Introduction
Allergic diseases, including asthma, atopic
dermatitis, sinusitis, food allergies and anaphylaxis are induced
by allergens and exhibit adaptive allergic inflammation. Mast cells
are the effector cells during the mediation of allergic
inflammation. Following exposure to external allergens, the
cross-linking of immunoglobulin (Ig) E antibodies occurs and leads
to aggregations of the IgE specific receptor (FcεR), which binds to
the surface of mast cells (1).
Subsequently, histamine is released and cytokines, chemokines,
proteases and eicosanoids are secreted following the degranulation
of mast cells (2). Histamine
released from degranulated mast cells immediately initiates
hypersensitivity and vasodilation and increases the permeability of
vessels that have been exposed to external allergens (3). Pro-inflammatory cytokines secreted in
mast cells, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β and IL-6 mediate the chronic inflammatory response
(4).
Signaling in mast cells following allergic
inflammation has been widely studied. Tyrosine-protein kinase Lyn
and tyrosine-protein kinase Syk activated by the aggregation of
FcεR induce the phosphorylation of phosphoinositide 3-kinase (PI3K)
and the phosphorylated PI3K stimulates protein kinase B (Akt) and
phospholipase C (PLC)γ (5).
Subsequently, Akt and protein kinase C (PKC) phosphorylate the
inhibitor of κB (IκB) kinase (IKK) (6). This phosphorylation of IKK stimulates
the phosphorylation of nuclear factor (NF)-κB inhibitor α (IκBα)
and results in the degradation of IκBα via the ubiquitin-proteasome
pathway (7). NF-κB bound to IκBα, an
important transcriptional factor in inflammation, is activated by
phosphorylation following the degradation of IκBα. Activated NF-κB
is translocated into the nucleus and regulates the expression of
pro-inflammatory cytokine genes, including TNF-α, IL-1β and IL-6,
as well as the production of caspase-1 (8,9).
Caspase-1 is a member of the cysteine-aspartic acid protease
(caspase) family and participates in the breakdown of proteins to
produce aspartic acid residues. Activated caspase-1 induces
inflammation by increasing the secretion of pro-inflammatory
cytokines (10). In addition, PLCγ
catalyzes the synthesis of inositol 1,4,5-trisphosphate (IP3). IP3
is usually bound to the receptors on the surface of the endoplasmic
reticulum (ER) and stimulates the transient movement of calcium
stored in the ER into the cytoplasm. The decrease in calcium
concentration in the ER causes an immediate influx of calcium into
the cell. Consequently, the intracellular calcium concentration
increases, promoting the degradation of mast cells and the
subsequent secretion of pro-inflammatory cytokines (11).
Formononetin is a natural isoflavone found in many
medicinal plants, including Pongamia pinnata (12), Astragalus membranaceus
(13), Ononis angustissima
(14) and Trifolium pretense
(15). Studies investigating
formononetin have demonstrated that it upregulates nitric oxide
synthase (16), inhibits
angiogenesis and tumor growth (17)
and induces the apoptosis of human osteosarcoma cells (18). Therefore, the present study
investigated the anti-allergic inflammatory effects of formononetin
and its mechanism of action.
Materials and methods
Reagents
Formononetin was supplied by Dalian Meilun Biology
Technology Co., Ltd. (Dalian, China). Anti-dinitrophenyl (DNP) IgE,
DNP-human serum albumin (HSA) and a total RNA purification kit were
obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Dulbecco's modified Eagle's medium (DMEM) was purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Fluo-3/AM was
purchased from the Beyotime Institute of Biotechnology (Nantong,
China). The luciferase assay system was supplied by Promega
Corporation (Madison, WI, USA). The Lipofectamine 2000 transfection
reagent was obtained from Invitrogen; Thermo Fisher Scientific,
Inc. and the caspase-1 fluorometric assay kit was supplied by
AmyJet Scientific Inc. (Wuhan, China). ELISA kits for TNF-α (cat
no. H052), IL-1β (cat no. H002), IL-4 (cat no. H005) and IL-6 (cat
no. H007) were purchased from the Nanjing Jiancheng Bioengineering
Institute (Nanjing, China).
Cell culture
RBL-2H3 cells were obtained from the Cell Bank of
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in DMEM with 10% fetal
bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin at
37°C in a humidified 5% CO2 atmosphere. Cells were
divided into a control group and experimental groups. The cells in
experimental groups were sensitized with anti-DNP IgE (10 µg/ml)
for 16 h and pretreated with concentrations of formononetin (0,
0.1, 1 and 10 µM) prior to treatment with of DNP-HSA (500 ng/ml).
The cells in the control group were maintained in the normal
aforementioned conditions.
Histamine release assay
The level of released histamine in the culture media
was measured to assess the degranulation of mast cells, using the
o-phthaldialdehyde spectrofluorometric method (19). RBL-2H3 cells (1×106/well)
were sensitized with anti-DNP IgE (10 µg/ml) and incubated
overnight. Following pretreatment with or without formononetin
(0.1, 1 and 10 µM) for 30 min according to previous studies
(3,6), DNP-HSA (500 ng/ml) was added to the
cells and they were incubated at 37°C for 2 h. The cells were
separated from the media by centrifugation at 400 × g for 5 min at
4°C. The fluorescence intensity was determined using a fluorescence
plate reader and the excitation and emission wavelengths were set
at 360 and 440 nm, respectively.
Secretion of TNF-α, IL-1β and
IL-6
RBL-2H3 cells were treated as aforementioned and the
supernatant was collected for analysis. TNF-α, IL-1β and IL-6
levels were determined using ELISA kits following the
manufacturer's instructions. The absorbance of each sample was
recorded on a microplate reader at 450 nm. The results were
expressed as pg/ml derived from standard curves.
RNA extraction and reverse
transcription polymerase chain reaction (RT-PCR)
Following stimulation with DNP-HSA in the presence
or absence of formononetin, the total cellular RNA of RBL-2H3 cells
was isolated using the total RNA purification kit following the
manufacturer's protocol. The first strand cDNA was synthesized from
2 µg total RNA using oligo (dT) primers. Following heating at 70°C
for 5 min and the chilling on ice, the reaction system was mixed
with avian myeloblastosis virus reverse transcriptase (AMV RT;
Promega Corporation, Madison, WI, USA) together with 5X AMV RT
reaction buffer and dNTP mix. The reaction system was then
incubated at 42°C for 60 min. PCR was performed to analyze the
expression of TNF-α, IL-1β, IL-6 and β-actin mRNA. The primer sets
for the cytokines used were as follows: TNF-α forward,
5′-TCCCAAATGGGCTCCCTCTC-3′ and reverse, 5′-AAATGGCAAACCGGCTGACG-3′;
IL-1β forward, 5′-GCTGTGGCAGCTACCTATGTCTTG-3′ and reverse,
5′-AGGTCGTCATCATCCCACGAG-3′; IL-6 forward,
5′-TGTGCAATGGCAATTCTGAT-3′ and reverse, 5′-GAGCATTGGAAGTTGGGGTA-3′,
as outlined in a previous study (20). The amplified products were separated
by electrophoresis with 2% agarose gel containing ethidium bromide
and visualized on a Motic Images Advanced 3.2 imager system (Motic
Incorporation, Ltd., Xiamen, China).
Level of intercellular calcium
The level of intracellular calcium was determined
using Fluo-3/AM molecular fluorescence probes following the
manufacturer's instructions. Briefly, RBL-2H3 cells were
pre-incubated with Fluo-3/AM at 37°C for 1 h and the dye was then
washed from the surface of cells. Cells were treated with or
without formononetin at 37°C for 30 min prior to stimulation with
DNP-HSA for 2 h. The excitation and emission wavelengths were set
at 488 and 525 nm, respectively and measured using a fluorescence
plate reader.
Protein extraction
The RBL-2H3 cells were pretreated prior to
stimulation with DNP-HSA. Nuclear and cytosolic proteins were
extracted, following a previously established protocol (17). Cells were lysed with ice-cold lysis
buffer [10 mM HEPES/KOH, 2 mM MgCl2, 0.1 mM EDTA, 10 mM
KCl, 1 mM dithiolthreitol (DTT), 0.5 mM phenylmethane sulfonyl
fluoride (PMSF), 5 µg/ml leupeptin/aprotinin], left on ice for 5
min, then vortexed and centrifuged at 1,200 × g for 5 min. The
supernatant, which consisted of cytosolic proteins, was collected.
Following washing with PBS, the pellets were suspended in another
ice-cold buffer (50 mM HEPES/KOH, 50 mM KCl, 300 mM NaCl, 0.1 mM
EDTA, 10% glycerol, 1 mM DTT, 0.5 mM PMSF, 5 µg/ml
leupeptin/aprotinin) and incubated on ice for 20 min. The solution
containing nuclear protein was vortexed and centrifuged at 15,000 ×
g for 5 min at 4°C. The supernatant consisting of nuclear protein
extracts was collected for analysis.
Western blot analysis
After determination of total protein using a BCA kit
(A045-4; Nanjing Jiancheng Bioengineering Institute), the collected
protein extracts were subjected to electrophoresis on 10% SDS-PAGE
(50 µg protein per lane) and transferred to a nitrocellulose
membrane. Membranes were then blocked with 5% nonfat milk for 1 h
at room temperature. The expression of nuclear NF-κB, IκBα and
caspase-1 was detected using anti-NF-κB (cat no. 3039), anti-IκBα
(cat no. 9246) and anti-caspase-1 (cat no. 2225) antibodies (all
1:1,000; all from Cell Signaling Technology Inc., Danvers, MA,
USA), which were incubated at 4°C overnight. The antibody of
β-actin (1:1,000; cat no. 21338; Nanjing Jiancheng Bioengineering
Institute) was used as the reference. The membranes were then
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat no. 112-035-044; Jackson ImmunoResearch Laboratories
Inc., West Grove, PA, USA) for 1 h at room temperature and an ECL
kit (cat no. W028-1; Nanjing Jiancheng Bioengineering Institute)
was used to visualize the immunoblots.
Cell transfection and dual-luciferase
(firefly luciferase and Renilla luciferase) reporter assay for
NF-κB
RBL-2H3 cells (1×106/well) were
co-transfected with 100 ng NF-κB luciferase reporter plasmid
pGL4.32 and 9.6 ng Renilla luciferase reporter vector
plasmid pRL-TK per well (Promega Corporation, Madison, WI, USA).
Transfection was performed over 24 h using Lipofectamine 2000
following the manufacturer's protocol. Medium was replaced with
fresh serum-free medium. Cells were treated with or without
formononetin prior to stimulation with DNP-HSA. Cells were then
washed with ice-cold saline buffer and lysed with lysis buffer
following the manufacturer's instructions. 20 h following
transfection, luciferase activity was determined using the
luciferase reporter assay system. Relative luciferase activity was
determined by normalizing the firefly luciferase activity vs. the
internal control Renilla luciferase.
Caspase-1 activity assay
Caspase-1 activity was measured using a fluorometric
assay kit following the manufacturer's instructions. Cells were
pretreated in the presence or absence of formononetin for 30 min
prior to stimulation with DNP-HSA. Cells were then lysed and
centrifuged at 10,000 × g for 1 min at room temperature. The
supernatant was incubated with the fluorescence substrate YVAD-AFC
at 37°C for 2 h. Fluorescence intensity was measured on the
fluorescence plate reader at an excitation wavelength of 400 nm and
an emission wavelength 505 nm.
Statistical analysis
Data are expressed as the mean ± standard deviation.
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was
used for statistical analysis. The experimental data from different
groups were analyzed by one way analysis of variance followed by a
Dunnett's t-test for multiple comparisons. Student's t-test was
used for single comparisons. P<0.05 was determined to indicate a
statistically significant difference.
Results
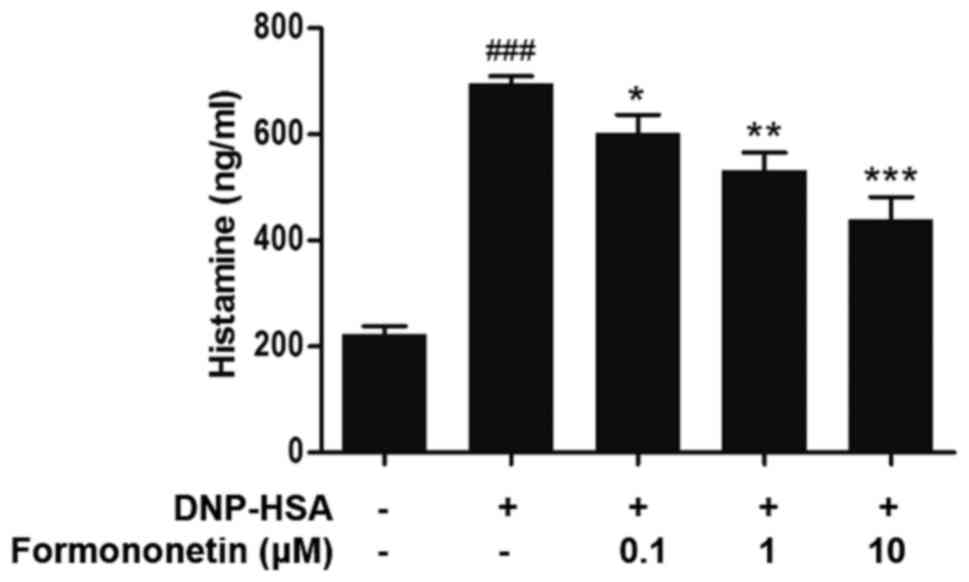
Effect of formononetin on histamine
release
The level of released histamine in the culture
medium was determined to assess the extent of mast cell
degranulation. Following stimulation with DNP-HSA, the release of
histamine was significantly increased compared with the control
group (P<0.001; Fig. 1). However,
when pretreated with increasing doses of formononetin following
stimulation with DNP-HSA, the level of released histamine was
significantly decreased in a dose-dependent manner (0.1 µm,
P<0.05; 1 µm, P<0.01; 10 µm, P<0.001; Fig. 1).
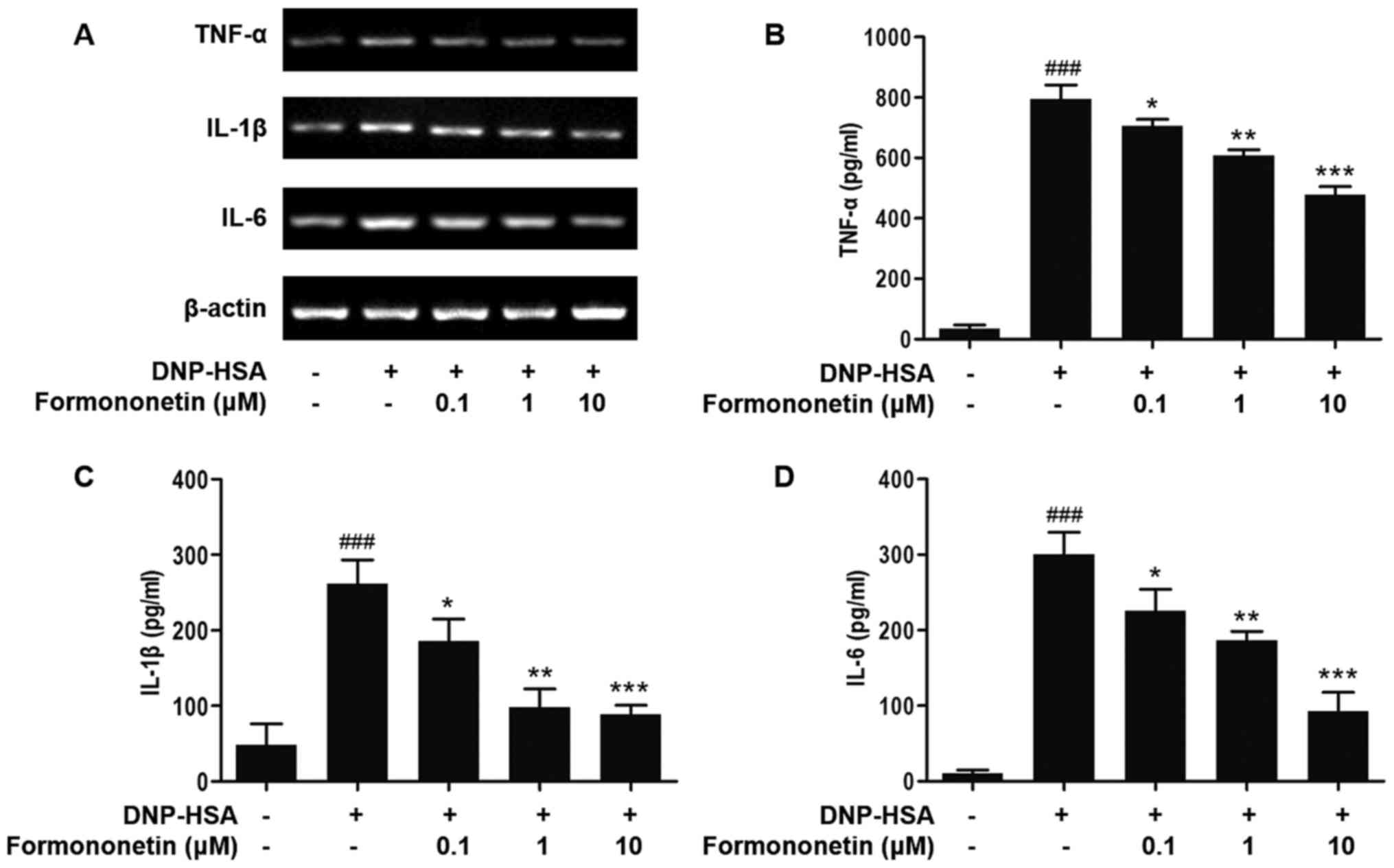
Effect of formononetin on the
secretion of pro-inflammatory cytokines
The expression of the pro-inflammatory cytokines
TNF-α, IL-1β and IL-6 was determined to evaluate the effect of
formononetin on inflammation (Fig.
2). The expression of TNF-α, IL-1β and IL-6 mRNA in RBL-2H3
cells stimulated by DNP-HSA was significantly increased compared
with the control group (all P<0.001; Fig. 2B-D). The expression of TNF-α, IL-1β
and IL-6 mRNA in RBL-2H3 cells induced by DNP-HSA was significantly
decreased in a dose-dependent manner following treatment with
increased dosages of formononetin, compared with RBL-2H3 cells
stimulated with DNP-HSA alone (0.1 µM, P<0.05; 1 µM, P<0.01;
10 µM, P<0.001; Fig. 2B-D).
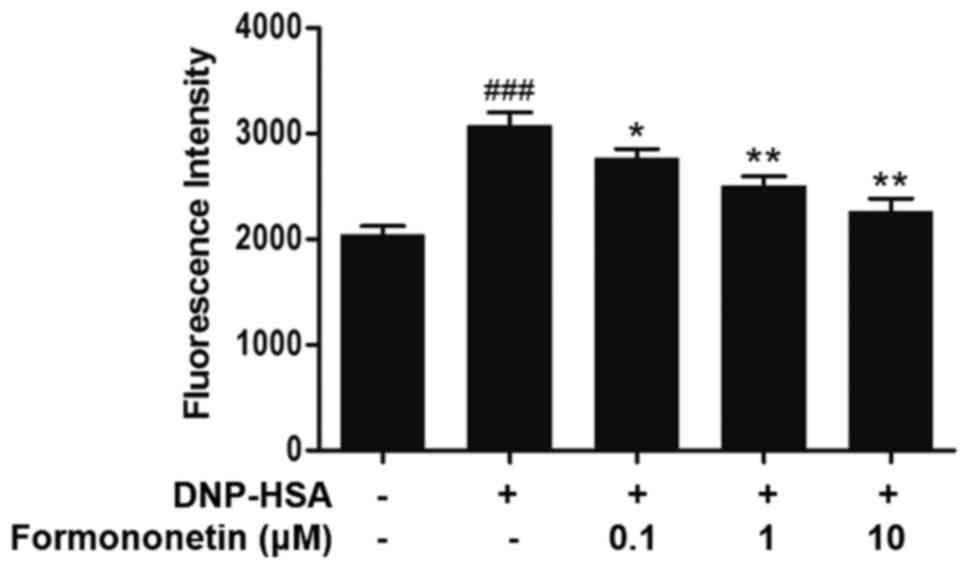
Effect of formononetin on the level of
intracellular calcium
The level of intracellular calcium was measured to
investigate the mechanism of action of formononetin on the release
of histamine. Following stimulation with DNP-HSA, the level of
intracellular calcium was significantly increased compared with the
control (P<0.001; Fig. 3). By
contrast, the level of intracellular calcium was significantly
decreased following treatment with increasing doses of formononetin
compared with the group treated with DNP-HSA alone (0.1 µM,
P<0.05; 1 and 10 µM, both P<0.01; Fig. 3).
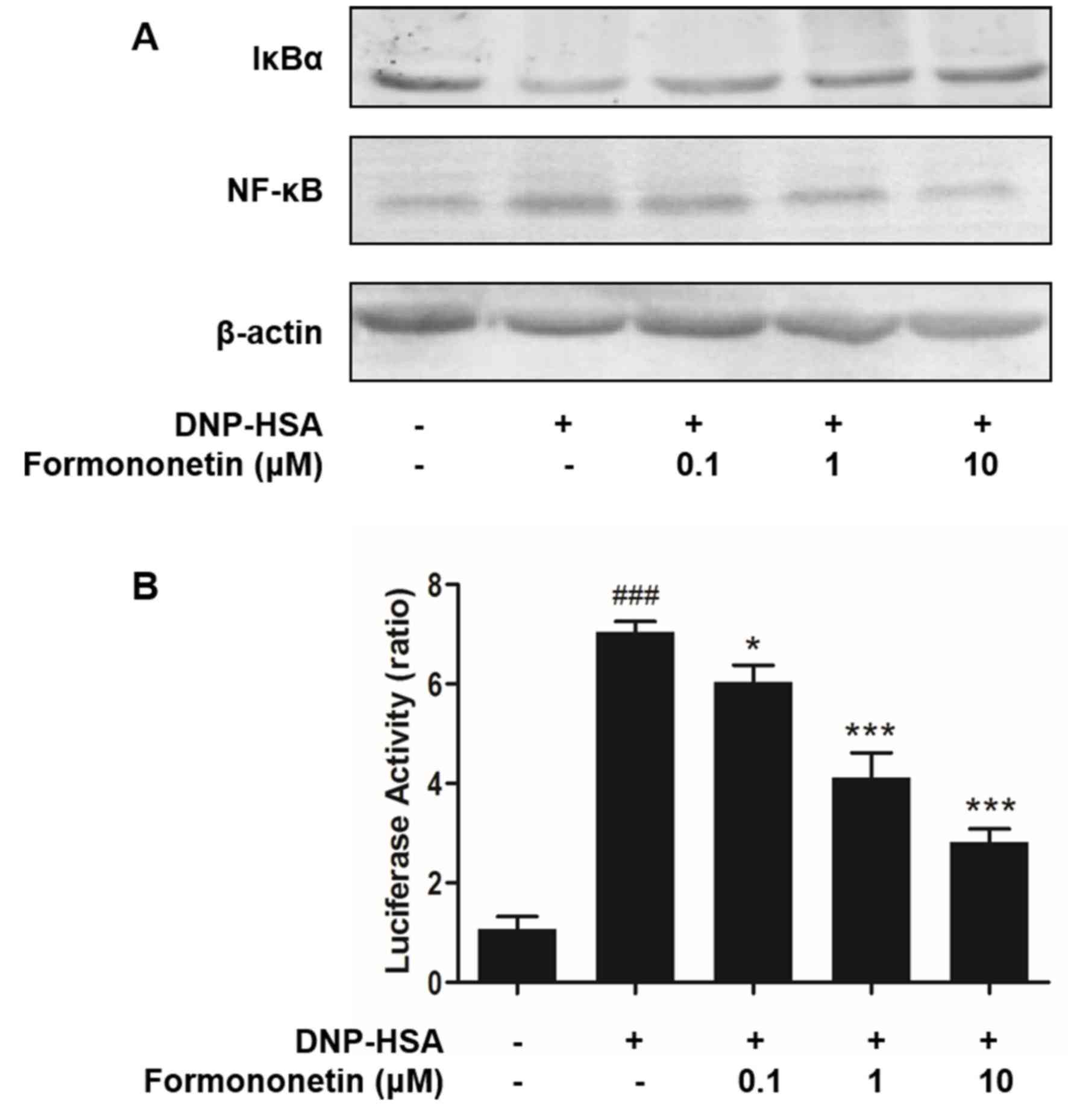
Effect of formononetin on the
activation of NF-κB
Activated NF-κB serves an important role in the
expression of pro-inflammatory cytokines. Thus, the effects of
formononetin on the activation of NF-κB and degradation of IκBα
were determined. Levels of degraded IκBα were reduced in RBL-2H3
cells stimulated by DNP-HSA; however, they were increased following
treatment with formononetin (Fig.
4A). By contrast, stimulation with DNP-HSA significantly
induced the activation of NF-κB (P<0.001; Fig. 4B), whereas treatment with
formononetin inhibited the activation of NF-κB and significantly
decreased the level of activated NF-κB in nuclei in a
dose-dependent manner (0.1 µM, P<0.05; 1 and 10 µM, both
P<0.001; Fig. 4B).
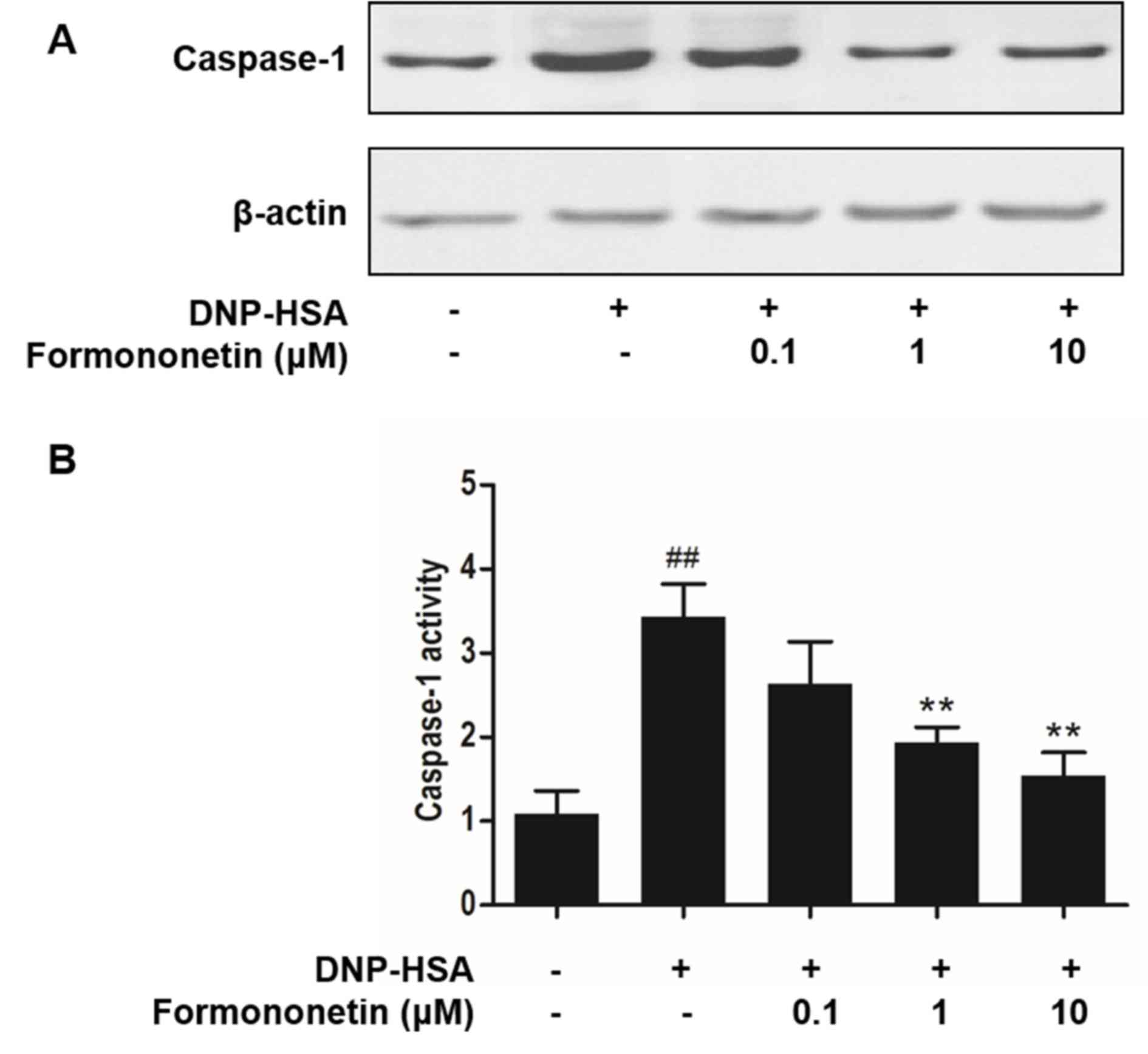
Effect of formononetin on caspase-1
activity
Caspase-1 promotes the production of
pro-inflammatory cytokines, thus, the activity of caspase-1
following treatment with formononetin was investigated to confirm
the mechanism of anti-inflammation. Stimulation with DNP-HSA
increased the expression (Fig. 5A)
and significantly increased the activity of caspase-1 (P<0.001;
Fig. 5B) compared with the control
group. However, increasing doses of formononetin inhibited the
expression (Fig. 5A) and
significantly inhibited the activity (>1 µM, P<0.01; Fig. 5B) of caspase-1 in RBL-2H3 cells.
Discussion
Allergic inflammation is mediated by mast cells and
causes allergic diseases. RBL-2H3 cells have been identified as a
suitable cell line to conduct investigations into allergic
inflammation in vitro (21).
The current study investigated the anti-allergic inflammatory
effects of formononetin and its mechanism of action in mast cells.
Histamine release serves an important role in the allergic
reactions that occur following induction by external allergens and
IgE-mediated signaling transduction. The level of released
histamine indicates the extent of mast cell degranulation (4). Following treatment with formononetin,
the increased level of released histamine in RBL-2H3 cells
stimulated by DNP-HSA was reversed in a dose-dependent manner.
These results indicate that formononetin treatment alleviates
chronic allergic reactions. Intracellular calcium affects the
release of histamine in mast cells and the expression of
pro-inflammatory cytokines (22).
Therefore, in the current study, the effect of formononetin on
intracellular calcium was determined to investigate its mechanism
of action in attenuating histamine release. The results
demonstrated that formononetin decreased intracellular calcium
levels, suggesting that formononetin decreases the level of
released histamine by inhibiting intracellular calcium.
TNF-α, IL-1β and IL-6 are effective mediators of
chronic inflammation (2). TNF-α
mediates the inflammatory response in the early phase of an
allergic reaction and IL-1β contributes to hypersensitivity and the
inflammatory response (23). IL-6 is
secreted in mast cells and is associated with acute allergic
reactions and a chronic inflammatory response (21). Inhibiting the secretion of these
pro-inflammatory cytokines improves inflammatory symptoms. The
current study has revealed that formononetin significantly reduces
the production of TNF-α, IL-1β and IL-6 in RBL-2H3 cells. In
addition to intracellular calcium, NF-κB is a key transcription
factor that influences the expression of these pro-inflammatory
cytokines (8). The phosphorylation
of IκKα is essential in the activation and translocation of NF-κB
(18). The results of the current
study demonstrated that formononetin inhibits the activation and
translocation of NF-κB. It was also demonstrated that formononetin
degrades IκKα via phosphorylation to produce free NF-κB. These
results suggest that the anti-inflammatory mechanism of action of
formononetin is associated with the regulation of NF-κB and
upstream IκKα. This is consistent with the results of a previous
study that demonstrated that treatment with formononetin in a
dose-dependent manner regulates NF-κB activation in 16HBE cells
(24).
Caspase-1 is a cysteine-aspartic acid protease that
converts pro-cytokines into mature forms, such as IL-1β (25). Inhibition of caspase-1 activity
attenuates inflammatory effects by reducing the secretion of
related cytokines. The current study demonstrated that formononetin
inhibits the activity of caspase-1 to attenuate inflammation. These
results may contribute to further understanding regarding the
anti-allergic inflammatory mechanism of formononetin.
In conclusion, the current study evaluated the
effects and mechanism of action of formononetin on allergic
inflammation in RBL-3H2 cells. Formononetin attenuates allergic
reactions by reducing histamine release and the inflammatory
response by inhibiting TNF-α, IL-1β and IL-6 secretion. The
mechanisms of this action include reducing intracellular calcium,
inhibiting caspase-1 activity and regulating the activation and
translocation of NF-κB and upstream phosphorylation of IκKα.
Therefore, the current study demonstrated that formononetin
prevents mast cell-mediated allergic inflammation.
References
|
1
|
Beghdadi W, Madjene LC, Benhamou M,
Charles N, Gautier G, Launay P and Blank U: Mast cells as cellular
sensors in inflammation and immunity. Front Immunol. 2:372011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HH, Bae Y and Kim SH: Galangin
attenuates mast cell-mediated allergic inflammation. Food Chem
Toxicol. 57:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bansal G, Xie Z, Rao S, Nocka KH and Druey
KM: Suppression of immunoglobulin E-mediated allergic responses by
regulator of G protein signaling 13. Nat Immunol. 9:73–80. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Je IG, Kim DS, Kim SW, Lee S, Lee HS, Park
EK, Khang D and Kim SH: Tyrosol suppresses allergic inflammation by
inhibiting the activation of phosphoinositide 3-kinase in mast
cells. PLoS One. 10:e01298292015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kalesnikoff J and Galli SJ: New
developments in mast cell biology. Nat Immunol. 9:1215–1223. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayden MS and Ghosh S: NF-κB in
immunobiology. Cell Res. 21:223–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lamkanfi M, Vande Walle L and Kanneganti
TD: Deregulated inflammasome signaling in disease. Immunol Rev.
243:163–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baba Y, Nishida K, Fujii Y, Hirano T,
Hikida M and Kurosaki T: Essential function for the calcium sensor
STIM1 in mast cell activation and anaphylactic responses. Nat
Immunol. 9:81–88. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Jiang Z, Li X, Hou Y, Liu F, Li N,
Liu X and Yang L: Natural therapeutic agents for neurodegenerative
diseases from a traditional herbal medicine Pongamia pinnata
(L.) Pierre. Bioorg Med Chem Lett. 25:53–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Sun YN, Yan XT, Yang SY, Kim S, Lee
YM, Koh YS and Kim YH: Flavonoids from Astragalus
membranaceus and their inhibitory effects on LPS-stimulated
pro-inflammatory cytokine production in bone marrow-derived
dendritic cells. Arch Pharm Res. 37:186–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghribi L, Waffo-Téguo P, Cluzet S, Marchal
A, Marques J, Mérillon JM and Ben Jannet H: Isolation and structure
elucidation of bioactive compounds from the roots of the Tunisian
Ononis angustissima L. Bioorg Med Chem Lett. 25:3825–3830.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tava A, Pecio Ł, Stochmal A and Pecetti L:
Clovamide and flavonoids from leaves of Trifolium pratense
and T. pratense subsp. nivale grown in Italy. Nat Prod Commun.
10:933–936. 2015.PubMed/NCBI
|
|
16
|
Sun T, Cao L, Ping NN, Wu Y, Liu DZ and
Cao YX: Formononetin upregulates nitric oxide synthase in arterial
endothelium through estrogen receptors and MAPK pathways. J Pharm
Pharmacol. 68:342–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu XY, Xu H, Wu ZF, Chen C, Liu JY, Wu GN,
Yao XQ, Liu FK, Li G and Shen L: Formononetin, a novel FGFR2
inhibitor, potently inhibits angiogenesis and tumor growth in
preclinical models. Oncotarget. 6:44563–44578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu W and Xiao Z: Formononetin induces
apoptosis of human osteosarcoma cell line U2OS by regulating the
expression of Bcl-2, Bax and MiR-375 in vitro and in vivo. Cell
Physiol Biochem. 37:933–939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Je IG, Kim HH, Park PH, Kwon TK, Seo SY,
Shin TY and Kim SH: SG-HQ2 inhibits mast cell-mediated allergic
inflammation through suppression of histamine release and
pro-inflammatory cytokines. Exp Biol Med (Maywood). 240:631–638.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bae Y, Lee S and Kim SH: Chrysin
suppresses mast cell-mediated allergic inflammation: Involvement of
calcium, caspase-1 and nuclear factor-κB. Toxicol Appl Pharmacol.
254:56–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HH, Park SB, Lee S, Kwon TK, Shin TY,
Park PH, Lee SH and Kim SH: Inhibitory effect of putranjivain A on
allergic inflammation through suppression of mast cell activation.
Toxicol Appl Pharmacol. 274:455–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka S, Mikura S, Hashimoto E, Sugimoto
Y and Ichikawa A: Ca2+ influx-mediated histamine
synthesis and IL-6 release in mast cells activated by monomeric
IgE. Eur J Immunol. 35:460–468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee NY, Chung KS, Jin JS, Bang KS, Eom YJ,
Hong CH, Nugroho A, Park HJ and An HJ: Effect of chicoric acid on
mast cell-mediated allergic inflammation in vitro and in vivo. J
Nat Prod. 78:2956–2962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen D, Xie X, Zhu Z, Yu X, Liu H, Wang H,
Fan H, Wang D, Jiang G and Hong M: Screening active components from
Yu-ping-feng-san for regulating initiative key factors in allergic
sensitization. PLoS One. 9:e1072792014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Besnard AG, Togbe D, Couillin I, Tan Z,
Zheng SG, Erard F, Le Bert M, Quesniaux V and Ryffel B:
Inflammasome-IL-1-Th17 response in allergic lung inflammation. J
Mol Cell Biol. 4:3–10. 2012. View Article : Google Scholar : PubMed/NCBI
|