Introduction
Acute renal ischemia reperfusion injury (IRI) refers
to a pathological and physiological phenomenon whereby cells in
ischemic tissues are damaged following the re-establishment of a
blood supply (1). Renal injury
following IRI does not improve and typically worsens over time. As
an organ with high perfusion rates, the kidneys are sensitive to
reperfusion following ischemia (2).
Therefore, renal IRI is likely to occur following kidney
transplant, kidney vascular surgery, extracorporeal shock wave
lithotripsy or resuscitation (3).
Renal IRI is associated with acute ischemic renal failure. In
addition, renal IRI may occur as a result of delayed renal graft
function or chronic renal allograft dysfunction (3).
Nuclear factor (NF)-κB, a key downstream factor of
the Toll-like receptor (TLR/NF-κB signaling pathway, mediates
essential functions by regulating inflammatory factors, cell
proliferation and differentiation (4). As a result, systemic inhibition of
NF-κB may reduce inflammation and have damaging consequences
(5). The results of a previous study
indicated that NF-κB serves important roles in inflammation of IRI
(6).
The number of studies investigating resveratrol
metabolins have increased in recent years. It has been demonstrated
that resveratrol exhibits neuroprotective effects in cerebral
ischemia (7). In addition, as the
contents of these metabolins in plants are low and their structures
are unstable, they are easily degraded when heated. Pterostilbene
(Pter) is a derivative of resveratrol (8). Resveratrol is rapidly metabolized to
produce Pter and piceid (9). The
selectivity and stability of Pter is superior when compared with
that of resveratrol (9). A previous
study demonstrated that, similar to resveratrol, Pter inhibits
oxidative stress, possesses anti-fungal properties and suppresses
cell proliferation (8). In addition,
Pter has been demonstrated to exhibit favorable effects in
Alzheimer's disease (10). The aim
of the present study was to investigate the protective effects of
Pter in acute renal IRI, and to explore the potential underlying
mechanisms involved.
Materials and methods
Animals
A total of 50 adult male Sprague Dawley (SD) rats
(weight, 250–300 g; age, 8–10 weeks old) were purchased from the
Center of Experimental Animal Research at the First Affiliated
Hospital of Zhengzhou University (Zhengzhou, China). All
experiments in the present study were approved by the Institutional
Animal Experimentation Committee of the Academic Medical Center of
the First Affiliated Hospital of Zhengzhou University. SD rats (10
rats/cage) were maintained in a temperature-controlled room at
22–23°C with a 12-h light/dark cycles, and provided with access to
food and water ad libitum.
Surgical preparation and experimental
protocol
SD rats were injected with 75 mg/kg ketamine
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 0.5 mg/kg
dexmedetomidine (Sigma-Aldrich; Merck KGaA) and 0.05 mg/kg
atropine-sulfate Sigma-Aldrich; Merck KGaA). For the duration of
the procedure, body temperatures were maintained at 37±0.5°C using
an external thermal heating pad. The left kidney was exposed and
immobilized using a Lucite kidney cup in the left flank. The renal
vessels were carefully separated, and the nerves and adrenal gland
were preserved. A polyethylene catheter was used to isolate, ligate
and cannulate the left ureter for urine collection. Following
stabilization for 30 min, the left kidney was treated with a
polyethylene catheter and blood flow was recovered. Following a
stabilization period of 30 min, rats were randomly divided into the
following 4 groups (n=10): IRI group, consisting of rats that
underwent the IRI procedure alone; Pter 10 group, consisting of
rats that underwent the IRI procedure and gavaged with 10 mg/kg/day
Pter at 24 h after IRI surgery; Pter 20 group, where rats underwent
the IRI procedure and gavaged with 20 mg/kg/day Pter at 24 h after
IRI surgery; Pter 30 group, where rats underwent the IRI procedure
and were gavaged with 30 mg/kg/day Pter at 24 h after IRI surgery.
A additional control group, consisting of SD rats (n=10) that had
not undergone the renal IRI procedure was included. Pter was
administered over the course of 2 weeks as described previously
(11,12).
Renal function
For the analysis of blood urea nitrogen (BUN) levels
and creatinine concentration, urine was collected after rats had
received 2 weeks of Pter treatment from the left ureter for 10 min.
BUN levels were determined using a Urea Nitrogen (BUN) diacetyl
monoxime test kit from Stanbio Laboratory L.P. (Boerne, TX, USA)
according to the manufacturer's instructions. The creatinine
concentration in urine samples was analyzed using a creatinine kit
from Tiangen Biotech Co., Ltd. (Beijing, China) according to the
manufacturer's instructions. Subsequently, total protein was
quantified using a BCA kit (Beyotime Institute of Biotechnology,
Nanjing, China).
Histological analysis
Following reperfusion and 2 weeks of Pter treatment,
renal tissue samples were harvested under anesthesia and fixed
using 4% paraformaldehyde for 24 h at room temperature. Tissue
samples were dehydrated, made transparent, waxed and embedded,
before being cut into 5-µM thick sections and stained with
hematoxylin-eosin at room temperature for 15–20 min. The sample
slices were examined using using a BX51 microscope (Olympus
Corporation, Tokyo, Japan).
Myeloperoxidase (MPO) level
Following reperfusion and 2 weeks of Pter treatment,
renal tissue samples were harvested and stored at −80°C. MPO levels
were detected using an MPO test kit (Beyotime Institute of
Biotechnology). Renal tissue samples were homogenized in cold 5 mM
sodium phosphate buffer and centrifuged at 12,000 × g for 5 min at
4°C. The concentration of MPO was determined using the Bradford
assay. MPO was expressed as U/g protein.
Western blot analysis
Following reperfusion and 2 weeks of Pter treatment,
renal tissue samples were collected and homogenized in RIPA buffer
(Beyotime Institute of Biotechnology) containing 1% protease
inhibitor cocktail. Tissue samples were centrifuged at 12,000 × g
for 5 min at 4°C, and the protein concentration of the sample
supernatant was determined using BCA kit (Beyotime Institute of
Biotechnology). A total of 50 µg protein per lane was loaded and
separated by 10% SDS-PAGE, and transferred to nitrocellulose
membranes (EMD Millipore; Billerica, MA, USA). The membranes were
blocked in Tris-buffered saline with Tween-20 (TBST) plus 5%
non-fat dry milk for 1 h at 37°C, and incubated overnight at 4°C
with primary antibodies against inducible nitric oxide synthase
(iNOS; dilution, 1:3,000, sc-649), TLR4 (dilution, 1:4,000;
sc-10741), NF-κB (dilution, 1:4,000; sc-109) and β-actin (sc-10731,
1:4,000, all from Santa Cruz Biotechnology). The membranes were
subsequently washed with TBST, and then probed with a goat
anti-rabbit IgG secondary antibody (dilution, 1:5,000; 7074; Cell
Signaling Technology, Inc., Danvers, MA, USA) at room temperature
for 1 h. β-actin was used as a loading control. The blots were
visualized with ECLPlus (Beyotime Institute of Biotechnology)
reagent and quantified using the Bio-Rad Laboratories Quantity One
software 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Determination of interleukin (IL)-10,
IL-1β, IL-6 and tumor necrosis factor (TNF)-α expression
levels
Following reperfusion and 2 weeks of Pter treatment,
whole blood samples were collected and centrifuged at 1,000 × g for
10 min at 4°C. Serum was used to measure IL-10 (EK0418), IL-1β
(EK0393), IL-6 (EK0412) and TNF-α (EK0526) levels using ELISA kits
(Boster Biological Technology Co., Ltd., Wuhan, China) according to
manufacturer's protocol. Samples were read using a
spectrophotometer at an absorbance of 450 nm.
Detection of caspase-3 activation
Following reperfusion and 2 weeks of Pter treatment,
renal tissue samples were harvested and homogenized in RIPA buffer
(Beyotime Institute of Biotechnology) containing 1% protease
inhibitor cocktail. Tissue samples were centrifuged at 12,000 × g
for 5 min at 4°C, and the protein concentrations of the sample
supernatants were determined using a BCA protein assay kit
(Beyotime Institute of Biotechnology). An equal quantity of total
protein (10 µg) from each sample was used to measure caspase-3
activity using caspase-3 activity kits (C1115; Beyotime Institute
of Biotechnology) in the dark. Subsequently, total protein was
quantified using a BCA kit (Beyotime Institute of Biotechnology).
Samples were read with a spectrophotometer at an absorbance of 405
nm. Caspase-3 activity was normalized to β-actin expression.
Statistical analysis
The results are expressed as the mean ± standard
deviation. SPSS software (version, 18.0; SPSS, Inc., Chicago, IL,
USA) was used to perform statistical analyses. One-way analysis of
variance was used for comparisons among multiple groups followed by
Tukey's test, and the independent samples t-test was used to
compare the difference between two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Pter protects against renal
function
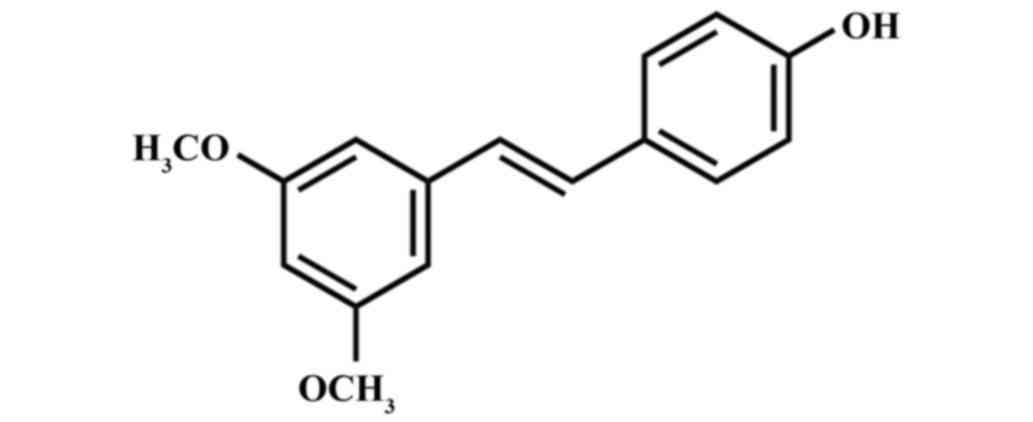
The chemical structure of Pter is indicated at
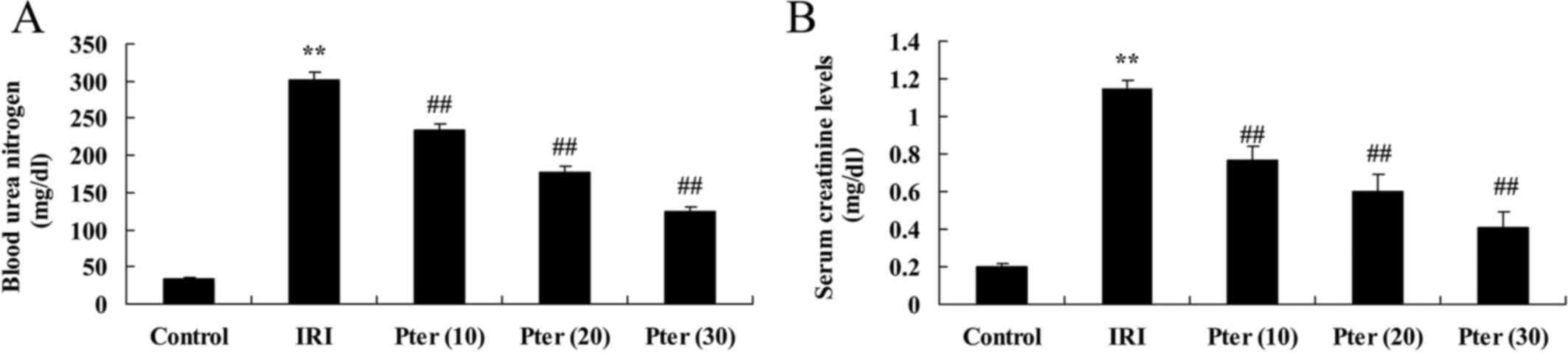
Fig. 1. When compared with the
control group, IRI induced a significant increase in the
concentration of BUN and creatinine in rat urine samples, which
indicated that IRI significantly impaired renal function
(P<0.01; Fig. 2). By contrast,
Pter treatment significantly attenuated the IRI-induced increase in
BUN and creatinine levels in rats (P<0.01; Fig. 2), which suggests that Pter may
protect against IRI-induced impairment of renal function.
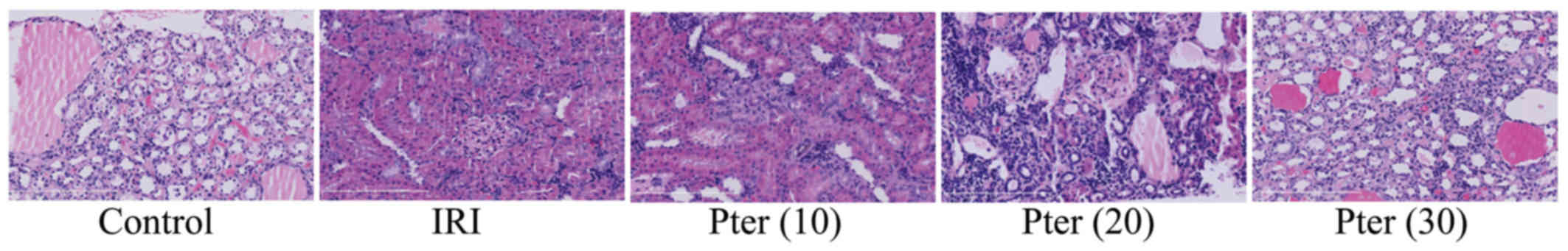
IRI induces morphological changes
Compared with the control group, IRI-induced renal
cell death was markedly observed in IRI-induced model rats
(Fig. 3). By contrast, Pter
treatment markedly reduced the IRI-induced increase in histological
scores (Fig. 3).
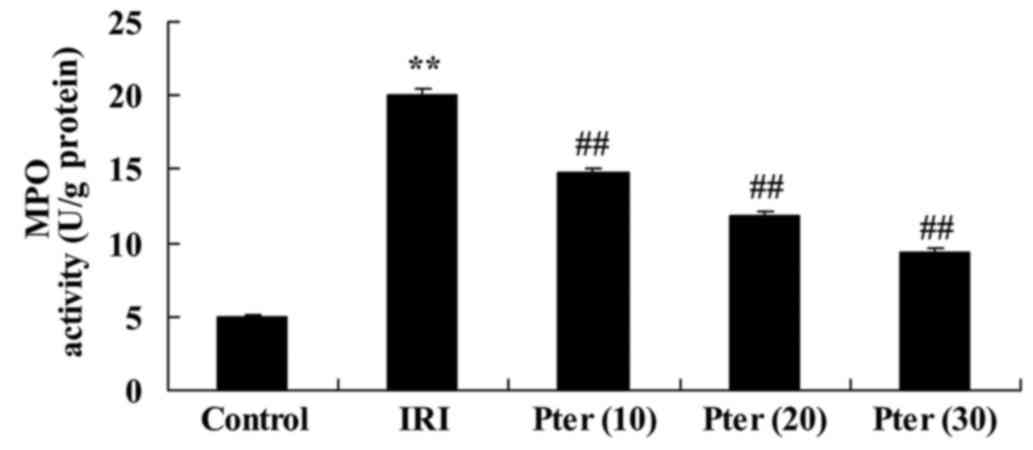
Pter protects against the IRI-induced
elevation in MPO levels
In the IRI-induced model group, MPO levels were
significantly increased when compared with the control group
(P<0.01; Fig. 4). Following the
administration of Pter, the IRI-induced increase in MPO levels was
significantly suppressed when compared with the untreated IRI model
group (P<0.01; Fig. 4).
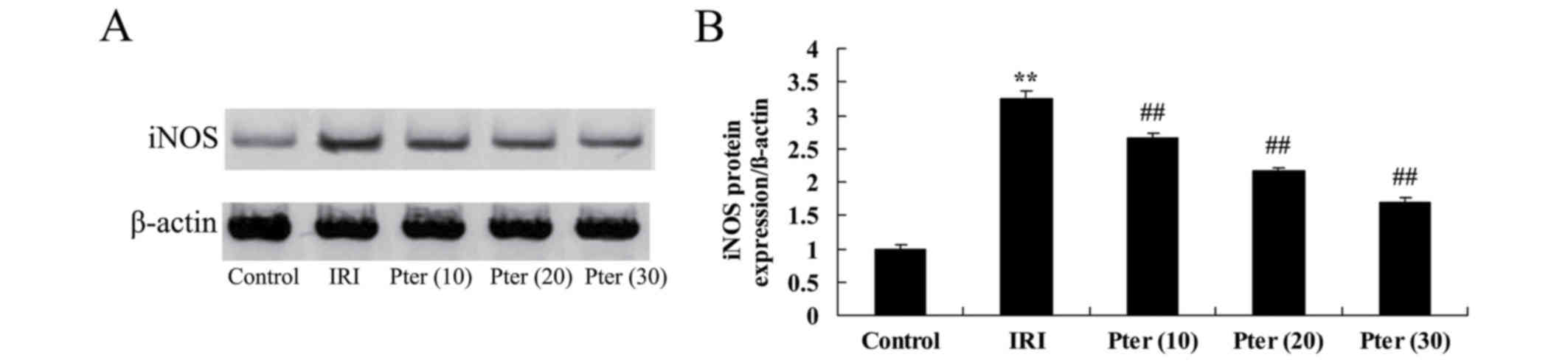
Pter protects against the IRI-induced
increase in iNOS protein expression levels
In order to investigate the effects of Pter on the
iNOS-mediated signaling pathway, iNOS protein expression levels in
the renal tissues of Pter-treated rats that had undergone renal IRI
were determined. Western blot analysis demonstrated that IRI
significantly increased iNOS protein expression levels in the IRI
model group when compared with the control group (P<0.01;
Fig. 5). By contrast, administration
of Pter significantly inhibited the IRI-induced elevation in iNOS
protein expression levels (P<0.01; Fig. 5).
Pter protects against IRI-induced
alterations in IL-10, IL-1β, IL-6 and TNF-α expression levels
To investigate the effects of Pter on inflammation,
IL-10, IL-1β, IL-6 and TNF-α expression levels in the renal tissues
of Pter-treated rats that had undergone renal IRI were determined.
Compared with the control group, IRI significantly increased the
expression levels of IL-1β, IL-6 and TNF-α, and significantly
decreased IL-10 expression levels (P<0.01; Fig. 6). By contrast, Pter treatment
significantly reversed the expression levels of these factors when
compared with the IRI model group (P<0.01; Fig. 6).
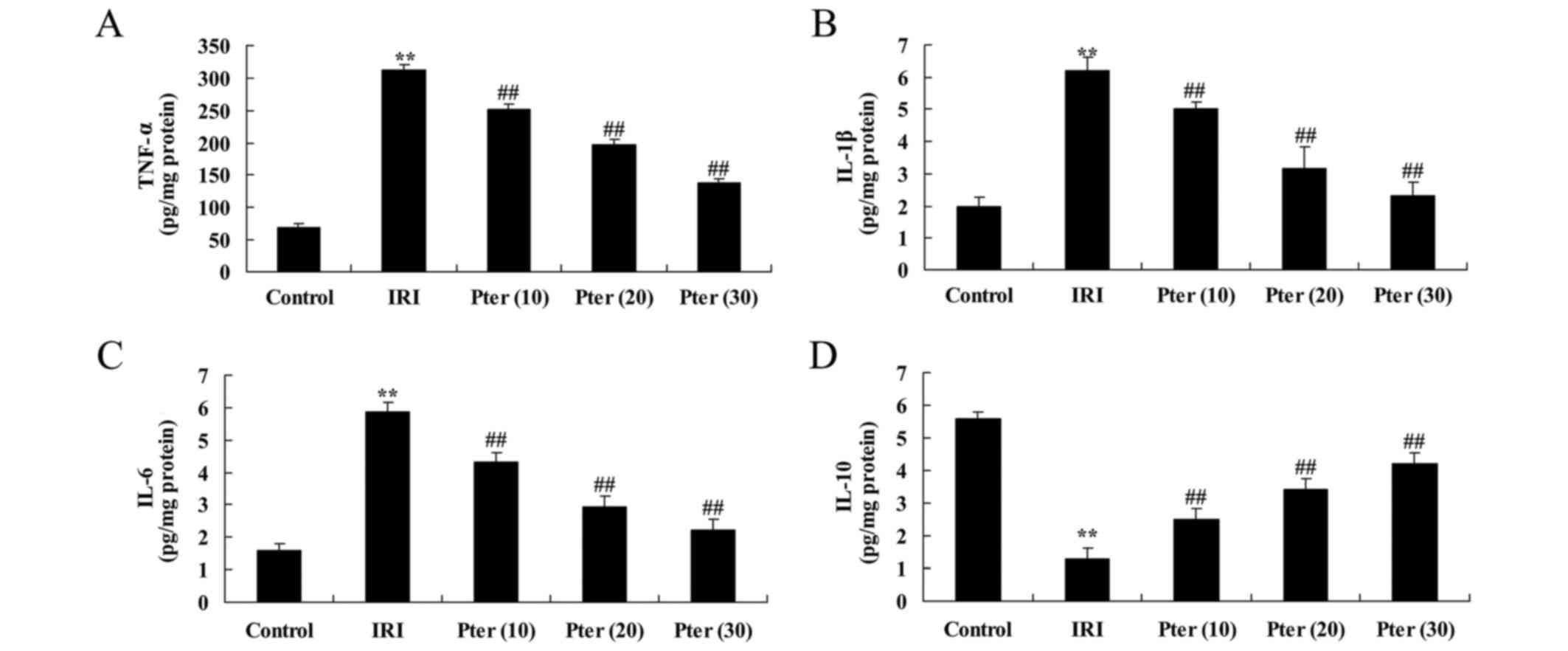 | Figure 6.Pter protected against IRI-induced
alterations in IL-10, IL-1β, IL-6 and TNF-α expression levels. The
levels of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-10 in rat renal
tissues. **P<0.01 vs. control group; ##P<0.01 vs.
IRI group. Pter, pterostilbene; IRI, ischemia reperfusion injury;
IL, interleukin; TNF-α, tumor necrosis factor-α; Pter (10), 10 mg/kg Pter-treated IRI group; Pter
(20), 20 mg/kg Pter-treated IRI
group; Pter (30), 30 mg/kg
Pter-treated IRI group. |
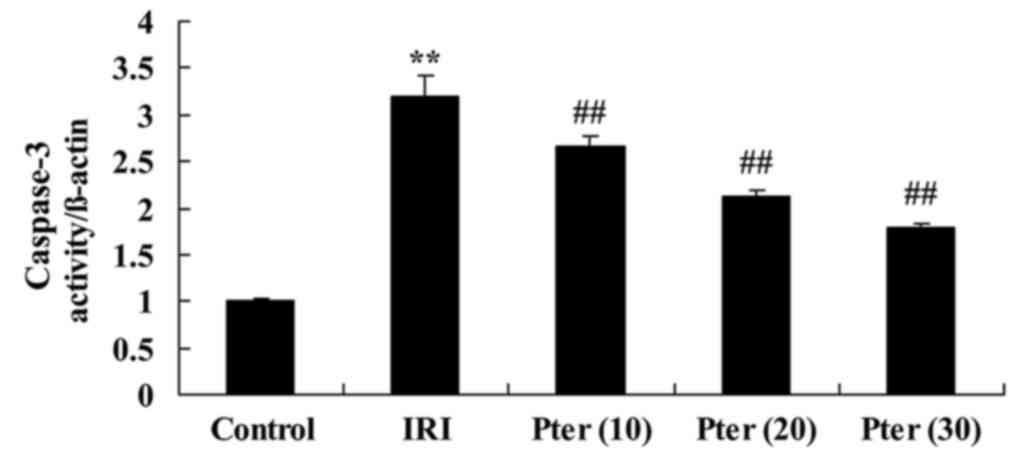
Pter protects against IRI-induced
caspase-3 activation
When compared with the control group, IRI
significantly increased caspase-3 activity in rats from the IRI
model group (P<0.01; Fig. 7).
However, treatment with Pter significantly inhibited the
IRI-induced increase in caspase-3 activity (P<0.01; Fig. 7).
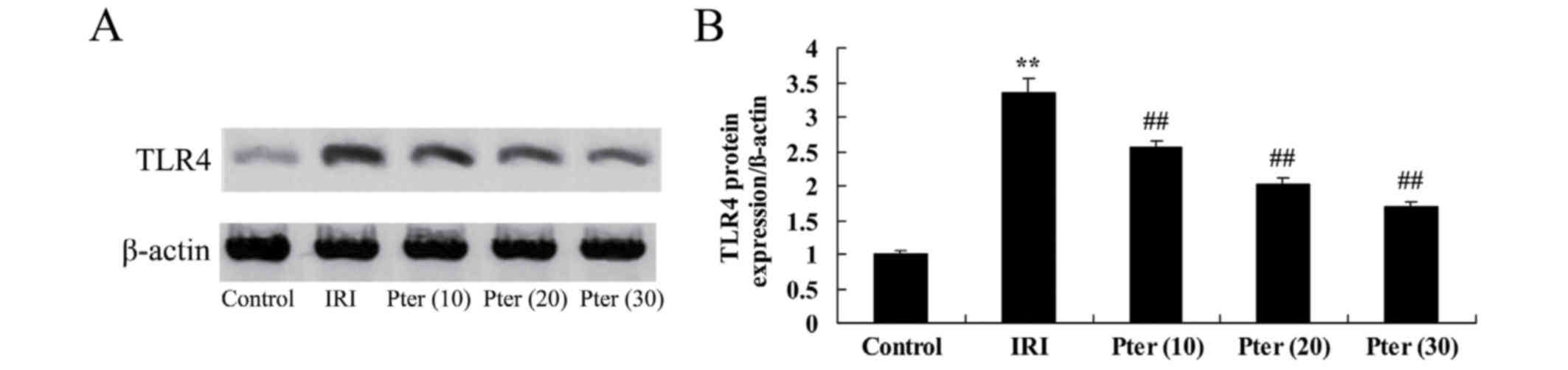
Pter protects against the IRI-induced
increase in TLR4 protein expression levels
In order to determine whether Pter influences TLR4
protein expression levels in rats following IRI, western blot
analysis was performed. As indicated in Fig. 8, TLR4 protein expression levels were
significantly increased in the IRI group when compared with the
control group (P<0.01). By contrast, Pter treatment
significantly reduced the IRI-induced increase in TLR4 protein
expression levels (P<0.01; Fig.
8).
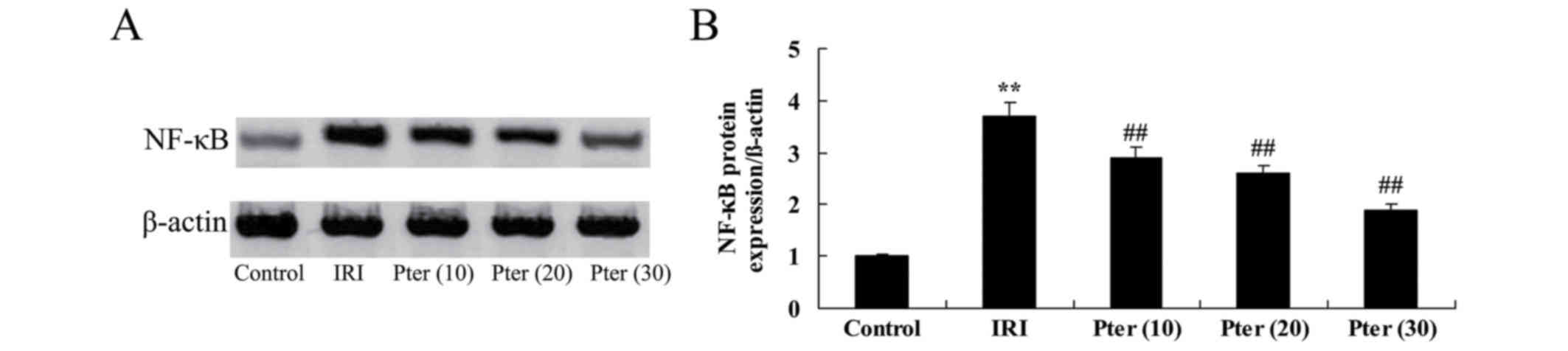
Pter protects against the IRI-induced
increase in NF-κB protein expression levels
To investigate the effects of Pter on NF-κB protein
expression levels in rats following IRI, the expression levels of
this protein were determined by western blot analysis. The results
indicated that NF-κB protein expression levels were significantly
increased in IRI model rats when compared with those in the control
group (P<0.01; Fig. 9). However,
administration of Pter significantly decreased NF-κB protein
expression levels in rats following IRI when compared with those in
the untreated IRI group (P<0.01; Fig.
9).
Discussion
A previous study investigated strategies for the
prevention of renal IRI (3).
Furthermore, alternative studies have included investigating the
protective mechanisms involved in renal endogenesis, including
pre-treatment and pre-conditioning, and have gained particular
attention (13). In addition,
previous study examining the drug-induced activation or inhibition
of various factors that protect renal tissues, have demonstrated
clinical significance to the outcome of renal transplants and
functional recovery, as well as patient survival following
transplantation (14). The aim of
the present study was to determine whether Pter significantly
inhibited IRI-induced alterations to BUN and creatinine
concentration levels, as well as histological scores in a rat model
of renal IRI.
As a typical enzyme of neutrophil granulocytes, MPO
is a dependable indicator of the level of neutrophil granulocyte
infiltration in tissues (15). In
addition, MPO activity is proportional to number of neutrophil
granulocytes, which may be used to indicate the level of
infiltration of these cells in the spinal cord (16). The quantitative results of previous
studies suggest that, among the different renal IRI mechanisms, the
participation of inflammatory cells, specifically neutrophil
granulocytes, may be important during renal IRI (16). When IRI occurs, neutrophil
granulocytes have been demonstrated to accumulate and migrate to
ischemic regions. In addition, the accumulation of blood platelets
impairs angiemphraxis. The presence of swollen vascular endothelial
cells leads to narrowing of the lumen and reduces blood flow. In
addition, increased numbers of neutrophil granulocytes may
stimulate the production of cytokines. iNOS, which is activated by
these inflammatory mediators, may subsequently produce NO, which is
involved in nerve injury and the production of oxygen radicals.
Following the adherence of hemameba and vascular endothelial cells,
the cytoskeleton may be reconstructed, which leads to an increase
in the space between endothelial cells and damages the endothelium
of the blood-brain barrier. MPO exists predominantly in the
azurophilic granules of neutrophil granulocytes. It has been
demonstrated that the expression of MPO in renal IRI tissues is
increased, which suggests that inflammation may be an important
mechanism underlying renal IRI. In the present study,
administration of Pter significantly suppressed MPO levels and iNOS
protein expression levels following renal IRI in rats. A previous
study reported that Pter attenuated inflammation via suppression of
MPO levels and the TLR4/NF-κB signaling pathway in rat hearts
following ischemia-reperfusion (17). In addition, Pan et al
(17) demonstrated that Pter
inhibited lipopolysaccharide-induced iNOS expression in murine
macrophages.
A previous study demonstrated that IL-10 exhibits
protective effects against IRI (18). In T-cells co-cultured with an
anti-CD3 monoclonal antibody and macrophages, the expression of
TNF-α and IL-6 were upregulated (19). Following stimulation of NF-κB
signaling pathways via death-1/B7-Hl, IL-10 was upregulated
(20). By employing the IL-10
monoclonal antibody for neutralization, the level of
pro-inflammatory factors, such as TNF-α and IL-6 are increased
(21). In the present study, Pter
treatment significantly suppressed the expression levels of IL-1β,
IL-6 and TNF-α, and significantly increased IL-10 expression levels
in renal IRI rats. Notably, Tsai et al (22) revealed that Pter inhibited mouse skin
carcinogenesis via inhibition of iNOS production and downregulated
the inflammatory response.
Apoptotic signaling pathways are dependent on
caspase enzymes, which serve important roles in the renal tubular
epithelium during renal IRI (20).
The caspase family of enzymes consist of key proteases involved in
cell apoptosis (20). Caspase-3 is
the predominant protease for the activation of cell apoptosis
(20). Caspase inhibitors inhibit
cell apoptosis and the inflammatory response by downregulating the
proteinase activities of caspase-1 and caspase-3 (23). In the present study, treatment with
Pter significantly inhibited the IRI-induced activation of
caspase-3 following renal IRI. Consistent with these observations,
Wang et al (24) demonstrated
that Pter attenuated inflammation via the TLR4/NF-κB signaling
pathway in ischemia-reperfusion rats.
NF-κB serves a key role in inflammation during renal
IRI in mice and NF-κB inhibition in specific T-cells may reduce IRI
when compared with NF-κB inhibition in non-specific T cells
(25). However, NF-κB exhibits a
wide range of functions, therefore, inhibition of NF-κB may not be
sufficient to prevent the damaging effects of renal IRI (26). In addition, the effects of NF-κB may
exhibit different effects in different organs. Although inhibition
of NF-κB may reduce systemic inflammation, it may exacerbate renal
IRI (26). The results of the
present study demonstrated that Pter significantly decreased NF-κB
protein expression levels in the renal tissues of rats that had
undergone renal IRI. Cichocki et al (27) reported that Pter inhibited
12-O-tetradecanoylphorbol-13-acetate-activated NF-κB and iNOS
expression in the mouse epidermis.
The TLR family consists of several members,
including TLR4, TLR2 and TLR9 (28).
The extent of renal injury in mice with TLR2, myeloid
differentiation primary response gene 88 and TLR4 deficiencies was
reduced when compared with that of wild-type mice (29). A previous study involving mice with a
deficiency in TLR4 demonstrated that the stimulation of TLR4 by
endogenous ligands serves an important role in mediating IRI
(30). When IRI occurs, TLR4
expression in renal tubular epithelial cells is upregulated, which
suggests that TLR4 may be involved in a positive feedback loop
(31). The results of the present
study suggested that Pter may significantly reduce the renal
IRI-induced increase in TLR4 protein expression levels in rats.
Similarly, Wang et al (24)
revealed that Pter attenuated inflammation in a rat model of
ischemia-reperfusion via the TLR4/NF-κB signaling pathway.
In conclusion, the results of the current study
demonstrated that Pter may protect against renal IRI in rats
potentially via inhibition of oxidative stress, iNOS expression and
inflammation. In addition, the protective effects of Pter may be
associated with inhibition of the TLR4/NF-κB signaling pathway.
Further studies are required to investigate the specific mechanisms
underlying the effects of Pter treatment during renal IRI.
Acknowledgements
The present study was supported by the Science and
Technology Development Project of Henan Province (grant no.
132300410110).
References
|
1
|
Zhang N, Cheng GY, Liu XZ and Zhang FJ:
Expression of Bcl-2 and NF-κB in brain tissue after acute renal
ischemia-reperfusion in rats. Asian Pac J Trop Med. 7:386–389.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novak KB, Le HD, Christison-Lagay ER, Nose
V, Doiron RJ, Moses MA and Puder M: Effects of metalloproteinase
inhibition in a murine model of renal ischemia-reperfusion injury.
Pediatr Res. 67:257–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ogawa T and Mimura Y: Antioxidant effect
of zinc on acute renal failure induced by ischemia-reperfusion
injury in rats. Am J Nephrol. 19:609–614. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu H, Steenstra R, de Boer EC, Zhao CY, Ma
J, van der Stelt JM and Chadban SJ: Preconditioning with
recombinant high-mobility group box 1 protein protects the kidney
against ischemia-reperfusion injury in mice. Kidney Int.
85:824–832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He W, Zhang Y, Zhang J, Yu Q, Wang P, Wang
Z and Smith AJ: Cytidine-phosphate-guanosine oligonucleotides
induce interleukin-8 production through activation of TLR9, MyD88,
NF-κB, and ERK pathways in odontoblast cells. J Endod. 38:780–785.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andreucci M, Faga T, Lucisano G, Uccello
F, Pisani A, Memoli B, Sabbatini M, Fuiano G and Michael A:
Mycophenolic acid inhibits the phosphorylation of NF-kappaB and
JNKs and causes a decrease in IL-8 release in H2O2-treated human
renal proximal tubular cells. Chem Biol Interact. 185:253–262.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Qiang X, Li Y, Yang X, Luo L, Xiao
G, Cao Z, Tan Z and Deng Y:
Pterostilbene-O-acetamidoalkylbenzylamines derivatives as novel
dual inhibitors of cholinesterase with anti-β-amyloid aggregation
and antioxidant properties for the treatment of Alzheimer's
disease. Bioorg Med Chem Lett. 26:2035–2039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McCormack D and McFadden D: A review of
pterostilbene antioxidant activity and disease modification. Oxid
Med Cell Longev. 2013:5754822013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McCormack D and McFadden D: Pterostilbene
and cancer: Current review. J Surg Res. 173:e53–e61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang J, Rimando A, Pallas M, Camins A,
Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA and
Casadesus G: Low-dose pterostilbene, but not resveratrol, is a
potent neuromodulator in aging and Alzheimer's disease. Neurobiol
Aging. 33:2062–2071. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pari L and Satheesh MA: Effect of
pterostilbene on hepatic key enzymes of glucose metabolism in
streptozotocin- and nicotinamide-induced diabetic rats. Life Sci.
79:641–645. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YY, Zhai W, Yang X, Ding J and Kan L:
Effects of Panax notoginseng saponins on proliferation, apoptosis
and cell cycle of K562 cells in vitro and the mechanisms. Nan Fang
Yi Ke Da Xue Xue Bao. 35:1103–1109. 2015.(In Chinese). PubMed/NCBI
|
|
13
|
Hilmi IA, Peng Z, Planinsic RM, Damian D,
Dai F, Tyurina YY, Kagan VE and Kellum JA: N-acetylcysteine does
not prevent hepatorenal ischaemia-reperfusion injury in patients
undergoing orthotopic liver transplantation. Nephrol Dial
Transplant. 25:2328–2333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freitas MC, Uchida Y, Lassman C, Danovitch
GM, Busuttil RW and Kupiec-Weglinski JW: Type I interferon pathway
mediates renal ischemia/reperfusion injury. Transplantation.
92:131–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asaga T, Ueki M, Chujo K and Taie S:
JTE-607, an inflammatory cytokine synthesis inhibitor,
attenuatesischemia/reperfusion-induced renal injury by reducing
neutrophil activation in rats. J Biosci Bioeng. 106:22–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lafci G, Gedik HS, Korkmaz K, Erdem H,
Cicek OF, Nacar OA, Yildirim L, Kaya E and Ankarali H: Efficacy of
iloprost and montelukast combination on spinal cord
ischemia/reperfusion injury in a rat model. J Cardiothorac Surg.
8:642013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan MH, Chang YH, Tsai ML, Lai CS, Ho SY,
Badmaev V and Ho CT: Pterostilbene suppressed
lipopolysaccharide-induced up-expression of iNOS and COX-2 in
murine macrophages. J Agric Food Chem. 56:7502–7509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan X, Huang WJ, Chen W, Xie HG, Wei P,
Chen X and Cao CC: IL-10 deficiency increases renal
ischemia-reperfusion injury. Nephron Exp Nephrol. 128:37–45. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang F, Lu M, Wang H and Ren T: Aspirin
attenuates angiotensin II-induced inflammation in bone marrow
mesenchymal stem cells via the inhibition of ERK1/2 and NF-κB
activation. Biomed Rep. 1:930–934. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsushima F, Tanaka K, Otsuki N, Youngnak
P, Iwai H, Omura K and Azuma M: Predominant expression of B7-H1 and
its immunoregulatory roles in oral squamous cell carcinoma. Oral
Oncol. 42:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung M, Sola A, Hughes J, Kluth DC,
Vinuesa E, Viñas JL, Pérez-Ladaga A and Hotter G: Infusion of
IL-10-expressing cells protects against renal ischemia through
induction of lipocalin-2. Kidney Int. 81:969–982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai ML, Lai CS, Chang YH, Chen WJ, Ho CT
and Pan MH: Pterostilbene, a natural analogue of resveratrol,
potently inhibits 7,12-dimethylbenz[a]anthracene
(DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse
skin carcinogenesis. Food Funct. 3:1185–1194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang BC and Ma J: Role of microRNAs in
malignant glioma. Chin Med J (Engl). 128:1238–1244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang C, Sun H, Song Y, Ma Z, Zhang G, Gu X
and Zhao L: Pterostilbene attenuates inflammation in rat heart
subjected to ischemia-reperfusion: Role of TLR4/NF-κB signaling
pathway. Int J Clin Exp Med. 8:1737–1746. 2015.PubMed/NCBI
|
|
25
|
Zhang J, Xia J, Zhang Y, Xiao F, Wang J,
Gao H, Liu Y, Rong S, Yao Y, Xu G and Li J: HMGB1-TLR4 signaling
participates in renal ischemia reperfusion injury and could be
attenuated by dexamethasone-mediated inhibition of the ERK/NF-κB
pathway. Am J Transl Res. 8:4054–4067. 2016.PubMed/NCBI
|
|
26
|
O'Neill S, Humphries D, Tse G, Marson LP,
Dhaliwal K, Hughes J, Ross JA, Wigmore SJ and Harrison EM: Heat
shock protein 90 inhibition abrogates TLR4-mediated NF-κB activity
and reduces renal ischemia-reperfusion injury. Sci Rep.
5:129582015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cichocki M, Paluszczak J, Szaefer H,
Piechowiak A, Rimando AM and Baer-Dubowska W: Pterostilbene is
equally potent as resveratrol in inhibiting
12-O-tetradecanoylphorbol-13-acetate activated NFkappaB, AP-1,
COX-2, and iNOS in mouse epidermis. Mol Nutr Food Res. 52 Suppl
1:S62–S70. 2008.PubMed/NCBI
|
|
28
|
Mankan AK, Lawless MW, Gray SG, Kelleher D
and McManus R: NF-kappaB regulation: The nuclear response. J Cell
Mol Med. 13:631–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang D, Sun Y, Shen Y, Li F, Song X, Zhou
E, Zhao F, Liu Z, Fu Y, Guo M, et al: Shikonin exerts
anti-inflammatory effects in a murine model of
lipopolysaccharide-induced acute lung injury by inhibiting the
nuclear factor-kappaB signaling pathway. Int Immunopharmacol.
16:475–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu D, Niu W, Luo Y, Zhang B, Liu M, Dong
H, Liu Y and Li Z: Endogenous estrogen attenuates hypoxia-induced
pulmonary hypertension by inhibiting pulmonary arterial
vasoconstriction and pulmonary arterial smooth muscle cells
proliferation. Int J Med Sci. 10:771–781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
D'Hooghe TM and Debrock S: Endometriosis,
retrograde menstruation and peritoneal inflammation in women and in
baboons. Hum Reprod Update. 8:84–88. 2002. View Article : Google Scholar : PubMed/NCBI
|