Introduction
In clinical practice, tooth loss is often caused by
trauma, infection and periodontal disease amongst other reasons.
The lack of stimulation of the surrounding ligament may lead to
physiological bone resorption and, as a result, alveolar ridge
height and width may be significantly reduced (1). In the physiological process of alveolar
bone reconstruction, the bone width has been demonstrated to be
decreased by 2.6–4.6 mm, and the bone height reduced by 0.4–3.9 mm
(2). According to a previous animal
study, the absorption of labial alveolar bone was greater than that
on the lingual side (3). In
addition, the loss of alveolar bone is extremely unfavorable for
dental restoration. An adequate quantity of bone is required as a
basis for repair, not only for the fixed denture but also for the
recoverable denture. Alveolar ridge preservation technology came
into being to save more bone following tooth removal (4). Furthermore, it has previously been
confirmed that alveolar ridge preservation technology may slow the
alveolar bone resorption process and reduce the absorption of
alveolar bone height and width (5).
Presently, the typically used method for alveolar
ridge preservation is to fill the alveolar fossa (6,7). The
goal of the present study was to maintain the alveolar ridge
height, width, and shape through the induction of osteogenesis.
Previous research has been conducted on alveolar fossa materials
such as granular or powdery hydroxyapatite (8), sponge-like polyethyl
ester/polypropylene (9), bioactive
glass (10) and injectable calcium
phosphate (11), amongst others.
Most of these biological materials are derived from heterogeneous
organisms, resulting in the increased risk of tooth socket
infection and economic burden for patients. It has also been
demonstrated that many graft materials have not been completely
degraded many years following implantation, and only slightly
promote osteogenic induction (5),
which also directly affects the formation of new bone and soft
tissue healing in the tooth extraction sockets. Calcium phosphate
cement and a variety of growth factors, including recombinant human
bone morphogenetic protein-2 (rhBMP-2) and basic fibroblast growth
factor (bFGF), have also been adopted to promote bone formation
(12–14). Although these growth factors showed
certain osteogenic ability, their relatively high price increased
the cost of this potential treatment (14). Finding a more safe and inexpensive
biological material for alveolar ridge preservation is a common
goal in current research.
Platelet-rich fibrin (PRF) is the second generation
of platelet concentrates. Compared with the first generation of
platelet-rich plasma (PRP), the preparation process is simple and
does not require any thrombin or coagulant (15). PRF is harvested from venous blood and
does not lead to immune rejection in clinical application (15,16).
PRF has a three-dimensional network structure with
is both flexible and durable (16).
PRF is rich in fibrin, platelets, white blood cells, growth
factors, cytokines, and other components conducive to tissue repair
(16–18). These cytokines include interleukin-1,
−4 and −6, and other growth factors including platelet-derived
growth factor, epithelial growth factor and vascular endothelial
growth factor (17,18). These components can be effective in
regulating the proliferation, differentiation and apoptosis of
repair-related cells, and subsequently regulating and promoting
tissue repair (19). Leukocytes are
able to release a large number of immune regulation-related
cytokines in the process of fibrinolysis, and this process is
persistent and progressive (20,21).
These cytokines may effectively reduce local inflammatory responses
effectively (22). The fibrous web
in the PRF, along with the large number of platelets and growth
factors, may also promote wound healing and accelerate bone
regeneration (23). In view of the
aforementioned organizational characteristics and biological
characteristics, PRF is safe, effective and more economical as a
transplant material for alveolar site preservation.
A number of previous literature reports have
confirmed that PRF may be used for alveolar ridge preservation and
is beneficial to clinical application (24–30).
Previous animal experiments have confirmed that, whether used in
conjunction with Bio-Oss or used alone in animal bone defect
models, PRF has exhibited good osteogenic ability and was able to
alleviative soft tissue inflammation (24,25). PRF
has also been used for clinical alveolar site preservation and
alveolar bone defect repair, which confirmed that it was able to
promote bone tissue regeneration (26–30).
In a number of previous studies, PRF was used in
combination with other bone substitute materials to realize its
osteogenic effects (19,24,25), but
it has been uncommon to use it separately for tooth extraction site
preservation. In the present study, PRF was used alone in the
extraction socket as a biological material.
From clinical, radiological and histological
perspectives, alveolar bone preservation and bone regeneration in
the experimental group were comprehensively assessed. The results
were compared with naturally healing tooth sockets. The purpose of
the present study was to explore the feasibility of using PRF alone
in preserving tooth extraction sites and providing a theoretical
basis for its clinical practice, which may ultimately maintain an
adequate quantity of bone for implant surgery.
Materials and methods
Case selection
A total of 28 patients were included in the present
study. They were outpatient patients from the Department of
Stomatology in East Hospital Affiliated to Tongji University
(Shanghai, China) and were selected between July 2015 and March
2017. Patient characteristics from both groups are presented in
Table I. Prior to surgery personal
data was collected and clinical trial specialist medical records
were completed. Patients gave informed written consent and were
included in the experimental or control group (n=14 each) according
to their wishes. The experimental protocol was reviewed and
approved by the Ethics Committee of East Hospital Affiliated to
Tongji University.
 | Table I.Patient demographics. |
Table I.
Patient demographics.
|
|
|
| Extracted tooth
position, n position, n | Classification of
extracted tooth, n |
|---|
|
|
|
|
|
|
|---|
| Group | Sex ratio,
female:male | Age, years (mean ±
SD) | Maxillary
molar | Mandibular | Fractured
tooth | Residual root and
crown |
|---|
| PRF | 6:8 |
33.2±3 | 7 | 7 | 9 | 5 |
| Control | 8:6 |
34.6±4 | 6 | 8 | 8 | 6 |
The inclusion criteria were as follows: i) Aged from
20 to 40 years, ii) upper and lower mandibular molars diagnosed as
fractured tooth or could not be retained for other reasons, as
these patients required implant surgery necessarily to repair their
missing teeth; iii) general good health and no uncontrolled
systemic disease; and iv) compliant patients. The presents study
recruited male and female patients in order to balance the
sexes.
The exclusion criteria were as follows: i) Tooth
extraction contraindications; ii) severe periodontal disease, as
patients with serious absorption of alveolar bone and alveolar bone
defects in tooth extraction sites were excluded; iii) implant
surgery contraindications, such as hypertension without good
control, diabetes, heart disease and other systemic diseases, and
bone metabolic diseases; iv) smoking and alcoholism; and v) severe
bruxism.
Preparation of radiation guide
Upper and lower mandibular research models were
prepared prior to tooth extraction. The crown of the target tooth
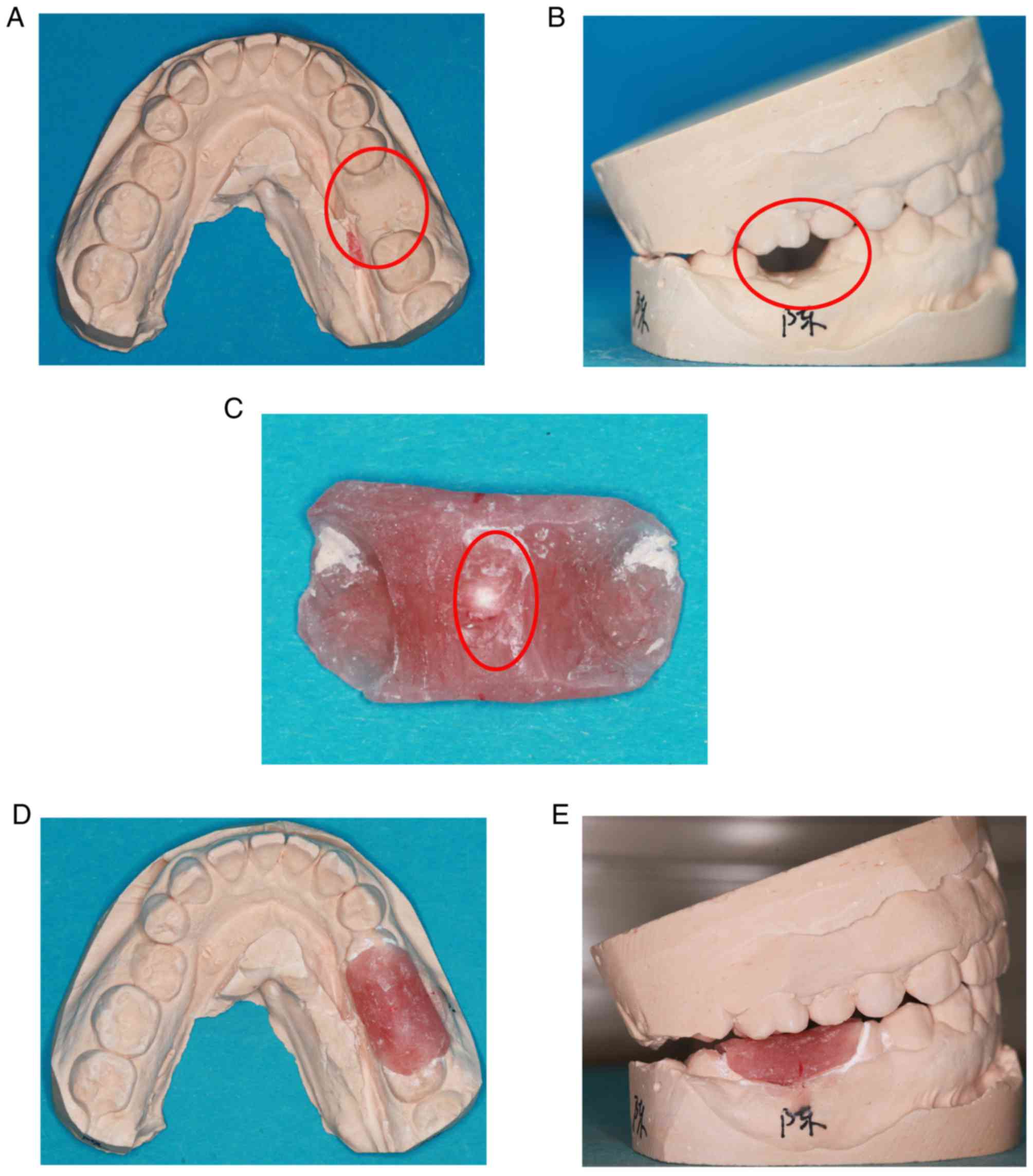
was abraded from the plaster model (Fig.
1A and B). The radiation guide plate was made from self-curing
plastic. The guide covered half of both adjacent teeth was removed
after the self-curing plastic had hardened. The radiation guide
plate was carefully polished to make it smooth. Subsequently, a
1-mm zirconium bead was placed in the center of the guide and fixed
(Fig. 1C). The guide was then placed
in the model to ensure that it was appropriate (Fig. 1D and E).
Preparation and structure of PRF
membrane
Following the provision of written informed consent,
two samples of venous blood (9 ml in each) were collected from
patients in the experimental group 5 min prior to tooth extraction.
The test tubes used did not contain any anticoagulant. The blood
samples were extracted in strict accordance with the PRF
manufacturing process (31). The
venous blood was rapidly centrifuged at 400 × g at room temperature
for 10 min using a specific table centrifuge, Hettich®
Universal 320 (Andreas Hettich GmbH & Co.KG, Tuttlingen,
Germany) and kept aside for 3–5 min. The sample was then divided
into three layers: The bottom layer of red blood cell (RBC) debris,
the middle layer of PRF gel and the top layer of supernatant. The
middle layer was removed using sterile tweezers, and the RBC layer
was removed from the PRF gel. Finally, the PRF gel was placed in
the PRF box (Process for PRF, Nice, France) for 10 sec under light
pressure. As a result, the tough and elastic PRF membrane was
obtained. The PRF membrane was immediately fixed with 4%
paraformaldehyde for 24 h at room temperature, dehydrated in
increasing gradients of alcohol, subjected to critical point drying
and gold sputter-coated to observe the ultrastructure. Samples were
then viewed and images were captured using scanning electron
microscopy (Quanta 250; FEI; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Minimally invasive tooth
extraction
Patients were asked to undergo an oral panoramic
radiograph prior to tooth extraction; this procedure was performed
using a using an Orthophos XG 5 (Dentsply Sirona, York, PA, USA).
An oral examination was performed, and local and systemic
conditions were assessed. All patients were treated with the
non-flap minimally invasive extraction technology following the use
of 1.8 ml of 4% articaine hydrochloride with epinephrine
(1:100,000) by subperiosteal infiltration (Ubistesin™ 1/100,000; 3M
ESPE AG, Seefeld, Germany) as local anesthesia. All inflammatory
granulation tissue was then removed from the extraction socket. In
the experimental group, PRF membranes were cut into a suitable size
and implanted into the extraction socket following tooth
extraction. The gum surrounding the tooth socket was loosened and
sutured following extraction. If the gum was not completely
sutured, one entire PRF membrane would be placed at the base of the
extraction socket and another PRF membrane covered the first
membrane. Therefore the first PRF membrane would be isolated from
the rest of the mouth. The extraction sockets in the control group
were left for natural healing after conventional suturing. The
gauze was held for 30–40 min following tooth extraction in the
experimental and control groups. Subsequently, three-dimensional
reconstruction of cone-beam computed tomography (CBCT) was taken
with the radiation guide that was previously prepared. CBCT was
taken with the respective radiating plate again at 3 months
following tooth extraction. Postoperative response following
extraction and local soft tissue healing condition were assessed at
3 and 7 days following tooth extraction. The healing of local
gingival tissue was recorded at 1 and 3 month following tooth
extraction.
CBCT scan
Scans were performed at 30–40 min (T1) and 3 months
(T2) following tooth extraction. Patients' posterior teeth
contacted with the pre-prepared radiating guide, and the upright
posture was taken. The operator was located in front of the patient
and adjusted the CBCT and the patient's positions so that the
horizontal alignment light overlapped the lips and vertical
calibration of the light in front of the condyle for 1.5 inches.
All procedures were performed by the same physician with the same
CBCT machine, and the following scan uniform parameters were set:
X-ray exposure settings, Voltage=120 V; Current=5 mA; scan window,
diameter=16 cm, height=13 cm, 25 stereo pixels, 26.9 sec; image
matrix size=640×640 pixels; and layer sweep thickness=0.250 mm. All
data were measured by the same radiologist in department of
Stomatology and analyzed by a blinded surveyor. Each measurement
was taken in triplicate to reduce the measurement error. Finally,
the mean value was adopted in the final statistical analysis. Data
measurement was completed using eXamVision software (version 1.9;
KaVo Dental GmbH, Biberach, Germany). Analysis was performed as
described in the following paragraph.
The blue line on the panorama view of the implant
screen was dragged so that the horizontal blue line coincided with
the maxillary or mandibular alveolar crest, and the vertical blue
line passed through the center of the positioning of the zirconium
beads. The sweep thickness and gap were adjusted to 1 mm (Fig. 2A). The blue line was positioned at
the gap of the tooth on the CBCT image immediately obtained
following tooth extraction (T1). A horizontal line S was drawn
through the center of the zirconium beads. The vertical distance of
the buccal alveolar crest to the line was recorded as h1, and the
palate/lingual alveolar crest to the line as h2. The horizontal
line S' and the vertical distance h1′ and h2′ were obtained by the
same method on the CBCT of T2. The buccal-lingual alveolar ridge
width w2 was measured by drawing a horizontal line through the
buccal or lingual alveolar ridge vertex position (a higher position
was chosen). The buccal-lingual alveolar ridge width w1 on the CBCT
image of T1 was obtained at the same distance from the vertebral
alveolar ridge (Fig. 2B-D). The
following differences were defined: the variation value of the
height of buccal alveolar crest=h1′-h1; the variation value of the
height of lingual/palatal alveolar crest=h2′-h2; the variation
value of the width of alveolar crest=w1-w2. All differences were
given as absolute values.
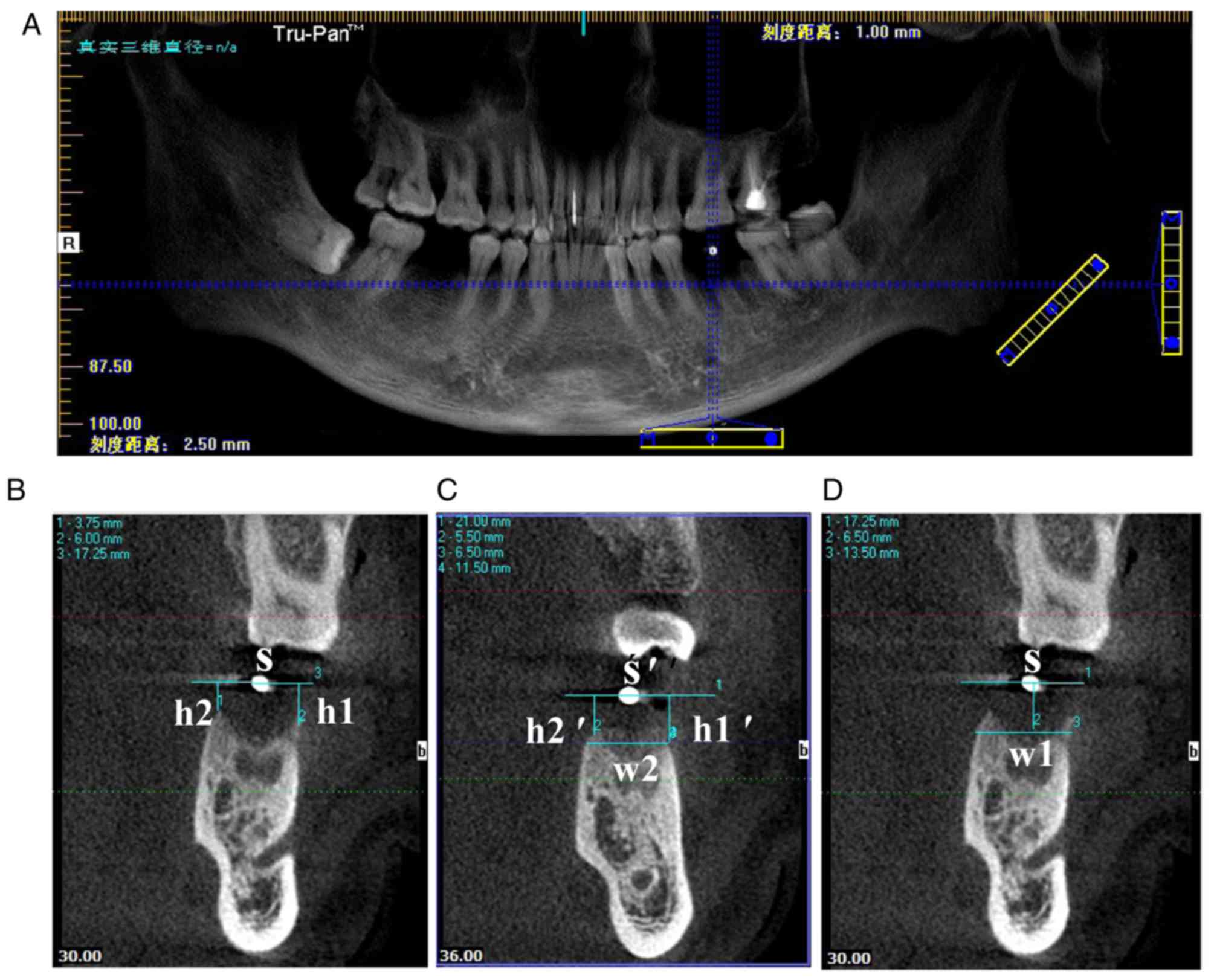 | Figure 2.CBCT data measurement. (A) The
horizontal blue line coincided with the maxillary or mandibular
alveolar crest, and the vertical blue line passed through the
center of the position of the zirconium beads. (B) The CBCT image
obtained at T1, where S, h1 and h2 were obtained. (C) On the CBCT
at 3 months following tooth extraction, the horizontal line S' and
the vertical distance h1′ and h2′ were obtained by the same method.
w2 was measured by drawing a horizontal line through the buccal or
lingual alveolar ridge vertex position. (D) w1 on the CBCT image of
T1 was obtained at the same distance from the vertebral alveolar
ridge. CBCT, cone-beam computed tomography; T1, immediately
following tooth extraction; S, line through center of zirconium
bead; h1, vertical distance of the buccal alveolar crest to the
line; h2, vertical distance of the palate/lingual alveolar crest to
the line; w1, buccal-lingual alveolar ridge width at T1; w2,
buccal-lingual alveolar ridge width at T2. |
Implant surgery
Blood routine check was performed prior to surgery.
Implant surgery was performed under local anesthesia with Ubistesin
1/100,000, following provision of preoperative informed consent.
Initially, the flap was cut in the center of the gums, and
granulation tissue was removed. A height of 3–4 mm alveolar bone
was then obtained in the center of the planting site using a ring
drill to prepare hard tissue sections, the inner diameter and the
outer diameter of which were 3.2 and 4.0 mm, respectively. Bone
samples (diameter, 3 mm; height, 3–4 mm) were separated and fixed
in Million's solution (Sinopharm Group Co., Ltd., Shanghai, China)
for 3 days (daily exchange) at room temperature, rinsed in flowing
tap water overnight, dehydrated using ethanol (40, 70, 95 and 100%)
every two day, soaked in xylene for 1 day at room temperature, and
embedded in methyl methacrylate. The samples were cut into
10-µm-thick sections following solidifying in a vacuum. The
sections were stained with Goldner's trichrome at room temperature.
Goldner's Trichrome staining procedure was as follows: The sections
were stained with Hematoxylin-Ferric Chloride Dye for 7 min, rinsed
with tap water two times, stained with Ponceau acid fuchsin for 7
min, rinsed with 1% acetic acid for 1 min, incubated with 1%
phosphomolybdic acid for 6 min, rinsed with 1% acetic acid for 1
min, incubated with 0.1% bright green solution staining for several
minutes, rinsed with 1% acetic acid for 1 min. Finally, the stained
sections were dried at 37°C overnight and sealed with neutral gum.
Then all stained sections were observed under a light microscope
and photographed using Leica Application Suite software (version
4.5) (both Leica Microsystems GmbH, Wetzlar, Germany). The novel
bone formation of the two groups was analyzed using SimplePCI 5.2.1
(Compix, Inc., Cranberry, PA, USA), and the osteoid area/tissue
area (OAr/TAr) was calculated. The corresponding types of implants
(NobelReplace Tapered Groovy; Nobel Biocare Services AG, Zürich,
Switzerland) were implanted following the step-by-step expansion of
the hole. The healing abutment was screwed into the implant with a
torque of 15 Ncm. Finally, the wound was sutured using 4–0
absorbable sutures.
Statistical analysis
Data analysis was performed using SPSS version 20.0
software (IBM Corp., Armonk, NY, USA). Data are presented as the
mean ± standard deviation. Statistical differences between the
groups were determined using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PRF membrane preparation and its
structure
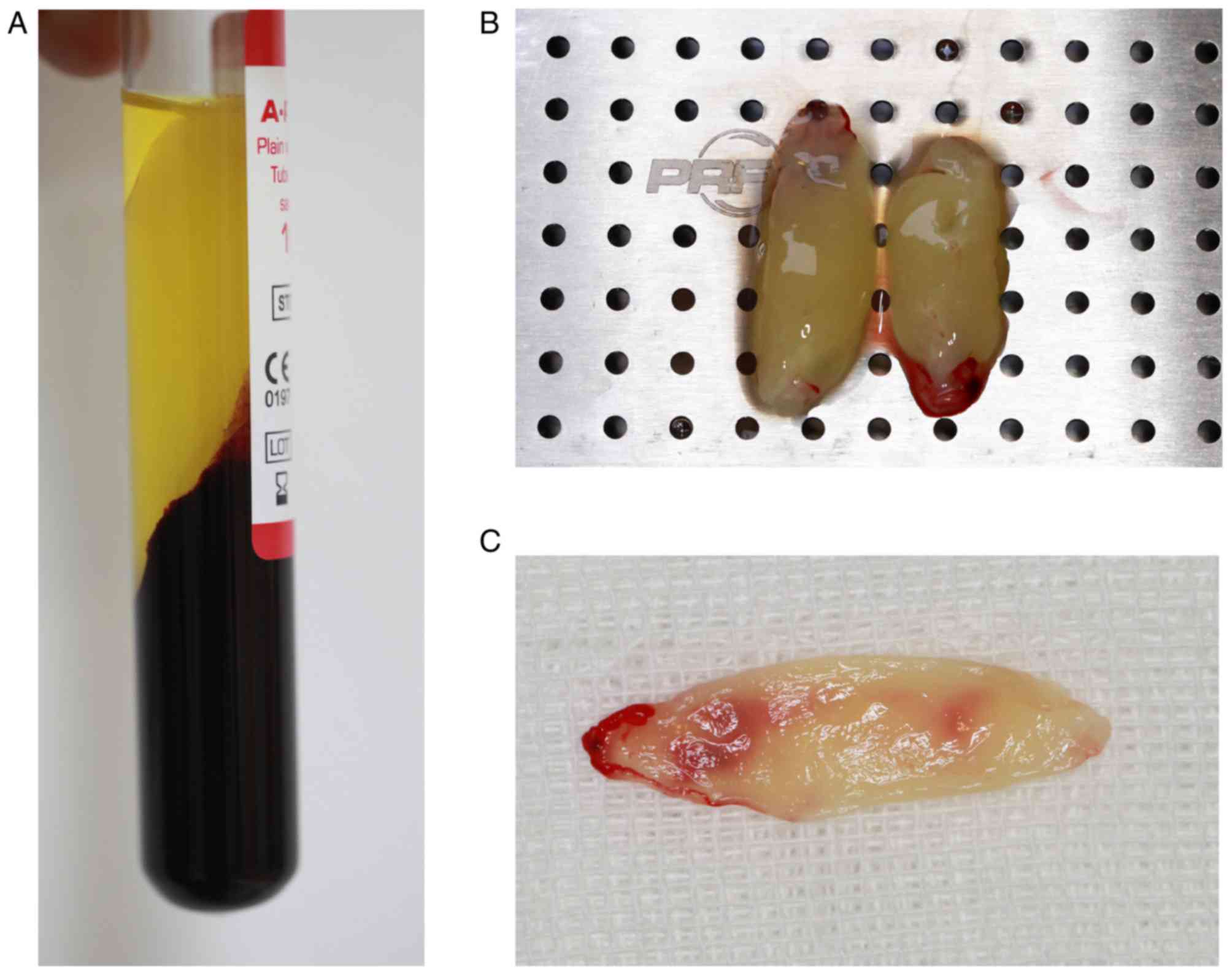
Venous blood exhibited three fractions following
centrifugation: The lower layer of RBCs, the middle layer of PRF
and the upper layer of platelet-poor plasma (Fig. 3A). The surface of the PRF layer was
smooth and flexible, and contained a light yellow liquid (Fig. 3B). PRF gels were extruded from the
liquid component by means of a lamination tool to form a membranous
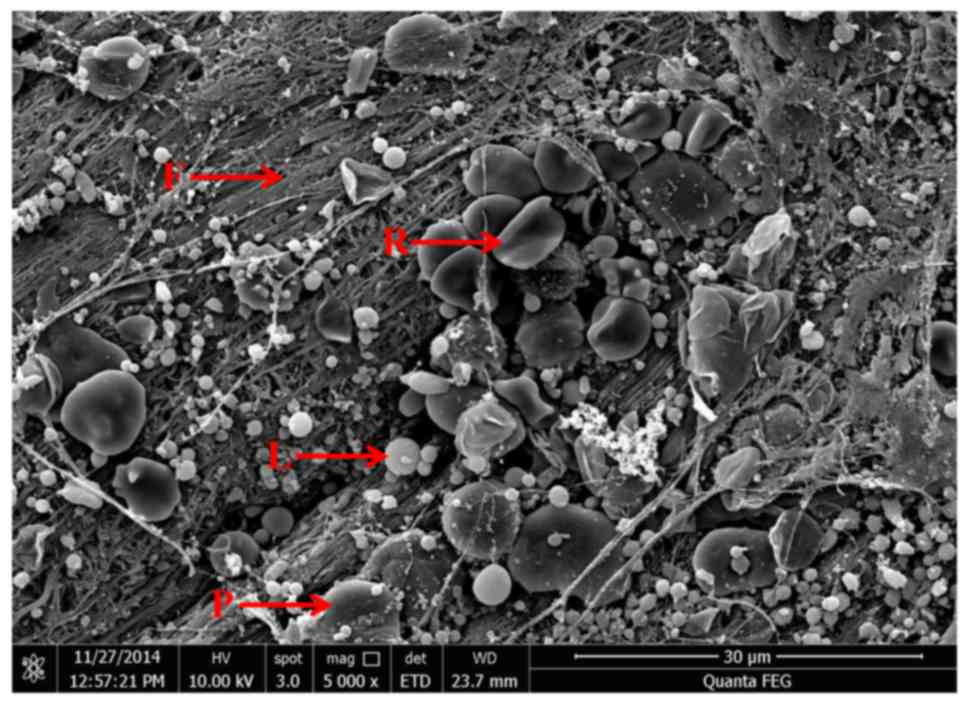
structure with durability and flexibility (Fig. 3C). As demonstrated in the scanning
electron micrograph (Fig. 4), fibrin
fibers tended to form a tight three-dimensional fibrin network
structure. RBCs were trapped within this fibrin matrix. Leukocytes
appeared as spherical structures with an irregular surface. The
platelets were often enmeshed in the fibrin network but sometimes
appeared as aggregates.
Minimally invasive extraction and the
process of PRF application
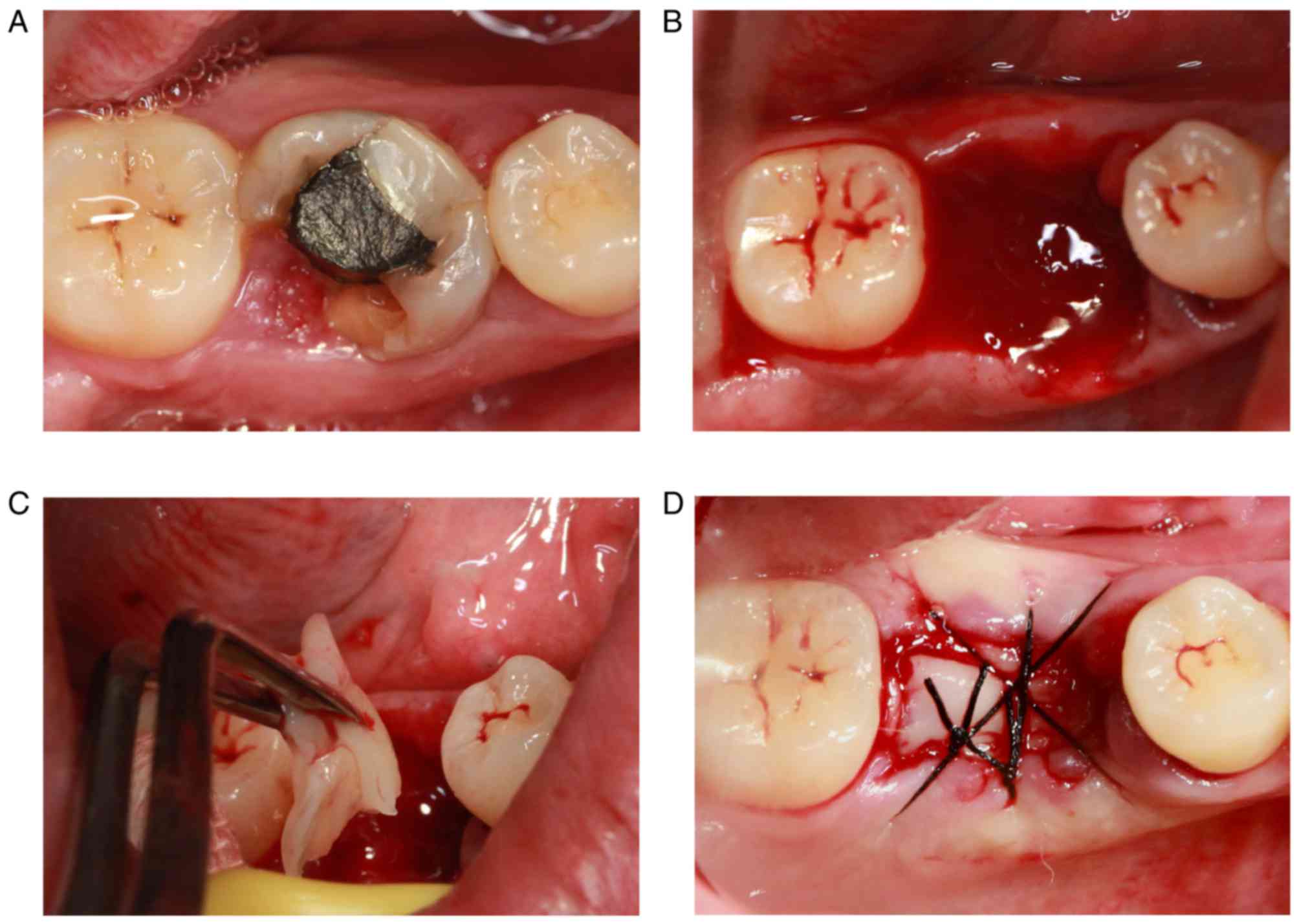
A representative case was chosen to demonstrate this
process. The first molar of the patient (a 28-year-old female) was
largely absent and the defect of the crown was <3 mm of the
subgingival tissue (Fig. 5A).
Therefore, the tooth could not be retained following a
comprehensive assessment. The broken teeth were removed via
minimally invasive tooth extraction surgery (Fig. 5B). The PRF membranes prepared were
cut into a suitable size and implanted into the extraction socket
following removal of the granulation tissue in the alveolar fossa
(Fig. 5C). Finally, the tooth
extraction socket was sutured (Fig.
5D). The extraction sockets in the control group were left for
natural healing.
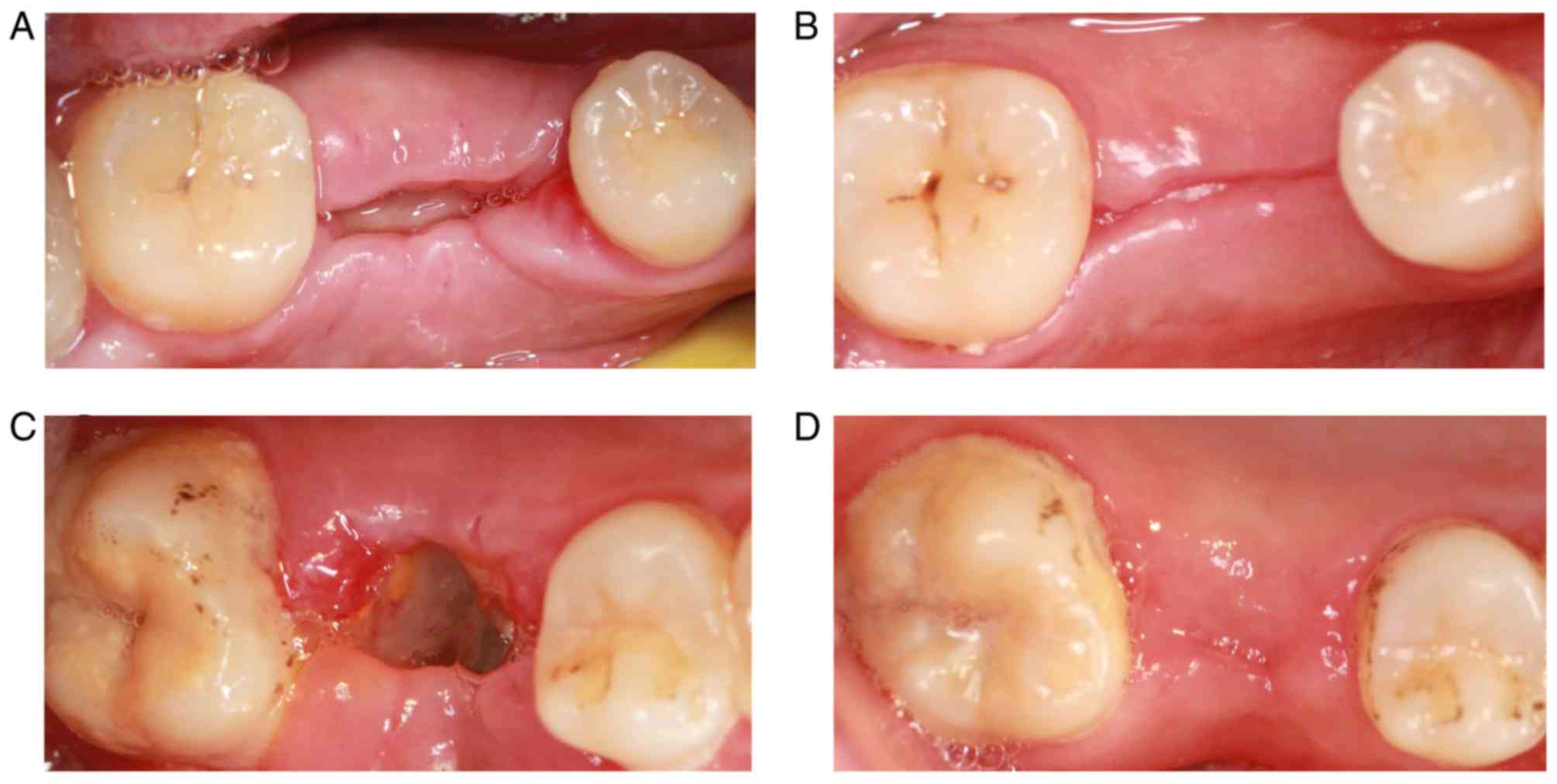
Postoperative follow-up results
The patients in the control group reported feeling
pain within 3 days following tooth extraction. The pain had
disappeared after 1 week. Patients in the experimental group
reported mild pain the day after surgery. The pain was not obvious
on the second day. The majority of patients felt no pain or
discomfort after 3 days. The PRF membrane was clearly visible at 7
days following tooth extraction, and PRF was not fully absorbed.
The tooth socket was partially closed, and the local gingival
tissue had no symptoms of swelling and infection (Fig. 6A). The local gingival tissue had
healed well by 3 months following tooth extraction, but a gap was
still visible (Fig. 6B). The
majority of tooth extractions were not closed at 7 days following
tooth extraction in the control group. Also, the white
pseudomembrane was observed in the tooth extraction, and the local
gingival tissue was slightly swollen (Fig. 6C). The tooth extractions were closed
by 3 months following tooth extraction. No signs of infection were
observed in the local gingival tissue (Fig. 6D).
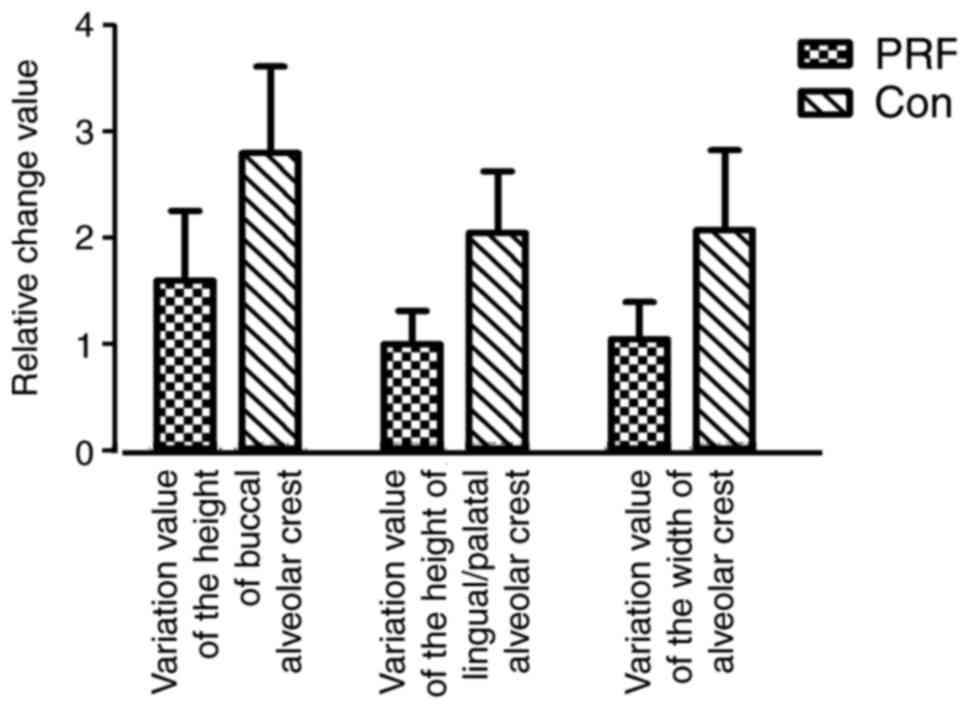
CBCT analysis results
The variation value of the height of buccal alveolar
crest and lingual/palatal alveolar crest, and the variation value
of the width of the alveolar crest in the two groups were measured
as detailed above. The variation value of the buccal alveolar crest
was markedly lower in the PRF group (1.6000±1.46416) compared with
the control group (2.8000±1.81487; Fig.
7). The variation value of the height of the lingual/palatal
alveolar crest was also lower in the PRF group (1.0000±0.70711)
compared with the control group (2.0500±1.29180; Fig. 7). The variation value of the width of
the alveolar crest was also lower in the PRF group (1.0500±0.77862)
compared with the control group (2.0760±1.67149; Fig. 7).
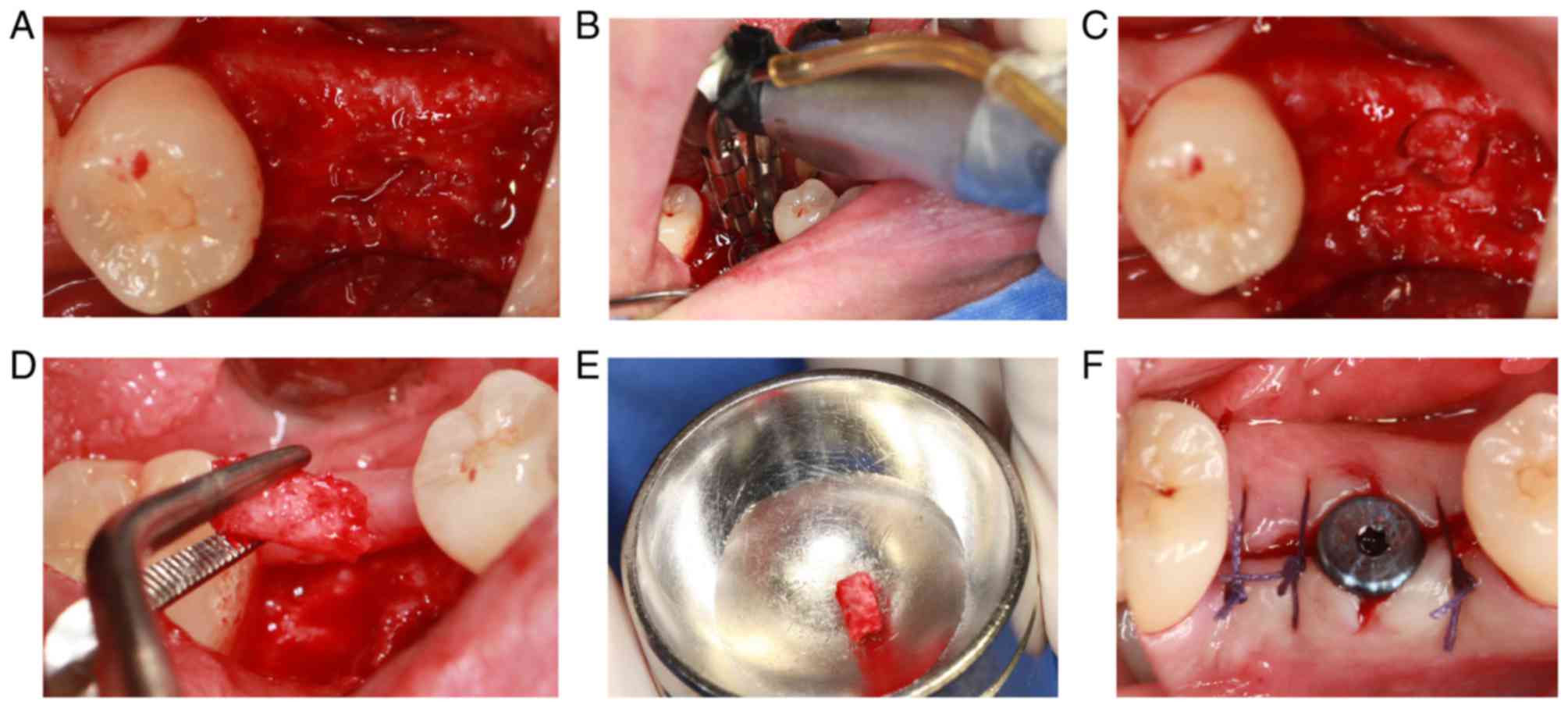
Implantation surgery
The implantation surgery was performed 3 months
following tooth extraction. After cutting the flap, the alveolar
bone surface was found to be smooth, with no obvious bone
depression, and granulation tissue was rare (Fig. 8A). During the operation, the alveolar
bone with a diameter of 2 mm and length of ~3 mm was drilled with a
circular drill at the planting center (Fig. 8B-E). The healing abutment was
inserted following implantation of the implant. Finally, the wound
was sutured (Fig. 8F).
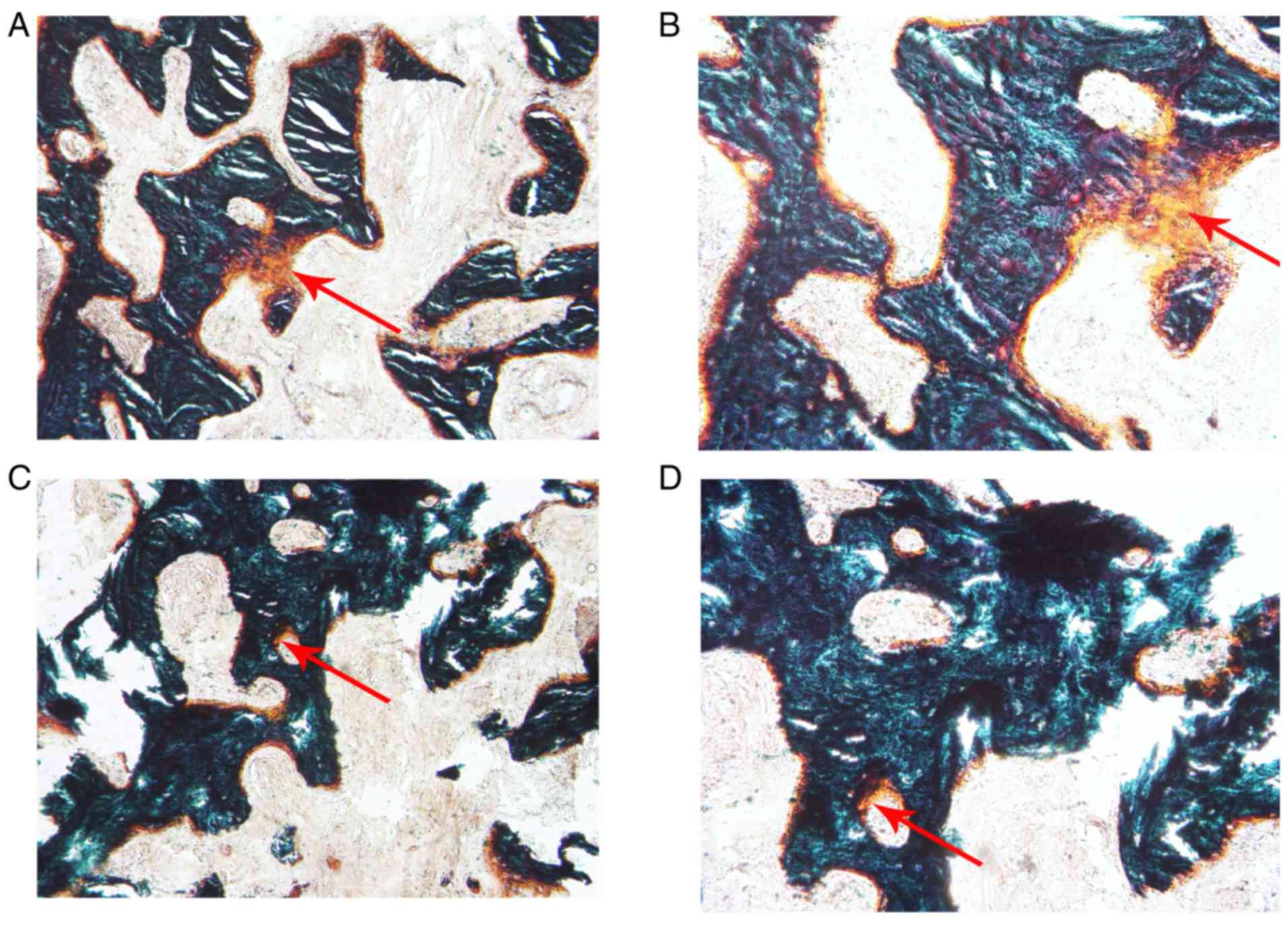
Histological analysis of new bone
formation in the two groups
The alveolar bone obtained during the implant
surgery was prepared for the hard tissue sections. Following
Goldner's trichrome staining, the new bone formation in the PRF
group (Fig. 9A and B) was relatively
abundant compared with that in the control group (Fig. 9C and D) when observed under the
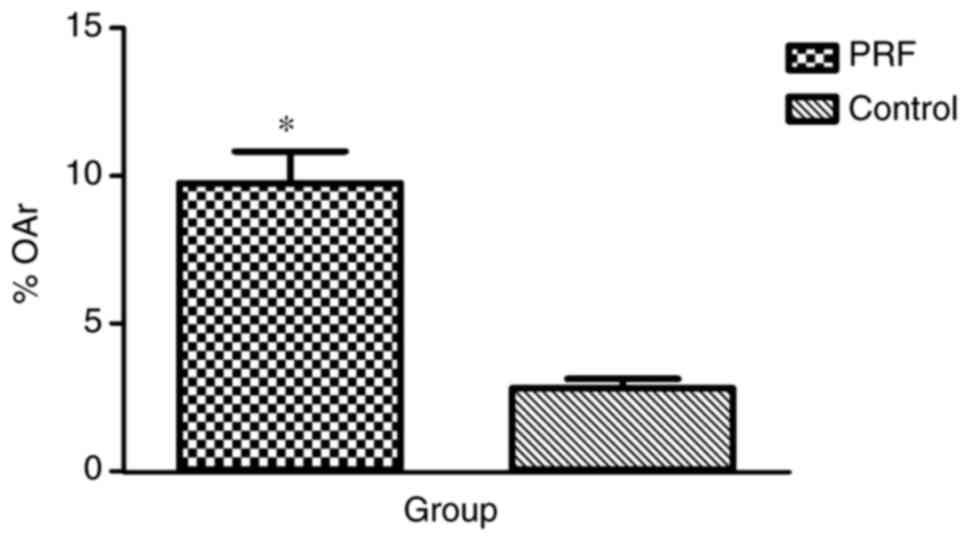
microscope. OAr/TAr in the experimental group (9.7624±4.0121%) was
significantly greater than that in the control group
(2.8056±1.2094%; P<0.01; Fig.
10).
Discussion
PRF was used alone for alveolar fossa in the present
study. The aim was to retain as much alveolar bone as possible;
therefore, minimally invasive surgery was adopted to remove the
teeth to minimize the alveolar bone destruction caused by tooth
extraction. A previous study confirmed that if flap surgery is used
in extraction surgery, the opened periosteal flap blocks the blood
supply of the buccal bone wall, further increasing buccal bone wall
absorption (32). In addition, the
use of flap surgery may increase the risk of gum gingival recession
and reduce the width of keratosis (33). Therefore, flap surgery was not
performed in the present study when the teeth were removed.
However, CBCT scanning was performed immediately following
extraction, instead of prior to tooth extraction, to reduce the
damage of the alveolar fossa caused by the tooth extraction
surgery. The following measures were taken to retain the PRF in a
relatively confined space: The gum surrounding the tooth socket was
loosened and sutured following extraction; if the gum was not
completely sutured, the entire PRF membrane would be covered, to
ensure that the PRF was relatively isolated from the rest of the
mouth.
Patients in the experimental group exhibited a
lesser postoperative reaction compared with the patients in the
control group, in terms of clinical manifestations. However, these
indicators may not serve as an ideal theoretical basis because of
variations between individuals. Furthermore, the healing of soft
tissue in the experimental group was improved compared with that in
the control group, and no symptoms of inflammation were observed,
indicating that PRF enhanced the healing of soft tissue, which was
consistent with previous studies (20,28,30). The
present results regarding enhanced soft tissue healing were due to
the properties of PRF, as detailed above.
In the present study, histological analysis
demonstrated that the novel bone formation in the PRF group was
significantly increased compared with that in the control group;
which suggested that PRF is able to stimulate bone regeneration. It
has previously been reported that bone graft healing is a
sequential process that consists of inflammation revascularization,
osteogenesis remodeling and incorporation into the host skeleton to
form a mechanically efficient structure, wherein the ingrowth,
proliferation and differentiation of osteoblasts occurs during the
initial 14 days (34,35). Furthermore, it has also been
demonstrated that PRF released autologous growth factors gradually
and expressed a durable effect on the proliferation and
differentiation of rat osteoblasts in vitro. The maximal
promoting effect of PRF occurred at day 14 (21). These findings suggest that the growth
factors contained in PRF may serve a role in promoting bone
regeneration, over a prolonged period of time.
In the present study, the changes in alveolar bone
height and bone mineral density were analyzed using CBCT
quantitative analysis software, whose radiation dose was relatively
low and the repeatability was strong. All procedures were performed
by the same doctor, and the patient position was maintained when
taking CBCT scans at different time points, to reduce human error.
Furthermore, the measurement data had a clear reference mark
through the preparation of the radiation guide, thereby reducing
the error in data measurement. Changes in alveolar bone have
previously been studied through the root tip; however, the results
were affected by the angle of shooting, position of the patient,
location of the film and magnification of the image (29). The results of imaging analysis
indicated that the variation values of the height of the buccal
alveolar crest and lingual/palatal alveolar crest, and the
variation value of the width of the alveolar crest were markedly
lower in the experimental group than in the control group. However,
the differences between the two groups were not statistically
significant. Therefore, it could not be concluded that PRF was
effective in reducing alveolar bone resorption and promoting bone
formation in the extraction socket. This finding was in accordance
with a previous clinical study (36). It is presumed that the effect of bone
regeneration was limited and could not maintain an alveolar ridge
shape following tooth extraction because of the low levels of the
cytokines in PRF. However, the present study corresponded with
previous studies where the loss of buccal marginal bone level was
more pronounced on the buccal than on the lingual counterpart
(37–39). Conversely, the present findings were
different from the results of a number of other previous studies;
in the present study the alveolar bone was preserved, which was not
the case in previous studies (26–30).
Possible reasons are as follows: i) The application of the
radiation guide promoted the accuracy of the measurement; ii)
examining the dimensional changes of the alveolar ridge via CBCT
ensured the reproducibility of the experiment and reduced human
error; iii) histological analysis of new bone formation was
performed; and iv) the teeth used in the present study were not
single-rooted, as are used in many other studies. Socket anatomy is
an important disturbing factor because of a variety in width and
height of the socket, thickness and densities of osseous plates,
presence of fenestrations and dehiscence, periodontal biotype, form
(single or multi rooted) and location of the socket (maxilla or
mandible). Therefore, it is difficult to quantify the real role of
a graft in the limitation of osseous resorption.
In the present study, patients were recruited by
following the inclusion and exclusion criteria. Therefore, the
number of patients was relatively small. The majority of teeth
included in the present study were broken, or had residual roots or
crowns (which could not be retained by assessment), resulting in
the reduction of sample size. Furthermore, experimental protocols
were interfered by a number of factors. Inevitably patients were
lost in the follow-up, resulting in the loss of corresponding data
and insufficiency of effective data. If some of the follow-up data
of a patient was missing, the patient was removed from the final
analysis. In the present study, 4 patients (2 patients in each
group) were lost in the follow-up; their information was not
included in the statistical analysis. Therefore, it was difficult
to demonstrate the final conclusion. However, the osteogenesis
effect of Bio-Oss is internationally recognized. Previous studies
have demonstrated that combining Bio-Oss and PRF in clinical
applications preserves alveolar bone and also shortens healing time
(19,40). However, no conclusive evidence is
currently available regarding the proportion required of both,
which may be the focus of future research.
In conclusion, PRF membrane used in the extraction
sockets was demonstrated to promote local soft tissue healing of
gums and reduce postoperative pain response; however, the effect of
PRF to reduce alveolar bone resorption was not significant.
However, PRF was able to increase the quality of the novel bone and
enhance the rate of bone formation due to the concentration of
growth factors. Despite certain limitations, the present study
demonstrated the potential for preserving the alveolar ridge
following tooth extraction. A larger sample size may be required to
obtain a conclusive result of the bone regeneration in extraction
sockets using the PRF membrane.
Acknowledgements
The present study was supported by the Science and
Technology Development Exhibition Innovation Fund of Pudong New
District (Shanghai, China; grant no. PKJ2013-Y15).
References
|
1
|
Wood DL, Hoag PM, Donnenfeld OW and
Rosenfeld LD: Alveolar crest reduction following full and partial
thickness flaps. J Periodontol. 43:141–144. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ten Heggeler JM, Slot DE and Van der
Weijden GA: Effect of socket preservation therapies following tooth
extraction in non-molar regions in humans: A systematic review.
Clin Oral Implan Res. 22:779–788. 2011. View Article : Google Scholar
|
|
3
|
Araujo MG and Lindhe J: Ridge alterations
following tooth extraction with and without flap elevation: An
experimental study in the dog. Clin Oral Implan Res. 20:545–549.
2009.
|
|
4
|
Wang RE and Lang NP: Ridge preservation
after tooth extraction. Clin Oral Implants Res. 23 Suppl
6:S147–S156. 2012. View Article : Google Scholar
|
|
5
|
Darby I, Chen ST and Buser D: Ridge
preservation techniques for implant therapy. Int J Oral Maxillofac
Implants. 24 Suppl:S260–S271. 2009.
|
|
6
|
Bartee BK: Extraction site reconstruction
for alveolar ridge preservation. Part. 1:Rationale and materials
selection. J Oral Implantol 27: 187–193. 2001.
|
|
7
|
Serino G, Biancu S, Iezzi G and Piattelli
A: Ridge preservation following tooth extraction using a
polylactide and polyglycolide sponge as space filler: A clinical
and histological study in humans. Clin Oral Implants Res.
14:651–658. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brugnami F, Then PR, Moroi H, Kabani S and
Leone CW: GBR in human extraction sockets and ridge defects prior
to implant placement: Clinical results and histologic evidence of
osteoblastic and osteoclastic activities in DFDBA. Int J
Periodontics Restorative Dent. 19:259–267. 1999.PubMed/NCBI
|
|
9
|
Serino G, Biancu S, Iezzi G and Piattelli
A: Ridge preservation following tooth extraction using a
polylactide and polyglycolide sponge as space filler: A clinical
and histological study in humans. Clin Oral Implan Res. 14:651–658.
2003. View Article : Google Scholar
|
|
10
|
Yilmaz S, Efeoğlu E and Kiliç AR: Alveolar
ridge reconstruction and/or preservation using root form bioglass
cones. J Clin Periodontol. 25:832–839. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gauthier O, Boix D, Grimandi G, Aguado E,
Bouler JM, Weiss P and Daculsi G: A new injectable calcium
phosphate biomaterial for immediate bone filling of extraction
sockets: A preliminary study in dogs. J Periodontol. 70:375–383.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Zou D, Zhang S, Zhao J, Pan K and
Huang Y: Repair of bone defects around dental implants with bone
morphogenetic protein/fibroblast growth factor-loaded porous
calcium phosphate cement: A pilot study in a canine model. Clin
Oral Implants Res. 22:173–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Howell TH, Fiorellini J, Jones A, Alder M,
Nummikoski P, Lazaro M, Lilly L and Cochran D: A feasibility study
evaluating rhBMP-2 absorbable collagen sponge device for local
alveolar ridge preservation or augmentation. Int J Periodontics
Restorative Dent. 17:124–139. 1997.PubMed/NCBI
|
|
14
|
Wang L, Huang Y, Pan K, Jiang X and Liu C:
Osteogenic responses to different concentrations/ratios of BMP-2
and bFGF in bone formation. Ann Biomed Eng. 38:77–87. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dohan Ehrenfest DM: How to optimize the
preparation of leukocyte- and platelet-rich fibrin (L-PRF,
Choukroun's technique) clots and membranes: Introducing the PRF
Box. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 110:275–280.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dohan Ehrenfest DM, Del Corso M, Diss A,
Mouhyi J and Charrier JB: Three-dimensional architecture and cell
composition of a Choukroun's platelet-rich fibrin clot and
membrane. J Periodontol. 81:546–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Pan S, Dangaria SJ, Gopinathan G,
Kolokythas A, Chu S, Geng Y, Zhou Y and Luan X: Platelet-rich
fibrin promotes periodontal regeneration and enhances alveolar bone
augmentation. Biomed Res Int. 2013:6380432013.PubMed/NCBI
|
|
18
|
Anitua E, Alkhraisat MH and Orive G:
Perspectives and challenges in regenerative medicine using plasma
rich in growth factors. J Control Release. 157:29–38. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choukroun J, Diss A, Simonpieri A, Girard
MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J and Dohan DM:
Platelet-rich fibrin (PRF): A second-generation platelet
concentrate. Part V: Histologic evaluations of PRF effects on bone
allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 101:299–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anilkumar K, Geetha A, Umasudhakar,
Ramakrishnan T, Vijayalakshmi R and Pameela E:
Platelet-rich-fibrin: A novel root coverage approach. J Indian Soc
Periodontol. 13:50–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He L, Lin Y, Hu X, Zhang Y and Wu H: A
comparative study of platelet-rich fibrin (PRF) and platelet-rich
plasma (PRP) on the effect of proliferation and differentiation of
rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 108:707–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part III: Leucocyte
activation: A new feature for platelet concentrates? Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 101:e51–e55. 2006. View Article : Google Scholar
|
|
23
|
Naik B, Karunakar P, Jayadev M and Marshal
VR: Role of Platelet rich fibrin in wound healing: A critical
review. J Conserv Dent. 16:284–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pripatnanont P, Nuntanaranont T,
Vongvatcharanon S and Phurisat K: The primacy of platelet-rich
fibrin on bone regeneration of various grafts in rabbit's calvarial
defects. J Craniomaxillofac Surg. 41:e191–e200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xuan F, Lee CU, Son JS, Jeong SM and Choi
BH: A comparative study of the regenerative effect of sinus bone
grafting with platelet-rich fibrin-mixed Bio-Oss® and
commercial fibrin-mixed Bio-Oss®An experimental study. J
Craniomaxillofac Surg. 42:e47–e50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoaglin DR and Lines GK: Prevention of
localized osteitis in mandibular third-molar sites using
platelet-rich fibrin. Int J Dent. 2013:8753802013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peck MT, Marnewick J and Stephen L:
Alveolar ridge preservation using leukocyte and platelet-rich
fibrin: A report of a case. Case Rep Dent.
2011:3450482011.PubMed/NCBI
|
|
28
|
Barone A, Ricci M, Romanos GE, Tonelli P,
Alfonsi F and Covani U: Buccal bone deficiency in fresh extraction
sockets: A prospective single cohort study. Clin Oral Implants Res.
26:823–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hauser F, Gaydarov N, Badoud I, Vazquez L,
Bernard JP and Ammann P: Clinical and histological evaluation of
postextraction platelet-rich fibrin socket filling: A prospective
randomized controlled study. Implant Dent. 22:295–303. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh A, Kohli M and Gupta N: Platelet
rich fibrin: A novel approach for osseous regeneration. J
Maxillofac Oral Surg. 11:430–434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part I: Technological
concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:e37–e44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fickl S, Zuhr O, Wachtel H, Bolz W and
Huerzeler M: Tissue alterations after tooth extraction with and
without surgical trauma: A volumetric study in the beagle dog. J
Clin Periodontol. 35:356–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beck TM and Mealey BL: Histologic analysis
of healing after tooth extraction with ridge preservation using
mineralized human bone allograft. J Periodontol. 81:1765–1772.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cypher TJ and Grossman JP: Biological
principles of bone graft healing. J Foot Ankle Surg. 35:413–417.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eriksson C, Ohlson K, Richter K,
Billerdahl N, Johansson M and Nygren H: Callus formation and
remodeling at titanium implants. J Biomed Mater Res A.
83:1062–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suttapreyasri S and Leepong N: Influence
of Platelet-rich fibrin on alveolar ridge preservation. J Craniofac
Surg. 24:1088–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schropp L, Wenzel A, Kostopoulos L and
Karring T: Bone healing and soft tissue contour changes following
single-tooth extraction: A clinical and radiographic 12-month
prospective study. Int J Periodontics Restorative Dent. 23:313–323.
2003.PubMed/NCBI
|
|
38
|
Araujo MG and Lindhe J: Dimensional ridge
alterations following tooth extraction. An experimental study in
the dog. J Clin Periodontol. 32:212–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fickl S, Zuhr O, Wachtel H, Stappert CF,
Stein JM and Hürzeler MB: Dimensional changes of the alveolar ridge
contour after different socket preservation techniques. J Clin
Periodontol. 35:906–913. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choukroun J, Diss A, Simonpieri A, Girard
MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J and Dohan DM:
Platelet-rich fibrin (PRF): A second-generation platelet
concentrate. Part IV: Clinical effects on tissue healing. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 101:e56–e60. 2006.
View Article : Google Scholar : PubMed/NCBI
|