Introduction
Benign prostatic hyperplasia (BPH) is caused by
non-malignant hyperplasia of the prostate. The prevalence of lower
urinary tract symptoms due to BPH increases with increasing age.
Moderate to severe symptoms occur in 40–80% of males 60–80 years.
Nearly all males develop microscopic BPH by the age of 90 years
(1,2). 5α-reductase inhibitors (5ARI) are able
to reduce the concentration of dihydrotestosterone (DHT) in
prostate tissue and thus shrink the prostate (3). Previous studies have revealed that
normal prostatic epithelial cells responded to hormone deprivation
by undergoing apoptosis (4–6). Over the course of treatment with
Finasteride, a 5α-reductase inhibitors, serum PSA level is reduced
by 50%, and over time total prostate volume decreases by 15–25% due
to apoptosis and shrinkage of the glandular epithelial compartment
in the transition and peripheral zones of the prostate (7). However, the molecular mechanism of
apoptosis in prostate epithelial cells in the process of androgen
deprivation (AD) remains unclear.
Macroautophagy (hereafter, autophagy) is an
evolutionarily conserved catabolic process, which is essential in
homeostasis and the stress-response as well as in macromolecular
turnover and development (8). The
association between apoptosis and autophagy is complex. In a number
of scenarios, apoptosis and autophagy are able induce cell death in
a coordinated or cooperative manner (9). In other cell types and the types of
stress, autophagy acts as an antagonist to block apoptotic cell
death by promoting cell survival (10). For example, pre-treatment of cells
with rapamycin, which induces autophagy and causes a decrease in
the mitochondrial mass by ~50% while reducing the susceptibility of
cells to MOMP-dependent apoptotic stimuli (11). In any given death scenario, the fate
of the cell is dependent on cellular settings, the nature of the
stimulus and the particulars of the cell growth microenvironment
(12). Li et al (13) previously reported that autophagy and
apoptosis perform in a mutually exclusive manner in LNCaP prostate
adenocarcinoma cells under androgen-deprivation conditions. They
also reported that the level of autophagy markedly increased, and
that the inhibition of autophagy by 3-methyladenine significantly
promoted the rate of apoptosis in PWR-1E cells under the conditions
of AD. Additionally, in vivo, autophagy was demonstrated to
significantly increase in hyperplasia of prostate tissue following
5-ARI treatment (14). To the best
of our knowledge, no study is currently available on the molecular
mechanism of apoptosis and autophagy of prostate epithelial cells
undergoing AD.
Beclin-1, which is the mammalian orthologue of yeast
Atg6, has a central role in autophagy (12). Wirawan et al (15) previously revealed that Beclin-1 was
cleaved by caspase in a cellular model system in which the
withdrawal of the growth factor interleukin (IL-3) induced
apoptotic cell death that was preceded by a phase of increased
autophagy. Additionally, cleavage of Beclin-1 by caspase produced
Beclin1-C, which was translocated to the mitochondria and amplified
apoptosis induced by IL-3 deprivation. This indicates that the
cleavage of Beclin-1 may contribute an amplifying loop for the
enhancement of apoptosis (16). The
present study proposes that the process of Beclin-1 protein
cleavage may serve a role in the apoptosis of benign epithelial
cells under AD, and explores whether the cleavage of the Beclin-1
protein leads to the activation of cell apoptosis. The present
study also evaluated whether the combination of AD and autophagy
inhibition (AI) had an additive or a synergistic effect on the
enhancement of apoptosis.
Materials and methods
Chemicals and antibodies
DHT and chloroquine (CQ) were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Antibodies against
microtubule-associated proteins 1A/1B light chain 3 (LC3) (cat no.
D3U4C), poly (ADP-ribose) polymerase 1 (PARP-1) (cat no. 46DH),
caspase-3 (cat no. 8G10) and GAPDH (cat no. 14C10) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA), and
antibodies against Beclin-1 were from BD Biosciences (cat no.
612112; Franklin Lakes, NJ, USA). Horseradish peroxidase-labeled
goat anti-rabbit secondary antibodies (cat no. GAR007) and anti-rat
secondary antibodies (cat no. GAM007) were purchased from
MultiSciences Biotech Co., Ltd (Hangzhou, China). The Annexin
V-fluorescein isothiocyanate/propidium iodide (FITC/PI) apoptosis
kit was purchased from Dojindo Molecular Technologies, Inc.
(Kumamoto, Japan).
Cell culture and treatments
BPH-1 cells (Sangon Biotech Co., Ltd., Shanghai,
China), an immortalized but non-transformed human prostate
epithelial cell line, which was immortalized by infection with the
ZipneoSV virus carrying the SV40 T antigen gene (17), were provided by the Department of
Urology of the Union Hospital of Fujian Medical University. The
cells were maintained at 37°C in RPMI 1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) that contained 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Prior to the
corresponding treatment, BPH-1 cells were washed twice with PBS to
eliminate the remnant serum. It has previously been demonstrated
that the total testosterone of a healthy adult human male was 10–35
nM (18). Li et al (13,14) have
previously reported that DHT treatment reduced the level of
autophagy, with the highest inhibition observed at 10 nM. BPH-1
cells that were cultured in RPMI 1640 medium with 10% fetal bovine
serum and 10 nM DHT at 37°C for 24 h served as the control group
(Cont). Cells cultured in phenol red-free RPMI 1640 medium with 10%
charcoal-stripped fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) served as the AD group. Cells cultured in RPMI
1640 medium with 10% fetal bovine serum, 1×10−8 mmol/l
DHT and 5×10−5 mmol/l CQ served as the AI group.
Finally, cells cultured in phenol red-free RPMI 1640 medium with
10% charcoal-stripped fetal bovine serum and 5×10−5
mmol/l CQ served as the AD + AI group. The cells in each group were
cultured for 24 h at 37°C.
Western blot analysis
Cells were cultured at 37°C in phenol red-free RPMI
1640 medium that contained 10% charcoal-stripped fetal bovine
serum, we defined the time for the replacement of the cell culture
medium as 0 h, and the total protein was extracted using a
radioimmunoprecipitation buffer (25 mM TrisNHCl pH 8, 150 mM NaCl,
1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) and protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA) every 4 h for a
total of 48 h. A BCA protein assay kit was then used as a protein
determination method (Thermo Fisher Scientific, Inc). Protein 10 µl
(1,500 µg/ml) per lane from each sample was then subjected to
electrophoresis on an 8% SDS-PAGE for PARP-1 detection, a 10%
SDS-PAGE for caspase-3 and Beclin-1 detection and a 15% SDS-PAGE
for LC3 detection, and transferred onto polyvinylidene fluoride
membranes. Following blocking with 5% nonfat dry milk for 90 min at
room temperature, membranes were incubated with primary antibodies
overnight at 4°C. The primary antibodies used were as follows:
PARP-1 polyclonal rabbit primary antibody (1:1,000); Caspase-3
primary antibody (1:1,000); LC-3 polyclonal rabbit primary antibody
(1:1,000; all Cell Signaling Technology); Beclin-1 polyclonal rat
primary antibody (1:2,000; BD Biosciences); and GAPDH polyclonal
rabbit primary antibody (1:2,000; both BD Biosciences) as a
reference protein. Membranes were then incubated with corresponding
horseradish peroxidase-conjugated secondary antibodies at 1:5,000
dilutions for 2 h at room temperature. Protein bands were
visualized on X-ray film using an enhanced chemiluminescence system
(Advansta, CA, USA). LC3-I and LC3-II are 18 and 16 kDa
respectively (19). The 35- and
37-kDa Beclin-1 fragments generated during apoptosis are C-terminal
Beclin-1 fragments resulting from caspase-mediated cleavage.
Densitometry analysis was performed using ImageJ v1.45 software
(National Institutes of Health, Bethesda, MD, USA).
Flow cytometry for the measurement of
the apoptosis rate of each group
Cells were seeded at 1×106 cells in 2.5
ml RPMI 1640 medium containing 10% FBS per well in 6-well plates
for 24 h at 37°C prior to exposure to the corresponding treatment.
Following incubation, the cells in each group were exposed to the
corresponding treatment for an additional 24 h, and the supernatant
medium from each well was transferred in a separate pre-labeled
centrifuge tube to collect non-adherent cells. Adherent cells from
the same well were then trypsinized and transferred to the same
centrifuge tube containing the non-adherent cells. Cells in each
centrifuge tube were then centrifuged at 2,000 × g at 4°C for 5
min, and the supernatant was discarded. The cells were re-suspended
in 100 µl Annexin V binding buffer (Dojindo Molecular Technologies,
Inc.). In total, 5 µl FITC-Annexin V and 5 µl PI (both from Dojindo
Molecular Technologies, Inc.) were added and the cells were
incubated at room temperature for 15 min in the dark. Subsequently,
400 µl Annexin V binding buffer was added and flow cytometry was
performed on a Beckman flow cytometer (Beckman Coulter, Inc., Brea,
CA, USA). Cells were considered to be apoptotic if they were
Annexin V+/PI− (early apoptotic) and Annexin
V+/PI− (late apoptotic). At least 10,000
events were recorded and the data were evaluated using Kaluza 1.3
software (Beckman Coulter, Inc.) for each analysis.
Statistical analysis
All experiments were repeated three times and
similar results were obtained. All data of normal distribution are
presented as the mean ± standard deviation and were analyzed by
one-way analysis of variance (ANOVA) or two-way ANOVA factorial
design. The data of a non-normal distribution were analyzed by the
Kruskal-Wallis H and the Wilcoxon rank-sum tests. When statistical
significance was identified, the differences between groups were
further analyzed by the Nemenyi test. P<0.05 was considered to
indicate a statistically significant difference. SPSS 11.5 (SPSS,
Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
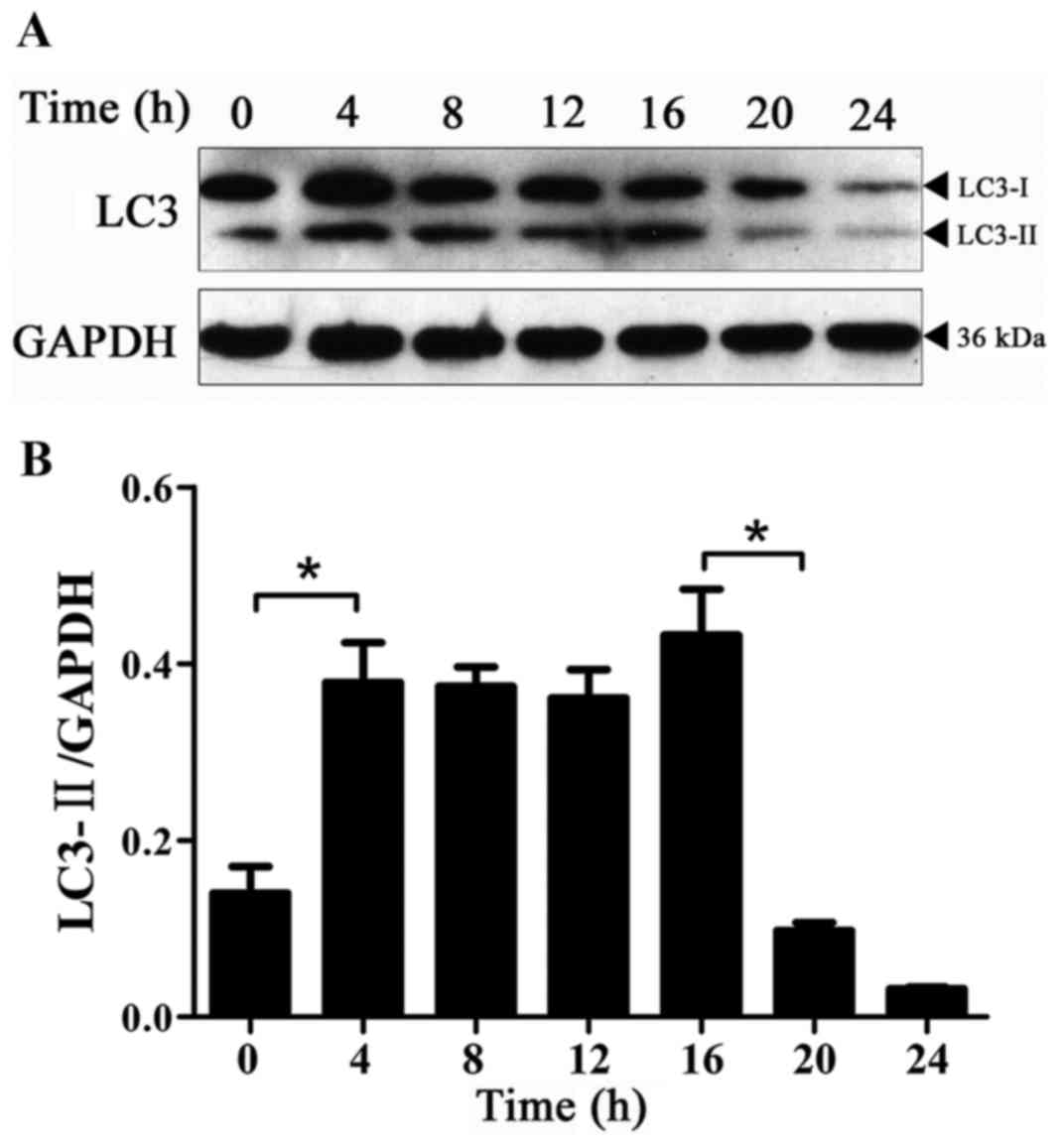
Expression of LC3-II protein with time
in BPH-1 cells under AD
Autophagy in BPH-1 cells under AD was monitored
using the levels of LC3-II compared with GAPDH protein as an
autophagy-specific marker and not to that of LC3-I (20). AD was associated with increased
autophagy during the first 4 h as evidenced by significantly
increased LC3-II levels (Fig. 1).
Consistent with a previous report (21), the basal levels of LC3-II could be
detected even in the presence of DHT (0-h time point in Fig. 1A), which reflects the regulation of
cellular homeostasis by autophagy. The expression of the LC3-II
protein was significantly decreased at 20 compared with 16 h
(Fig. 1), and this low level was
maintained at 24 h. These results indicated that there was a basal
level of autophagy activation at 0 h; autophagy was significantly
activated at 4–16 h and the level of autophagy significantly
decreased from the beginning of the 20-h time point.
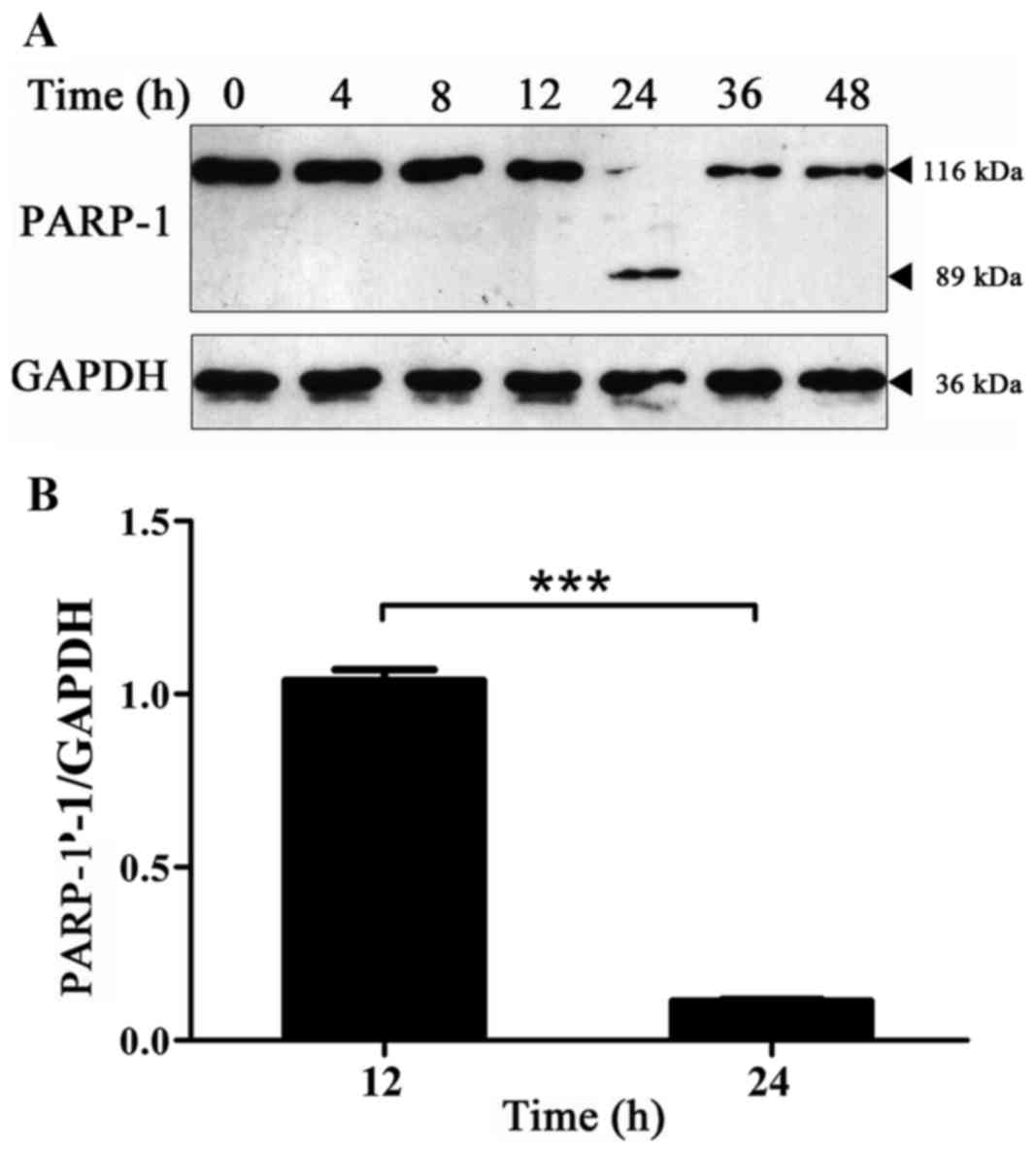
Expression of PARP-1 protein changes
with time in BPH-1 cells under AD
Protein synthesis and tissue degradation decreased
in prostate epithelial cells under AD. Additionally, AD was able to
activate cell apoptosis-associated genes, which increase the level
of apoptosis of prostate epithelial cells (22). The expression level of the PARP-1
protein in BPH-1 cells that were cultured in an androgen-deprived
medium for 0, 4, 8, 12, 24, 36 and 48 h was detected by western
blot analysis, which was used to detect the level of apoptosis of
BPH-1 cells under AD. The hydrolysis fragments of PARP-1 protein
were captured at 24 h and the expression level of PARP-1 protein
was almost undetectable at other time points (Fig. 2A). The cause of this phenomenon is
not clear, it is speculated that it may be that the transformation
of the PARP-1 protein is rapid in this cell type and the type of
stress. Thus, the hydrolysis fragments of PARP-1 protein could be
detected only when the hydrolysis of PARP-1 was at a high enough
level. Therefore, PARP-1 protein fragments were not used for
quantitative analysis, but the expression of PARP-1 protein is
presented. The results revealed that compared with 12 h, the
expression level of the PARP-1 protein was significantly decreased
at 24 h (Fig. 2). These results
indicate that there was a significant increase in cell apoptosis at
24 h.
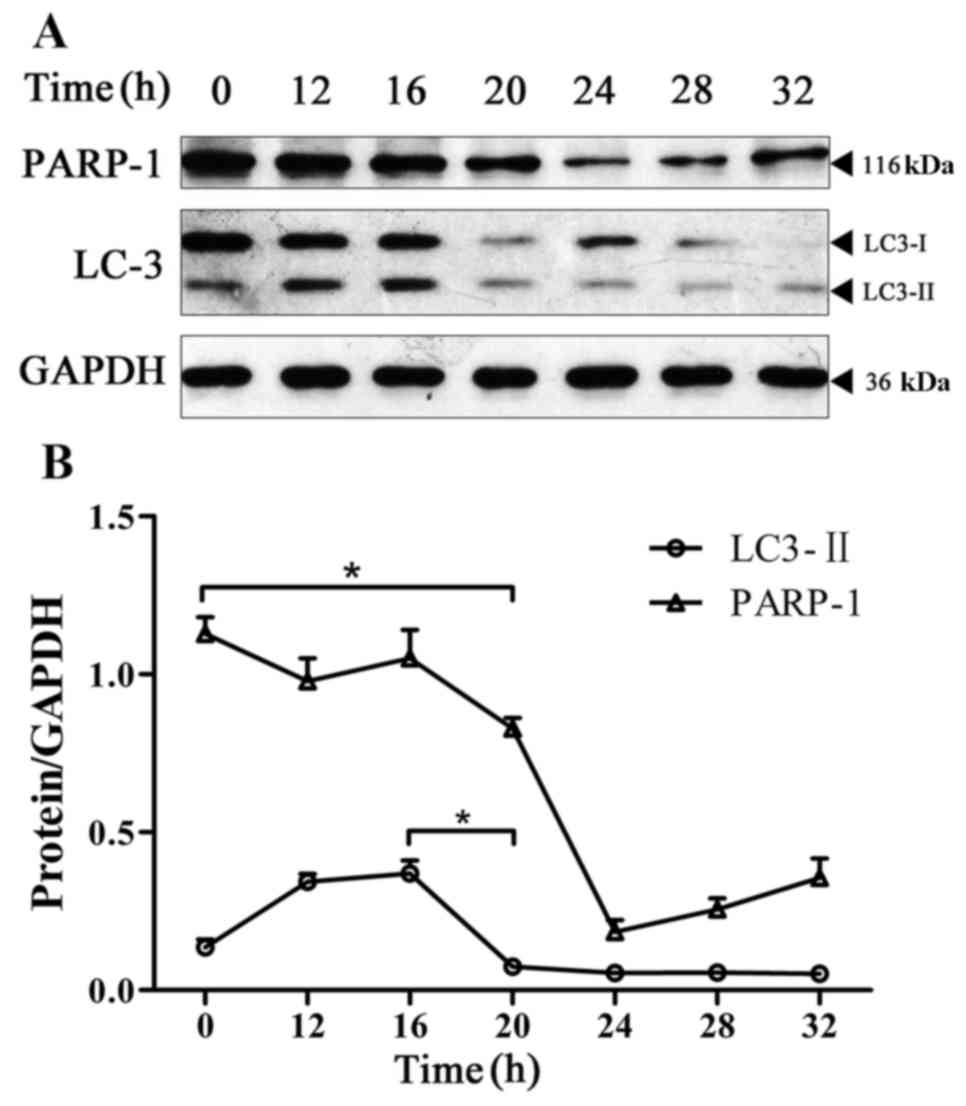
Change in the expression level of
LC3-II and PARP-1 in BPH-1 cells under AD
In the absence of nutrients or cytokines, cell
autophagy typically serves a role in cell protection. The
protective effect of autophagy on cells is limited, and the main
manifestations of apoptosis and autophagy are considered to be
mutually antagonistic (10). The
present study monitored the changes in apoptosis and autophagy in
BPH-1 cells under AD by detecting the expression levels of LC3-II
and PARP-1 in the BPH-1 cells that were cultured in an
androgen-deprived medium for 0, 12, 16, 20, 24, 28 and 32 h. The
results revealed that the expression of LC3-II was significantly
decreased at 20 h compared with its expression at 16 h. However,
the expression level of PARP-1 was significantly decreased starting
at the 20-h time point (Fig. 3).
These results indicate that under the conditions of AD, the
activation of autophagy occurs prior to apoptosis in BPH-1 cells,
and the activation of apoptosis occurs when the level of autophagy
has decreased.
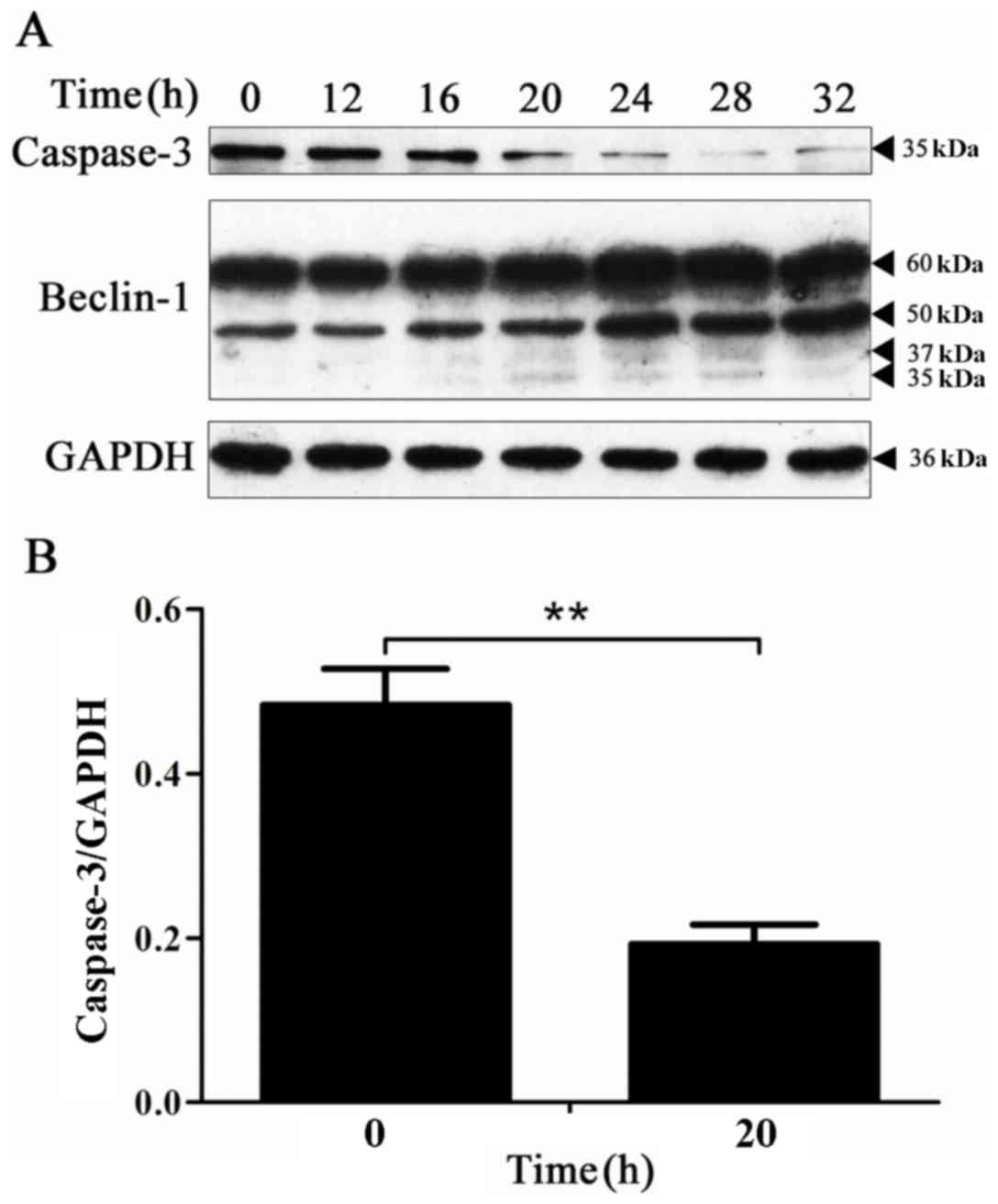
Caspase-3 protein activation is
accompanied by the hydrolysis of the Beclin-1 protein in the
process of apoptosis
To determine the expression of the caspase-3 protein
and the hydrolysis of the Beclin-1 protein over time, the
expression of the caspase-3 and Beclin-1 proteins at the
corresponding time points was detected by western blot analysis.
The results revealed that the expression level of the caspase-3
protein was markedly decreased at 20 h, and the 35 and 37-kDa
hydrolysis fragments of the Beclin-1 protein appeared at the
beginning of the 20-h time point (Fig.
4). These results indicate that in the process of BPH-1 cell
apoptosis, the Beclin-1 protein is hydrolyzed.
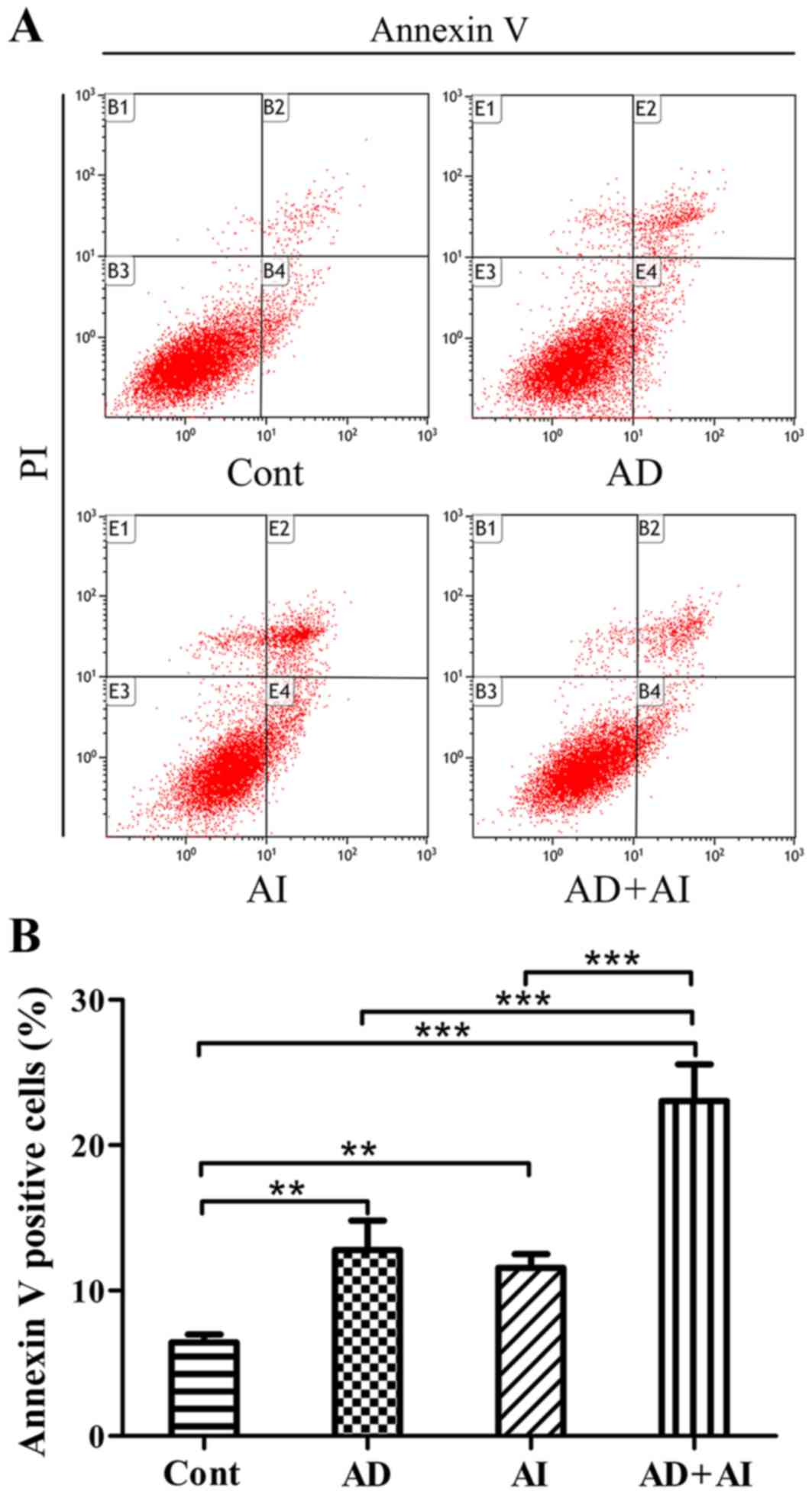
Effect of AD, AI and AD + AI on the
level of apoptosis of prostate epithelial cells
The apoptosis rate of each group was monitored using
flow cytometry. The results revealed that compared with the Cont
group, the apoptosis level of the cells in the AD, the AI and the
AD + AI groups were significantly increased (Fig. 5). Furthermore, compared with the AD
or AI groups, the apoptosis rate of the BPH-1 cells was
significantly increased in the AD + AI group (Fig. 5). The analysis of the two-way ANOVA
factorial design indicated that AD and the inhibition of autophagy
may have a synergistic effect. These results indicate that AD or
inhibition of autophagy significantly increase the level of
apoptosis of BPH-1 cells. Additionally, AD and the inhibition of
autophagy may produce synergistic effects and further increase the
rate of apoptosis in BPH-1 cells.
Discussion
When cells suffer extracellular or intracellular
stress, including growth factor deprivation, starvation,
endoplasmic reticulum stress and pathogen infection, autophagy is
always upregulated to serve a role in cytoprotection (23). Androgens serve a critical role in
prostate development, growth and pathogenesis. The use of 5ARIs and
castration are effective therapies for BPH and prostate cancer,
respectively (3). To investigate the
variation of autophagy in human prostate epithelial cells under AD,
a human prostate epithelial cell line, BPH-1, was used, which was
cultured in a medium in which the androgens had been removed. The
autophagy of BPH-1 cells was monitored at different time points
using the processing of cytosolic microtubule-associated protein
LC3-I into LC3-II as an autophagy-specific marker (8). The present results demonstrated that
autophagy is evidently activated in the early stages of androgen
removal, which implies that autophagy is upregulated in response to
AD. The results of flow cytometry demonstrated that inhibition of
autophagy may further improve the level of apoptosis of BPH-1 cells
under AD. These results indicate that autophagy functions as a
survival mechanism under AD.
In clinical practice 5-ARI is often used to
maximally induce prostate cell apoptosis by blocking the alteration
of testosterone to DHT for the treatment of BPH and prostate cancer
(3). The association between
apoptosis and autophagy is complex in that, depending on the cell
type and stimulus, different sensitivity thresholds may dictate
whether autophagy or apoptosis may develop; they customarily
exhibit some degree of mutual inhibition (12). Initially, the significant activation
of apoptosis was confirmed to occur at 24 h in BPH-1 cells under
AD. Subsequently, the combined apoptotic and autophagic results of
the present study demonstrated that once apoptosis was initiated in
BPH-1 cells, the LC3-II levels were markedly diminished starting at
20 h. These results further demonstrate that autophagy serves a
cell-protective function by mutual inhibition with apoptosis in
BPH-1 cells in androgen-deficient conditions.
Li et al (13,14)
previously reported that the blockage of autophagy by
3-methyladenine led to increased apoptosis of LNCaP or PWR-1E cells
in serum-free medium compared with the medium with DHT. However,
the interactive mechanism between cell apoptosis and autophagy
remains unclear in this process. Wirawan et al (15) previously reported that the withdrawal
of the obligatory growth factor IL-3 from Ba/F3 cells, which are
dependent on IL-3 for growth, induced apoptotic cell death that was
preceded by a phase of increased autophagy. The authors identified
that Beclin-1, a component of the autophagy-inducing complex, was a
direct substrate of the caspases and that cleavage of Beclin-1 was
observed in response to the mitochondrial pathways of apoptosis.
Based on these findings, it was hypothesized that the cleavage of
Beclin-1 sensitizes cells to apoptotic signals, inhibits cell
autophagy and can form an amplifying loop for inducing massive
apoptotic cell death (16). The
present study investigated whether there was a similar occurrence
in BPH-1 cells. As expected, cleavage of Beclin-1 coincided with
caspase-3 maturation starting at 20 h. The previous experimental
results confirmed that the level of apoptosis was increased and
that the autophagy level was decreased starting at 20 h, which
indicated a similar molecular mechanism present in BPH-1 cells
under AD.
Clinically, 5-ARI has been known to reduce the
volume of the prostate; however, the reduced volume is only ~20%,
which reduces the long-term risk of the need for surgical therapy
by ~55% (24). The treatment options
for BPH include medical therapy and surgery. Surgical therapy has
certain risks and numerous complications; therefore, it is
important to improve the therapeutic effect of drugs to delay the
progression of the disease and reduce the risk of surgery (25). Inhibition of autophagy may be a novel
therapeutic strategy. According to a report of testosterone levels
in the serum from adult human males the normal range is 10–35 nM
(18). In the current study, BPH-1
cells were cultured in medium containing 1×10−8 mmol/l
DHT as the control group. The results of flow cytometry revealed
that compared with the normal Cont group, the apoptosis level of
the cells in the AD and AI groups were significantly increased. The
results indicated that AD was able to induce apoptosis, and the
inhibition of autophagy could induce apoptosis in prostate
epithelial cells. Notably, the rate of apoptosis of prostate
epithelial cells induced by AD or AI was similar. Additionally, the
experimental data derived from flow cytometry revealed that the
cells that are subject to AD and AI are in accordance with the
two-way ANOVA factorial design. Furthermore, the data analysis
results indicated that AD and the inhibition of autophagy could
exhibit a synergistic effect.
Such results indicate that the combination of AD and
AI was able to induce apoptosis of prostate epithelial cells more
effectively and are most likely associated with the amplifying loop
mechanism derived from the caspase-mediated cleavage fragments of
Beclin-1. These results contribute to the further study for the
application of autophagy in the treatment of BPH. The present study
focuses on the BPH-1 cell line only and therefore, further
validation is required in other prostate epithelial and stromal
cells, and within studies using animals.
Acknowledgements
The present study was sponsored by Medical Elite
Cultivation Program of Fujian, P.R.C (no. 2014-ZQN-ZD-33).
References
|
1
|
Dhingra N and Bhagwat D: Benign prostatic
hyperplasia: An overview of existing treatment. Indian J Pharmacol.
43:6–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roehrborn CG and McConnell J: Benign
prostatic hyperplasia: Etiology, pathophysiology, epidemiology and
natural historyCampbell's Urology. Walsh PC, Retik AB, Vaughan ED
Jr and Wein AJ: 8th edition. WB Saunders Co; Philadelphia, PA: pp.
1297–1336. 2002
|
|
3
|
Moore A, Butcher MJ and Köhler TS:
Testosterone replacement therapy on the natural history of prostate
disease. Curr Urol Rep. 16:512015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silva IS, Morsch DM, Urnauer L and
Spritzer PM: Androgen-induced cell growth and c-myc expression in
human non-transformed epithelial prostatic cells in primary
culture. Endocr Res. 27:153–169. 2009. View Article : Google Scholar
|
|
5
|
Furuya Y and Isaacs JT: Differential gene
regulation during programmed death (apoptosis) versus proliferation
of prostatic glandular cells induced by androgen manipulation.
Endocrinology. 133:2660–2666. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raffo AJ, Perlman H, Chen MW, Day ML,
Streitman JS and Buttyan R: Overexpression of bcl-2 protects
prostate cancer cells from apoptosis in vitro and confers
resistance to androgen depletion in vivo. Cancer Res. 55:4438–4445.
1995.PubMed/NCBI
|
|
7
|
Roehrborn CG: Benign prostatic
hyperplasia: An overview. Rev Urol. 7 Suppl 9:S3–S14.
2005.PubMed/NCBI
|
|
8
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fimia GM and Piacentini M: Regulation of
autophagy in mammals and its interplay with apoptosis. Cell Mol
Life Sci. 67:1581–1588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ravikumar B, Berger Z, Vacher C, O'Kane CJ
and Rubinsztein DC: Rapamycin pre-treatment protects against
apoptosis. Hum Mol Genet. 15:1209–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Jiang X, Liu D, Na Y, Gao GF and Xi
Z: Autophagy protects LNCaP cells under androgen deprivation
conditions. Autophagy. 4:54–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Yang X, Wang H, Xu E and Xi Z:
Inhibition of androgen induces autophagy in benign prostate
epithelial cells. Int J Urol. 21:195–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wirawan E, Vande Walle L, Kersse K,
Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R,
Verspurten J, Declercq W, et al: Caspase-mediated cleavage of
Beclin-1 inactivates Beclin-1-induced autophagy and enhances
apoptosis by promoting the release of proapoptotic factors from
mitochondria. Cell Death Dis. 1:e182010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Djavaheri-Mergny M, Maiuri MC and Kroemer
G: Cross talk between apoptosis and autophagy by caspase-mediated
cleavage of Beclin 1. Oncogene. 29:1717–1719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayward SW, Dahiya R, Cunha GR, Bartek J,
Deshpande N and Narayan P: Establishment and characterization of an
immortalized but non-transformed human prostate epithelial cell
line: BPH-1. In Vitro Cell Dev Biol Anim. 31:14–24. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sedelaar JP and Isaacs JT: Tissue culture
media supplemented with 10% fetal calf serum contains a castrate
level of testosterone. Prostate. 69:1724–1729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altman BJ, Wofford JA, Zhao Y, Coloff JL,
Ferguson EC, Wieman HL, Day AE, Ilkayeva O and Rathmell JC:
Autophagy provides nutrients but can lead to chop-dependent
induction of bim to sensitize growth factor-deprived cells to
apoptosis. Mol Biol Cell. 20:1180–1191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Isaacs JT: Antagonistic effect of androgen
on prostatic cell death. Prostate. 5:545–557. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nickel JC, Gilling P, Tammela TL, Morrill
B, Wilson TH and Rittmaster RS: Comparison of dutasteride and
finasteride for treating benign prostatic hyperplasia: The Enlarged
Prostate International Comparator Study (EPICS). BJU Int.
108:388–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McVary KT, Roehrborn CG, Avins AL, Barry
MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan
SA, Penson DF, et al: Update on AUA guideline on the management of
benign prostatic hyperplasia. J Urol. 185:1793–1803. 2011.
View Article : Google Scholar : PubMed/NCBI
|