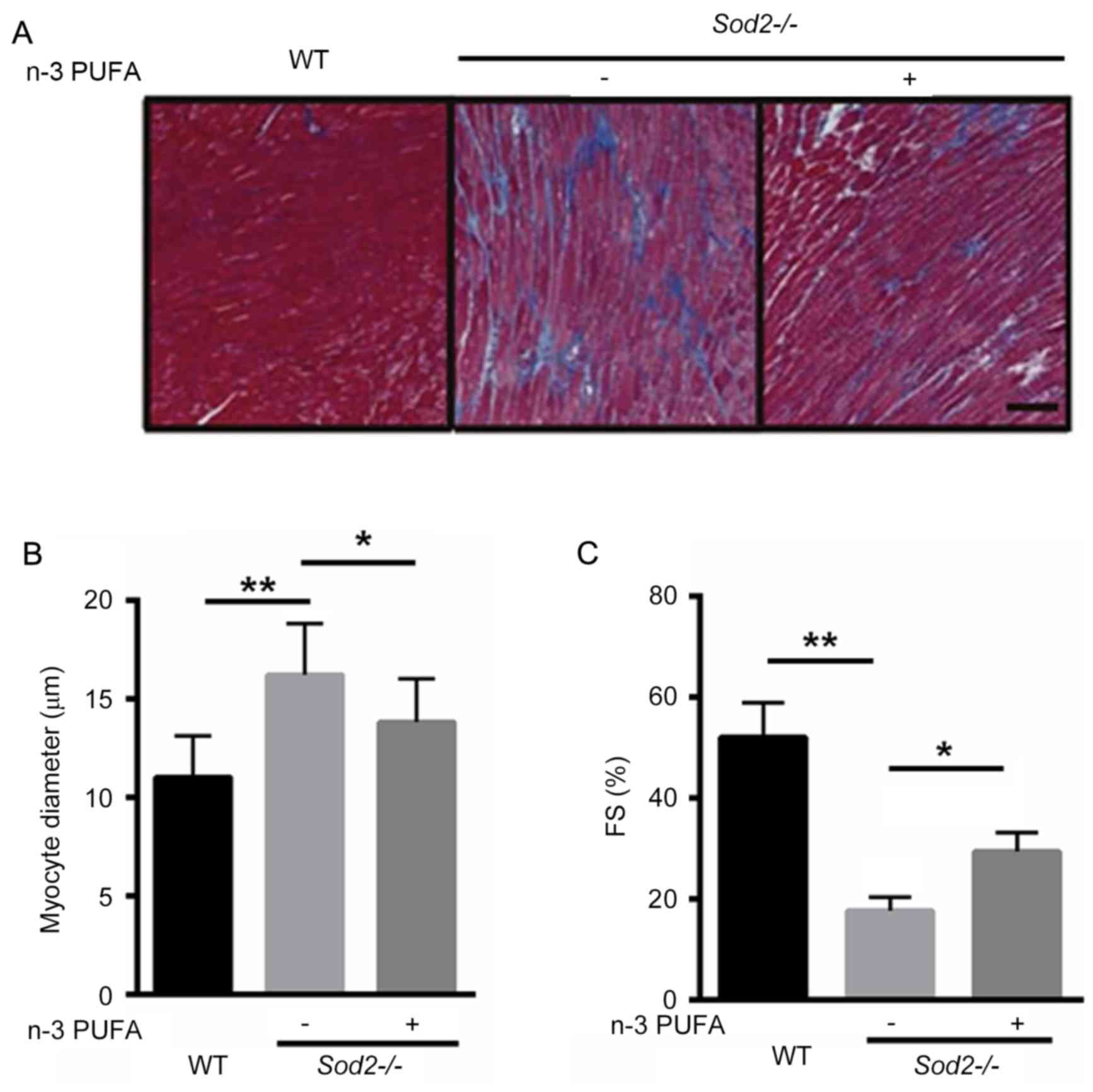
n-3 PUFA ameliorates cardiac
enlargement in H/M-Sod2−/− mice
To identify whether n-3 PUFA prevents cardiac
enlargement in H/M-Sod2−/− mice, Masson's trichrome
staining demonstrated that interstitial fibrosis was markedly
improved after n-3 PUFA treatment, suggesting that n-3 PUFA was
able to partially reverse cardiac fibrosis in the
H/M-Sod2−/− mice (Fig.
2A). Furthermore, the cardiomyocyte diameter was significantly
decreased in H/M-Sod2−/− mice treated with n-3 PUFA,
compared with untreated mice, indicating that cardiac hypertrophy
was ameliorated by n-3 PUFA treatment (Fig. 2B). Following n-3 PUFA treatment, the
percentage of FS was significantly enhanced, compared with
untreated mice (Fig. 2C). These data
suggested that n-3 PUFA treatment ameliorated cardiac dysfunction
in H/M-Sod2−/− mice.
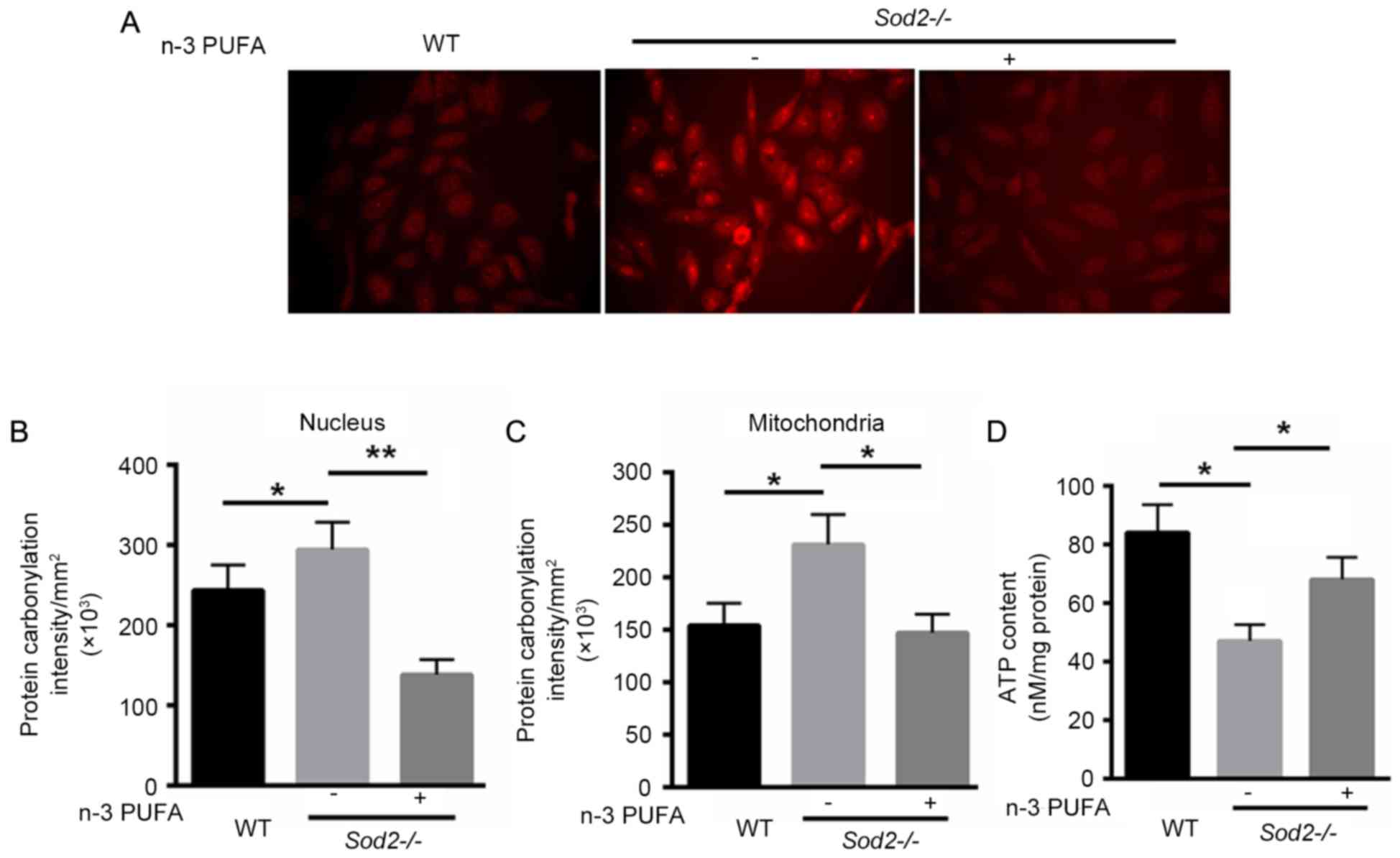
n-3 PUFA decreases ROS production in
H/M-Sod2−/− mice
The ROS production in primary cardiomyocytes
isolated from H/M-Sod2−/− and WT mice was identified. As
shown in Fig. 3A, DHE staining
revealed that n-3 PUFA treatment markedly decreased the ROS
production in primary cardiomyocytes isolated from
H/M-Sod2−/−. Subsequently, oxidative protein damage was
investigated in nuclei and mitochondria. Protein carbonylation was
significantly decreased in the nuclei and mitochondria of primary
cardiomyocytes of H/M-Sod2−/− mice treated with n-3 PUFA
(Fig. 3B and C). Furthermore, n-3
PUFA treatment recovered 21% more ATP content compared with the
untreated H/M-Sod2−/− mice (Fig. 3D). These data indicated that n-3 PUFA
decreases ROS generation, protein damage and the ATP content in
H/M-Sod2−/− mice.
n-3 PUFA reduces primary cardiomyocyte
apoptosis
In order to investigate the potential role of n-3
PUFA on cell apoptosis, Hoechst staining and flow cytometry were
applied to determine apoptosis of primary cardiomyocytes isolated
from H/M-Sod2−/− mice. Following n-3 PUFA treatment,
primary cardiomyocytes isolated from H/M-Sod2−/− mice
after apoptosis were evidently decreased compared with the
untreated group (Fig. 4A and B).
Notably, the phosphorylation levels of c-Jun N-terminal kinases
(JNK), nuclear factor (NF)-κB and cleaved-caspase 3 were
significantly decreased with n-3 PUFA treatment, whereas the
expression of NADPH oxidase 4 (NOX4) was significantly increased
(Fig. 4C).
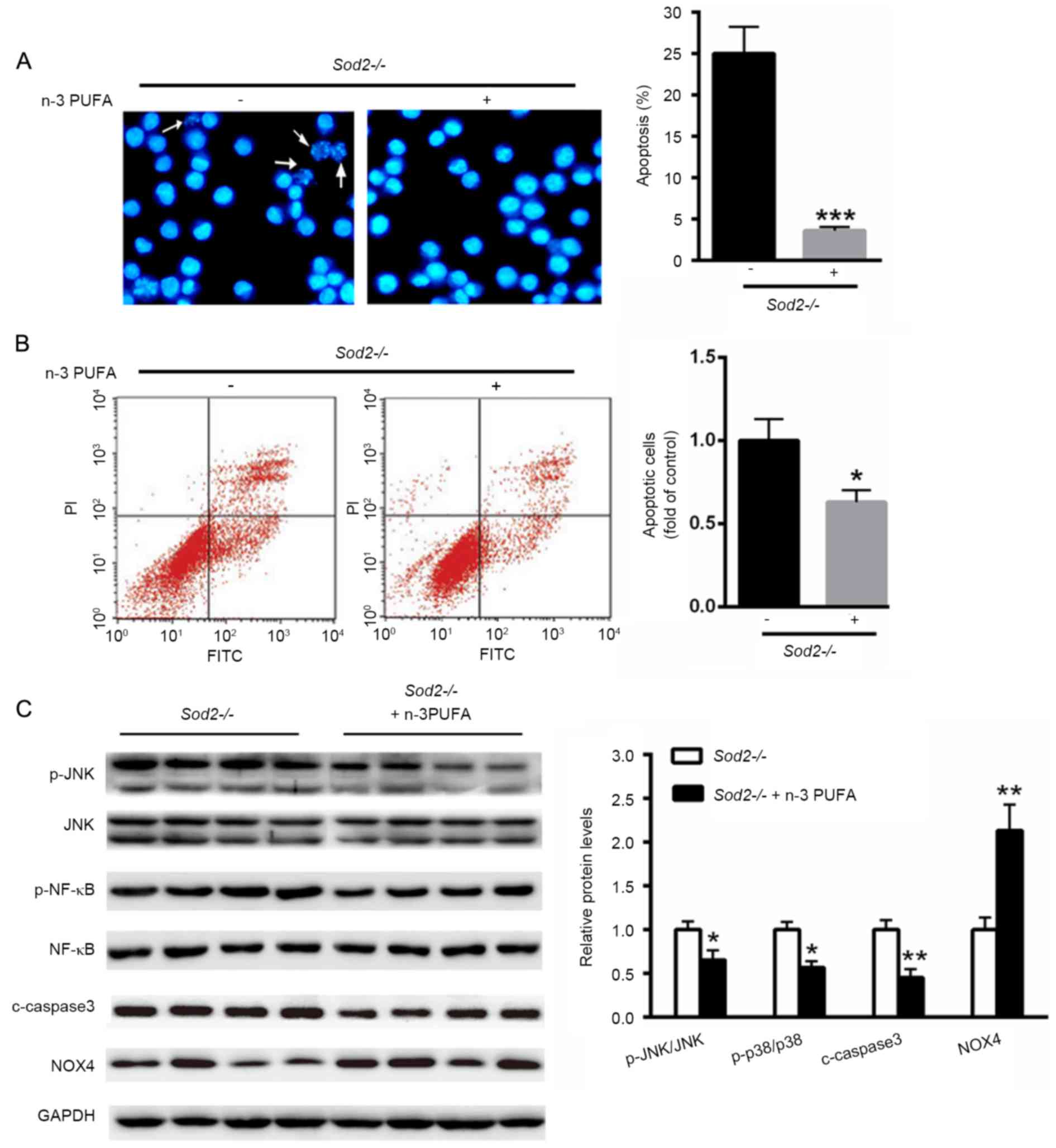 | Figure 4.n-3 PUFA reduces primary cardiomyocyte
apoptosis. (A) Hoechst staining (white arrows indicate apoptotic
cells) and (B) flow cytometry were applied to determine apoptosis
of primary cardiomyocytes isolated from H/M-Sod2−/−
mice. (C) Western blot analysis of the changed protein patterns
after n-3 PUFA treatment in the hearts of H/M-Sod2−/−
mice. n=5; *P<0.05, **P<0.01 and ***P<0.001 vs. WT mice.
n-3 PUFA, omega-3 polyunsaturated fatty acids;
H/M-Sod2−/−, heart/muscle-specific manganese-superoxide
dismutase-deficient mice; WT, wild type; FITC, fluorescein
isothiocyanate; PI, propidium iodide; JNK, c-Jun N-terminal
kinases; NF, nuclear factor; c, cleaved; NOX4, nicotinamide adenine
dinucleotide phosphate oxidase 4. |
Discussion
The present study identified that n-3 PUFA treatment
was able to partially abolish cardiac enlargement and dysfunction
in H/M-Sod2−/− mice and that the protective effects of
n-3 PUFA predominantly originated from reduced ROS production and
cardiomyocyte apoptosis. Together, these data indicate that
decreased oxidative damage contributes to the reduction of cardiac
enlargement in H/M-Sod2−/− mice.
Knockout of the Mn-SOD gene in the heart and muscle
may lead to cardiac oxidative stress, which leads to contractile
dysfunction, fibrosis and myocyte damage (23). According to echocardiographic
analysis, hearts from H/M-Sod2−/− mice were
significantly enlarged. Furthermore, EF and FS were also found to
be reduced in the hearts of H/M-Sod2−/− mice, indicating
severe DCM and cardiac dysfunction of these mice. Notably, n-3 PUFA
treatment was demonstrated to improve histological abnormalities in
DCM hearts of H/M-Sod2−/− mice, such as fibrosis,
compared with hearts from the control group. This observation is in
accordance with the Masson's trichrome-stained section analysis and
suggests that fibrosis is improved in DCM hearts.
ROS are widely associated with age-related diseases,
such as Alzheimer's and Parkinson's disease, and heart failure
(24,25). During mitochondrial respiration,
small amounts of mitochondrial ROS production may be cleared by
scavenging systems. In the presence of NOX4, superoxide anions were
significantly decreased in dilated cardiomyopathy, suggesting the
protective role of NOX4 on ROS production in HF (26,27). In
accordance with the above observations, n-3 PUFA treatment was
found to significantly increase the protein level of NOX4 in DCM
hearts of H/M-Sod2−/− mice, suggesting the protective
role of n-3 PUFA in DCM. These results indicate that mitochondrial
dysfunction may be improved by n-3 PUFA treatment in DCM.
It is widely reported that abnormal ROS production
and apoptosis are important in the pathology of DCM (28,29).
Therefore, to prevent diabetic cardiomyopathy, it is important to
simultaneously inhibit oxidative stress and apoptosis. The present
study explored the effects of n-3 PUFA on cardiomyocyte apoptosis.
It was observed that n-3 PUFA treatment led to a significant
reduction of cell apoptosis in primary cardiomyocytes isolated from
H/M-Sod2−/− mice compared with the untreated group.
Accordingly, the protein level of cleaved-caspase 3 was
significantly decreased following n-3 PUFA treatment. Furthermore,
it was also shown that n-3 PUFA demonstrated anti-inflammatory
effects since the phosphorylation levels of JNK and NF-κB were
significantly decreased.
In conclusion, oxidative stress was shown to
increase in the DCM model of H/M-Sod2−/− mice. Notably,
the results indicate that n-3 PUFA may be used as an antioxidant to
protect hearts from DCM, primarily by reducing ROS production and
cardiomyocyte apoptosis. Finally, the present study may assist in
the development of a novel therapy and prevention for DCM in human
patients.
References
|
1
|
Arumugam S, Thandavarayan RA, Veeraveedu
PT, Nakamura T, Arozal W, Sari FR, Giridharan VV, Soetikno V,
Palaniyandi SS, Harima M, et al: Beneficial effects of edaravone, a
novel antioxidant, in rats with dilated cardiomyopathy. J Cell Mol
Med. 16:2176–2185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elliott PM: Classification of
cardiomyopathies: Evolution or revolution? J Am Coll Cardiol.
62:2073–2074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cecchi F, Tomberli B and Olivotto I:
Clinical and molecular classification of cardiomyopathies. Glob
Cardiol Sci Pract. 2012:42012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elliott P, Andersson B, Arbustini E,
Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B,
McKenna WJ, et al: Classification of the cardiomyopathies: A
position statement from the European society of cardiology working
group on myocardial and pericardial diseases. Eur Heart J.
29:270–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lynch TL IV, Sivaguru M, Velayutham M,
Cardounel AJ, Michels M, Barefield D, Govindan S, dos Remedios C,
van der Velden J and Sadayappan S: Oxidative stress in dilated
cardiomyopathy caused by MYBPC3 mutation. Oxid Med Cell Longev.
2015:4247512015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samarghandian S, Farkhondeh T and Samini
F: Honey and health: A review of recent clinical research.
Pharmacognosy Res. 9:121–127. 2017.PubMed/NCBI
|
|
7
|
Zhang P, Yi LH, Meng GY, Zhang HY, Sun HH
and Cui LQ: Apelin-13 attenuates cisplatin-induced cardiotoxicity
through inhibition of ROS-mediated DNA damage and regulation of
MAPKs and AKT pathways. Free Radic Res. 51:449–459. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kono Y, Nakamura K, Kimura H, Nishii N,
Watanabe A, Banba K, Miura A, Nagase S, Sakuragi S, Kusano KF, et
al: Elevated levels of oxidative DNA damage in serum and myocardium
of patients with heart failure. Circ J. 70:1001–1005. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gopal DM and Sam F: New and emerging
biomarkers in left ventricular systolic dysfunction-insight into
dilated cardiomyopathy. J Cardiovasc Transl Res. 6:516–527. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
White M, Ducharme A, Ibrahim R, Whittom L,
Lavoie J, Guertin MC, Racine N, He Y, Yao G, Rouleau JL, et al:
Increased systemic inflammation and oxidative stress in patients
with worsening congestive heart failure: Improvement after
short-term inotropic support. Clin Sci (Lond). 110:483–489. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Willcox BJ, Curb JD and Rodriguez BL:
Antioxidants in cardiovascular health and disease: Key lessons from
epidemiologic studies. Am J Cardiol. 101:75D–86D. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Firuzi O, Miri R, Tavakkoli M and Saso L:
Antioxidant therapy: Current status and future prospects. Curr Med
Chem. 18:3871–3888. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rizos EC and Elisaf M: Omega-3 supplements
and cardiovascular disease. Re: Sperling LS, Nelson JR. History and
future of omega-3 fatty acids in cardiovascular disease. Curr Med
Res Opin 2015: 32(2);301–311. Curr Med Res Opin. 32:583–584. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sperling LS and Nelson JR: History and
future of omega-3 fatty acids in cardiovascular disease. Curr Med
Res Opin. 32:301–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colussi G, Catena C and Sechi LA: ω-3
Polyunsaturated fatty acids effects on the cardiometabolic syndrome
and their role in cardiovascular disease prevention: An update from
the recent literature. Recent Adv Cardiovasc Drug Discov. 9:78–96.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawakami S, Matsuda A, Sunagawa T, Noda Y,
Kaneko T, Tahara S, Hiraumi Y, Adachi S, Matsui H, Ando K, et al:
Antioxidant, EUK-8, prevents murine dilated cardiomyopathy. Circ J.
73:2125–2134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimizu T, Nojiri H, Kawakami S, Uchiyama
S and Shirasawa T: Model mice for tissue-specific deletion of the
manganese superoxide dismutase gene. Geriatr Gerontol Int. 10 Suppl
1:S70–S79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita N, Hiroe M, Ohta Y, Horie T and
Hosoda S: Chronic effects of metoprolol on myocardial
beta-adrenergic receptors in doxorubicin-induced cardiac damage in
rats. J Cardiovasc Pharmacol. 17:656–661. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cittadini A, Strömer H, Katz SE, Clark R,
Moses AC, Morgan JP and Douglas PS: Differential cardiac effects of
growth hormone and insulin-like growth factor-1 in the rat. A
combined in vivo and in vitro evaluation. Circulation. 93:800–809.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen T, Aneas I, Sakabe N, Dirschinger RJ,
Wang G, Smemo S, Westlund JM, Cheng H, Dalton N, Gu Y, et al: Tbx20
regulates a genetic program essential to adult mouse cardiomyocyte
function. J Clin Invest. 121:4640–4654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ieda M, Tsuchihashi T, Ivey KN, Ross RS,
Hong TT, Shaw RM and Srivastava D: Cardiac fibroblasts regulate
myocardial proliferation through beta1 integrin signaling. Dev
Cell. 16:233–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Remmen H, Williams MD, Guo Z, Estlack
L, Yang H, Carlson EJ, Epstein CJ, Huang TT and Richardson A:
Knockout mice heterozygous for Sod2 show alterations in cardiac
mitochondrial function and apoptosis. Am J Physiol Heart Circ
Physiol. 281:H1422–H1432. 2001.PubMed/NCBI
|
|
23
|
Dayal S, Baumbach GL, Arning E,
Bottiglieri T, Faraci FM and Lentz SR: Deficiency of superoxide
dismutase promotes cerebral vascular hypertrophy and vascular
dysfunction in hyperhomocysteinemia. PLoS One. 12:e01757322017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klein JA and Ackerman SL: Oxidative
stress, cell cycle, and neurodegeneration. J Clin Invest.
111:785–793. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ide T, Tsutsui H, Kinugawa S, Suematsu N,
Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K and
Takeshita A: Direct evidence for increased hydroxyl radicals
originating from superoxide in the failing myocardium. Circ Res.
86:152–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ago T, Matsushima S, Kuroda J, Zablocki D,
Kitazono T and Sadoshima J: The NADPH oxidase Nox4 and aging in the
heart. Aging (Albany NY). 2:1012–1016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuroda J, Ago T, Matsushima S, Zhai P,
Schneider MD and Sadoshima J: NADPH oxidase 4 (Nox4) is a major
source of oxidative stress in the failing heart. Proc Natl Acad Sci
USA. 107:pp. 15565–15570. 2010, View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang F, Lin X, Yu L, Li W, Qian D, Cheng
P, He L, Yang H and Zhang C: Low-dose radiation prevents type 1
diabetes-induced cardiomyopathy via activation of AKT mediated
anti-apoptotic and anti-oxidant effects. J Cell Mol Med.
20:1352–1366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu H, Zhen J, Yang Y, Gu J, Wu S and Liu
Q: Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by
inhibiting endoplasmic reticulum stress-induced apoptosis in a
streptozotocin-induced diabetes rat model. J Cell Mol Med.
20:623–631. 2016. View Article : Google Scholar : PubMed/NCBI
|