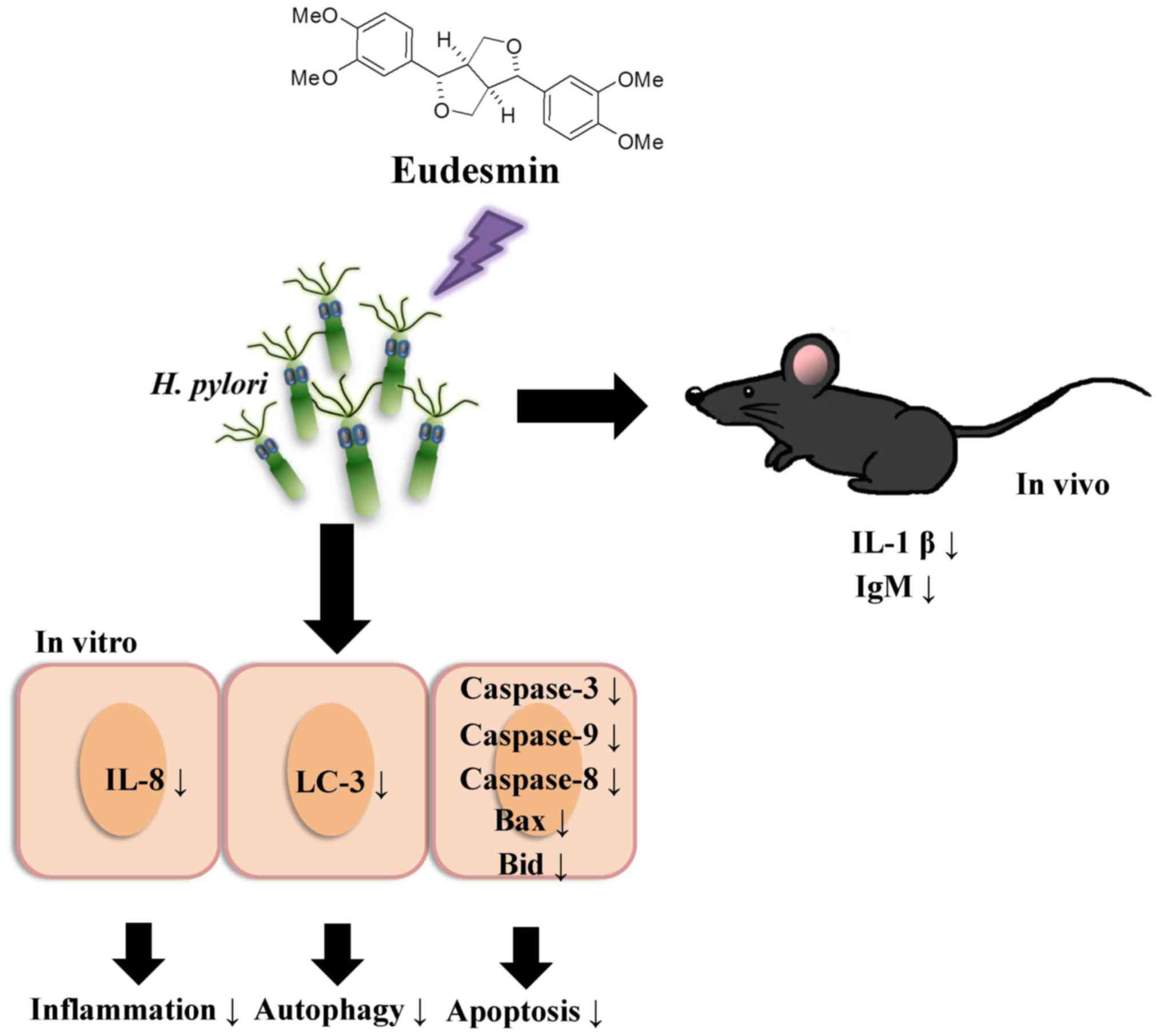
Introduction
Helicobacter pylori (H. pylori) infection is
one of the most prevalent bacterial infections worldwide, and is a
primary cause of gastritis, gastroduodenal ulcers and malignancies
(1,2). H. pylori adhere to the gastric
mucosa, inducing the production of reactive oxygen species (ROS)
which damage the epithelium (3). The
response of the gastric mucosal epithelium to H. pylori
infection is a multistep progression reflecting the interaction of
several factors, including bacterial virulence, specific
receptor-linked signaling pathways and the host immune response
(4,5). The first-line therapy for H.
pylori infection is antibiotics, but the increasing emergence
of antibiotic-resistant H. pylori strains has led to a
decline in eradication rates (6,7).
Therefore, developing alternative treatments for H. pylori
infection is important.
Previous studies of novel H. pylori
treatments have decreased H. pylori-triggered ROS production
and apoptosis but enhanced autophagy (8,9).
Apoptosis and autophagy are recognized to be non-inflammatory
programmed cell-death (PCD) pathways (10,11).
Apoptosis and autophagy serve vital roles in tissue homeostasis and
in disease development in infected patients (9,10).
Apoptosis (type I PCD) includes the cell-surface death receptor
pathway and the mitochondrial pathway. The Fas/CD95 receptor, Fas
ligand and downstream caspase-8 initiate the process of apoptosis
in the death receptor-dependent pathway. The mitochondrial pathway
is characterized by an increase in mitochondrial membrane
permeability and the release of cytochrome c into the
cytoplasm. Cytochrome c then initiates the formation of the
apoptosome, which activates caspase-9. Finally, caspase-8 or −9
activate caspase-3, thus triggering apoptosis (8–10).
Autophagy is a catabolic process encompassing the pathways for
intracellular macromolecule degradation (9,12).
Autophagy begins with the sequestration of cytoplasmic organelles
in a membrane vacuole, forming an autophagosome, which then fuses
with a lysosome, where the cellular materials are degraded and
recycled. Increased autophagic activity is associated with cell
death, and autophagy is now considered type II PCD (9,12).
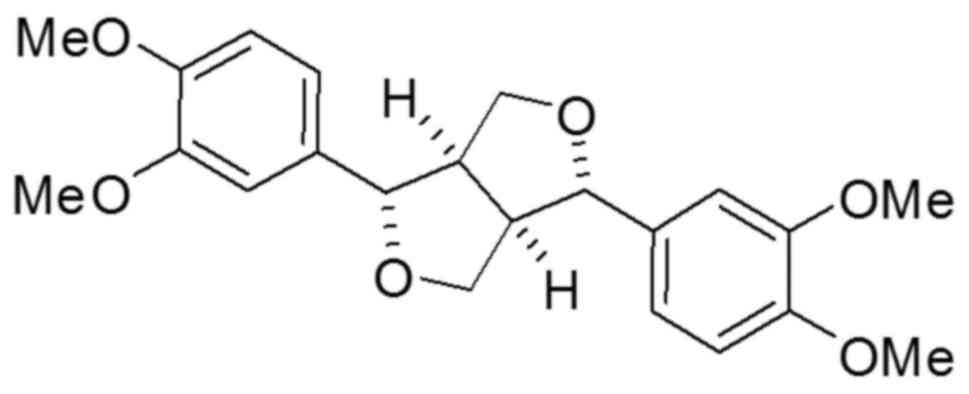
Eudesmin (Fig. 1) has
previously been demonstrated to exert weak toxicity in mice and no
toxicity in human macrophages (13).
In immunological studies, eudesmin inhibits tumor necrosis factor
(TNF)-α production and T cell proliferation (14). A previous study reported that
eudesmin-induced vascular relaxation of rat aorta could be
facilitated by the endothelial histamine receptor-mediated release
of nitric oxide and prostanoids (15). (+)-Eudesmin can induce neurite
outgrowth from PC12 cell neurons by stimulating signaling upstream
of the mitogen-activated protein kinase, protein kinase C and
protein kinase A pathways (16).
However, there are no applicable studies on eudesmin regarding the
response of epithelial cells to H. pylori infection. In the
present study, the effects of eudesmin, extracted from Fatsia
polycarpa Hayata, on H. pylori-induced epithelial
damage, as well as H. pylori colonization in vitro
[human gastric adenocarcinoma (AGS) cells] and in vivo
(C57BL/6 mice) was investigated.
Materials and methods
Isolation and identification of
eudesmin
The leaves of Fatsia polycarpa Hayata were
collected in November 2009 from study sites in Hehuan mountain
(2105 m above sea level), Hehuanshan, Taiwan. Air-dried leaves of
F. polycarpa Hayata (7 kg total) were extracted with
methanol over three times, following standard extraction procedures
(17). The isolated compound was
identified by 1H NMR and 13C NMR spectroscopy
using a Varian Inova 600 (Bruker Daltonics Inc., Billerica, MA,
USA) and electrospray ionisation mass spectrometry using a Bruker
Daltonics Esquire HCT (Bruker Daltonics Inc.,) as (+)-eudesmin,
through comparison of spectra data in previously reported
literature (18). Eudesmin was
dissolved in 0.1% of dimethyl sulfoxide (DMSO) for cell culture
experiments.
Bacterial strains, human cell lines
and culture conditions
The H. pylori reference strain 26695 (ATCC
700392) was obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). The antibiotic-resistant (metronidazole
and clarithromycin) H. pylori strains V633, V1254, V1354 and
V2356 were clinical isolates from previous studies (19,20).
H. pylori were grown on Brucella agar (BD Biosciences,
Franklin Lakes, NJ, USA) supplemented with 5% sheep blood under
microaerophilic conditions at 37°C for 48–72 h. Brucella blood agar
plates containing H. pylori supplement SR0147E (Thermo
Fisher Scientific Oxoid Ltd., Basingstoke, UK) were used to examine
the H. pylori load in infected mice under same culture
condition. Salmonella enterica serovar Typhimurium (ATCC
6994), Escherichia coli (ATCC 25922) and Streptococcus
aureus (ATCC 25923) were obtained from the ATCC and
Pseudomonas aeruginosa (BCRC 13984) was obtained from the
Bioresource Collection and Research Centre (Hsinchu, Taiwan). These
bacteria were grown in Luria-Bertani medium (BD Biosciences) at
37°C for 48–72 h. Human AGS cells (CRL-1739; ATCC, Manassas, VA,
USA) were purchased from ATCC and were cultured in RPMI-1640 medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin.
Antimicrobial activity of eudesmin on
Gram-negative and Gram-positive bacteria
The minimum inhibitory concentrations (MICs) of
eudesmin were tested by a two-fold serial dilution method. Eudesmin
was serially diluted with 0.1% DMSO to achieve concentrations of
500, 250, 125, 62.5, 31.25 and 15.625 µM. Equal volumes of
bacterial suspension [1×106 colony forming units
(CFUs)/ml] and diluted eudesmin samples were mixed and added to a
96-well plate, with an additional well containing broth only that
acted as a negative control. The plate was incubated at 37°C for 24
h, following which the well containing the lowest concentration of
eudesmin presenting with no visible bacterial growth was considered
the MIC. The minimum bactericidal concentrations (MBCs) of eudesmin
were then obtained. All samples with concentrations of eudesmin
that exhibited complete inhibition of visual bacterial growth were
identified and 50 µl of each culture was transferred onto a
Mueller-Hinton agar plate supplemented with 5% sheep blood and
incubated for 48–72 h at 37°C. The complete visual absence of
bacterial colonies on the agar surface in the lowest eudesmin
concentration was defined as the MBC. Each assay was repeated three
times.
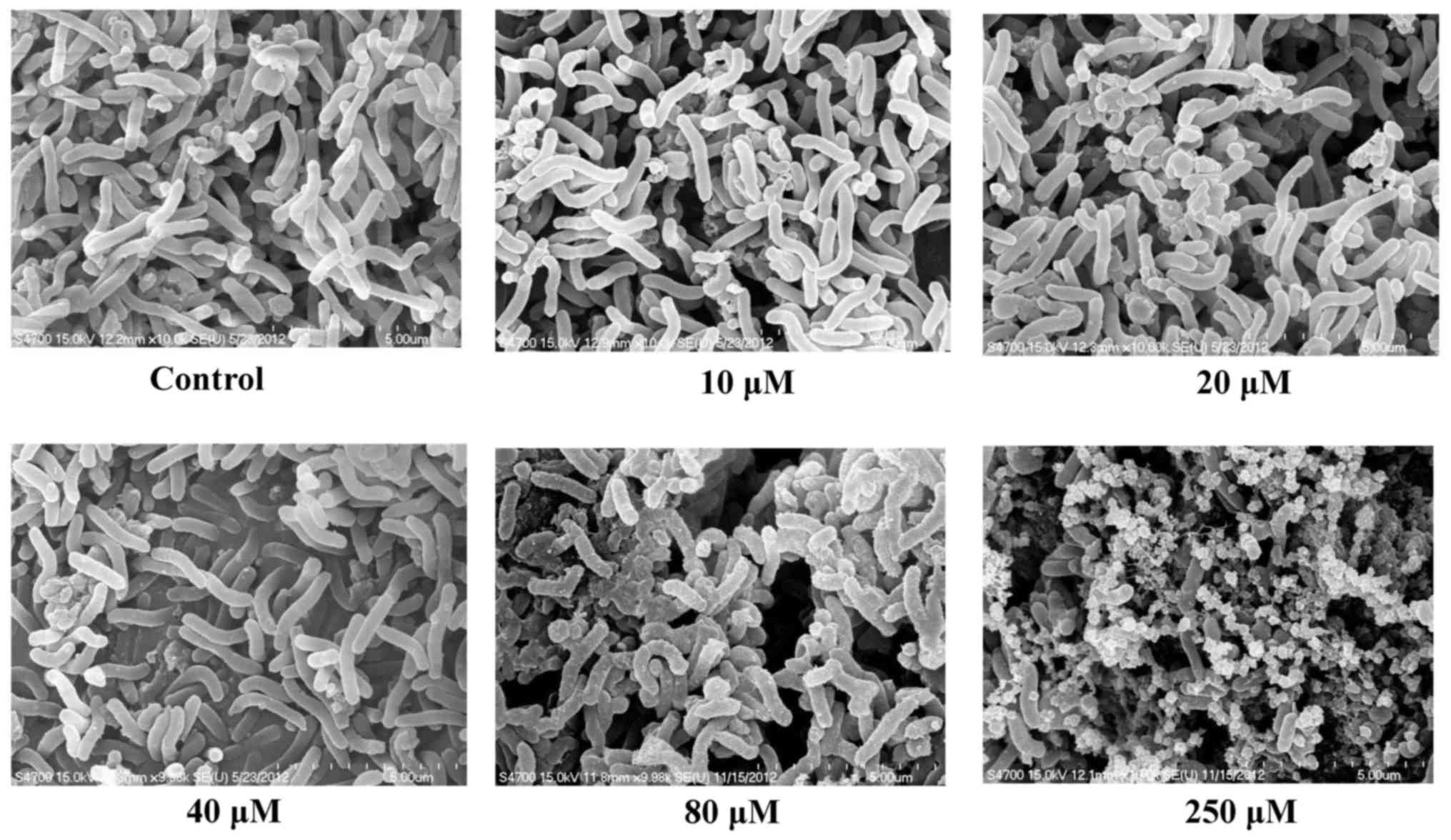
Scanning electron microscopy
H. pylori cultures were treated with 0
(control), 10, 20, 40, 80 and 250 µM eudesmin on Brucella blood
agar plates under microaerophilic conditions at 37°C for 6 h.
Bacterial colonies were scraped from the plates and then washed
twice in phosphate-buffered saline (PBS) and bacteria were
collected by centrifugation at 15,000 × g for 10 min at room
temperature. The pellets were then transferred to cover glasses and
fixed using 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4)
at 4°C for 2 h. Following rinsing with buffer, specimens were
post-fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer for
1.5 h at room temperature. Specimens were subsequently dehydrated
with a graded series of ethanol up to 100% ethanol. Following two
exchanges of 100% acetone, specimens were critical point dried and
sputter coated with gold. Specimens were then observed under a
scanning electron microscope (Hitachi S-4700; Hitachi, Ltd., Tokyo,
Japan) at 15 kv. Different areas (≥3) were randomly selected for
image capture at magnification of ×10,000 and representative images
were selected.
Cell viability assay
AGS cells were seeded into 96-well plates at a
density of 1×104 cells/well and cultured in RPMI 1640
medium in an incubator containing 5% CO2 at 37°C for 18
h. Eudesmin in 0.1% DMSO at concentrations of (5, 10, 20, 40, 80,
160, 320 and 640 µM) was then added to cells and then cultured at
37°C in CO2 for 24 h. A control group, which was treated
only with 0.1% DMSO, underwent the same procedures. To determine
cell viability, the trypan blue exclusion test was used, where
results represent the percentage of cells surviving treatment.
Equal volumes of 10 µl cell suspension in PBS (pH 7.4) and trypan
blue (Thermo Fisher Scientific, Inc.) were mixed. Subsequently,
stained (dead) and unstained (surviving) cells were counted using a
hemocytometer.
Association activity assay
AGS cells and PBS-suspended H. pylori 26695
at a multiplicity of infection (MOI) ratio of 100 were co-cultured
in antibiotic-free RPMI-1640 medium supplemented with 10% FBS. The
6 concentrations of eudesmin (0, 10, 20, 40, 80 and 250 µM) were
added to the culture. A control group, which was treated only with
0.1% DMSO, underwent the same procedures. Cell-associated bacteria
were quantified 6 h later, following infection of the host (AGS)
cells by osmotic lysis. Cell culture supernatants were removed by
centrifugation at 1,500 × g for 5 min at room temperature, cells
were washed with PBS twice and osmotic lysis was performed to
calculate the total quantity of bacteria remaining. For this
purpose, sterile water was added to the infected cells following
washing, the cell lysates were re-suspended in PBS, and then plated
using serial dilutions on the Brucella blood agar plates. These
plates were cultured for 100 µl from each dilution at 37°C for 48
h. Bacterial cell numbers were then determined by manual colony
counting. The association activity of H. pylori was
determined as the mean of triplicate readings at each concentration
of eudesmin. The bacteria associated with host cells included
adherent and invading bacteria. The results are expressed as a
percentage of the association activity of H. pylori in
comparison with the control group.
Confocal fluorescence microscopy
AGS cells were grown on glass coverslips
(~5×106 cells/dish) for 18 h at 37°C. H. pylori
26,695 cells were then added to cultures at an MOI ratio of 100 and
grown at 37°C for 12 h. Eudesmin (10, 20, 40, 80 and 250 µM) was
added to the cells, while only 0.1% DMSO was added to the control
group. Cells were washed twice with PBS and fixed in 4%
paraformaldehyde in PBS for 30 min at room temperature. Cells were
then washed twice in PBS and quenched with PBS (pH 7.4) containing
0.02% Triton X-100 for 30 min, prior to blocking for 30 min at room
temperature in PBS with 5% non-fat dry milk. Coverslips were
incubated with anti-microtubule-associated protein 1A/1B-light
chain 3, isoform B (LC-3B) antibody (cat. no. NB600-1384, dilution
1:200; Novus Biologicals, LLC, Littleton, CO, USA) at room
temperature for 1 h. Cells were then washed in PBS for 5 min three
times and incubated with secondary anti-rabbit fluorescein
isothiocyanate-labeled antibody (cat. no. NB730-F, dilution
1:10,000; Novus Biologicals, LLC) at room temperature in PBS for 1
h. Then, coverslips were washed twice in PBS and incubated with
LysoTracker Red DND-99 (cat. no. L7528; Thermo Fisher Scientific,
Inc.) at a dilution of 1:1,000 in PBS for 1 h, followed by
incubation with 300 nM 4′,6-diamidino-2-phenylindole (cat. no.
D1306; Thermo Fisher Scientific, Inc.) for 5 min at room
temperature. Fluorescent signatures were then visualized using a
confocal spectral microscope (Leica SP2; Leica Microsystems GmbH,
Wetzlar, Germany).
Preparation of cell extracts and
western blot analysis
AGS cells were seeded onto 6-well plates at a
density of 5×105 cells/well for 18 h. The cells
co-cultured with PBS-resuspended H. pylori at MOI of 100
were treated with varying concentrations of eudesmin (10, 20, 40,
80 and 250 µM) or 0.1% DMSO alone (control group) for 6 h in
antibiotic-free RPMI 1640 supplemented with 10% FBS. Infected cells
were then lysed with ice-cold lysis buffer (0.5 M Tris-HCl, pH 7.4,
10% SDS and 0.5 M dithiothreitol). Protein concentration was
determined using the Bradford method (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). A total of 20 µg protein samples were loaded on
each lane and separated on 12% SDS-PAGE using the Hoefer miniVE
system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Proteins
were transferred to a Hybond-P PVDF membrane (GE Healthcare
Bio-sciences) according to the manufacturer's instructions.
Following the transfer, the membrane was washed with PBS and
blocked for 1 h at 37°C with 5% non-fat dry milk and 0.1% Tween-20
in PBS (PBST). The primary antibodies [mouse anti-β-actin (cat. no.
MAB1501; EMD Millipore, Billerica, MA, USA), rabbit anti-caspase-8
(cat. no. 25901; Abcam, Cambridge, UK), rabbit anti-BH3 interacting
domain death agonist (Bid; cat. no. 2002; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit anti-Bcl-2-associated X
protein (Bax; cat. no. 2772; Cell Signaling Technology, Inc.),
rabbit anti-cytochrome c (cat. no. SC-7159), and rabbit
anti-caspase-9 (cat. no. SC-7885) (both from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) or rabbit anti-caspase-3
(cat. no. AB1899; EMD Millipore)] were added at a dilution of
1:1,000 in PBST. Blots were washed for 5 min three times in PBST
and then incubated with the peroxidase-conjugated secondary
antibodies [horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G (IgG) (cat. no. SC-2005; Santa Cruz Biotechnology,
Inc.) or goat anti rabbit IgG (cat. no. 7074; Cell Signaling
Technology, Inc.)] at a dilution of 1:10,000 in PBS. Following
removal of the secondary antibody, blots were washed with PBST
(three times, 5 min each) and then developed using a Pierce
ECL-Western blotting substrate (Pierce; Thermo Fisher Scientific,
Inc.). Densities of the obtained immunoblots were quantified by
Kodak digital science 1D (version 2.03; Kodak, Rochester, NY,
USA).
Mouse model of H. pylori
infection
A total of 60 male C57BL/6 mice, obtained from the
National Laboratory Animal Center (Taipei, Taiwan), weighing 20–22
g, were maintained in a pathogen-free environment and used when
they reached 4 weeks of age. Mice were randomly divided into 6
groups (n=10). Mice were housed in an air-conditioned room (25±2°C)
with a relative humidity of 40–70% and were subjected to a 12-h
light/dark cycle. Mice had ad libitum access to tap water
and a standard laboratory rodent diet. All animal-based
experimental protocols were approved by the Institutional Animal
Care and Use Committee of China Medical University (approval no.
101-96-N; Taichung, Taiwan) and performed according to the ethical
rules and laws of China Medical University. Mice were fed a basal
diet (Prolab RMH 2500, 5P14; LabDiet, St. Louis, MO, USA) for 1
week prior to use in the study. To establish H. pylori
infection, all mice, apart from those in the control group, were
infected with 1×109 CFU H. pylori 26695 every
other day for a total of 3 doses using stomach tubes. All test
samples (5, 10, 20 and 40 µM eudesmin) were dissolved in water and
administered orally every day at a volume of 0.2 ml per mouse for 3
days using a stomach tube. The control group received the basal
diet, without infection and administered water instead of
treatment. The infection group was infected and received the basal
diet but without eudesmin treatment. Stomach and blood samples were
collected from all groups the day after the last treatment was
administered and subsequently, the mice were humanely sacrificed by
CO2 asphyxiation. Blood was taken directly from the
heart via microsyringe to determine the expression of IL-1b
and IgM. The stomach samples were homogenized in 1.0 ml of sterile
saline, with the aid of a tissue homogenizer, at 4°C. The
homogenates were then subjected to mRNA isolation using the total
RNA Miniprep Purification kit (GMbiolab Co., Ltd., Taichung,
Taiwan) described later on. After standing for 5 min at room
temperature, supernatant of the homogenate were processed for H.
pylori load.
ELISA evaluation of immune responses
of H. pylori infected tissue and cells
Detection of interleukin (IL)-8 in the supernatant
of H. pylori 26695 infected human AGS cells was conducted
using a human IL-8 ELISA Ready-SET-Go!® kit
(eBioscience, Inc., San Diego, CA, USA). IL-1β and immunoglobulin M
(IgM) in H. pylori infected mice blood were measured using a
mouse IL-1 β ELISA Ready-SET-Go! kit (eBioscience, Inc.) and goat
anti-mouse IgM horseradish peroxidase-conjugated antibody (cat. no.
A90-101P; Bethyl Laboratories, Inc., Montgomery, TX, USA), at a
dilution of 1:10,000 in PBS, respectively. All kits were performed
following the manual instructions. Each sample was analyzed
individually. Results were calculated as the mean of triplicate
readings and expressed as fold-change compared with the control
group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
During cell culture, AGS cells and PBS-suspended
H. pylori 26695 at an MOI ratio of 100 were co-cultured in
antibiotic-free RPMI-1640 medium supplemented with 10% FBS. The 6
concentrations of eudesmin were added to the culture and 0.1% DMSO
alone was added to the control group. Following 3 h infection,
total mRNA was isolated from the AGS cells following the method
previously described for detecting cytotoxin associated gene A
(cagA) gene (21) and
expression of the vacuolating cytotoxin A (vacA) gene
(designed in this study) was also detected. For mice models of
H. pylori infection, the total mRNA of stomach tissue sample
homogenates was isolated using the total RNA Miniprep Purification
kit (GMbiolab Co., Ltd.) and reverse transcription (RT) was
performed using the Fast-Run HotStart RT-qPCR (AMV) kit (Protech
Technology Enterprise Co., Ltd., Taipei, Taiwan). The kits were
used following the manufacturer's instructions. The oligonucleotide
primers used for RT corresponded with the murine gene sequences.
All oligonucleotide primers used were synthesized by Mission
Biotech Co., Ltd. (Taipei, Taiwan). RT-qPCR was performed at the
following conditions: 10 min at 95°C; 40 cycles of 15 sec at 95°C;
and 1 min at 60°C using 2X Power SYBR Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and 200 nM forward (F)
and reverse (R) primers (vacA F, 5′-CTGGAGCCGGGAGGAAAG-3′
and R, 5′-GGCGCCATCATAAAGAGAAATTT-3′; cagA F,
5′-ATAATGCTAAATTAGACAACTTGAGCGA-3′ and R,
5′-TTAGAATAATCAACAAACATCACGCCAT-3′; 16S RNA of H. pylori F,
5′-GTGTGGGAGAGGTAGGTGGA-3′ and R, 5′-TGCGTTAGCTGCATTACTGG-3′). Each
assay was run on an Applied Biosystems 7300 Real-Time PCR system
(Thermo Fisher Scientific, Inc.) and the fold-changes in expression
were derived using the comparative ΔΔCq method (22). 16S RNA of H. pylori served as
an internal control for sample loading and mRNA integrity, as
previously described (21).
Statistical analysis
The differences between the mean values of groups
were evaluated by one-way analysis of variance followed by Duncan's
test using SAS version 9.1 software (SAS Institute Inc., Cary, NC,
USA). The results were then presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of eudesmin on H. pylori in
vitro
The anti-H. pylori properties of eudesmin
were tested against the reference strain 26695 and clinical
isolates from H. pylori-positive patients who failed
following typical antibiotic treatment in previous studies
(19,20). All clinical isolate strains exhibited
resistance against amoxicillin, clarithromycin and metronidazole
(Table I). The MBC of eudesmin
against the tested H. pylori strains are summarized in
Tables I and II. Eudesmin exhibited the best
bactericidal activity against antibiotic resistant strain v1254
(MBC, 2.5 µM) and strain 26695 (MBC, 10 µM). The bactericidal
activity of eudesmin against Gram-negative (Pseudomonas
aeruginosa, Salmonella enterica serovar Typhimurium and
Escherichia coli) and Gram-positive bacteria
(Streptococcus aureus) were also tested (Table II). The MBCs of eudesmin against all
bacteria tested, excluding H. pylori, were >320 µM.
Eudesmin exhibited a strong bacterial activity against the
morphology of H. pylori in a dose-dependent manner (Fig. 2). Eudesmin at concentration of 250 µM
markedly damaged the architecture of H. pylori. Furthermore,
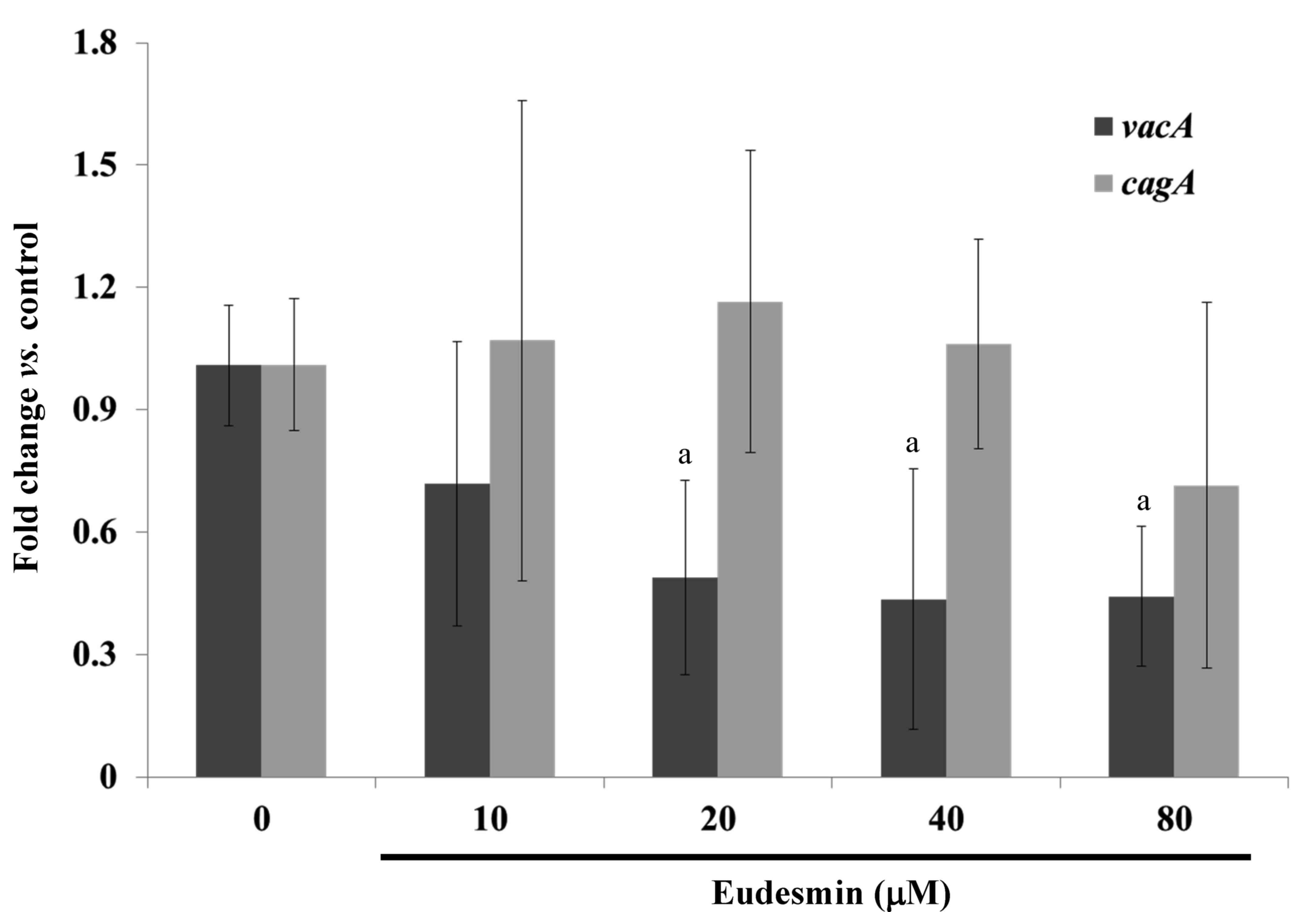
eudesmin significantly decreased the expression of vacA but
not cagA, in H. pylori at concentrations ≥20 µM
(P<0.05; Fig. 3). These genes are
well-characterized virulence factors of H. pylori.
 | Table I.Minimal bactericidal concentrations
of eudesmin, amoxicillin, clarithromycin, and metronidazole against
various H. pylori strains. |
Table I.
Minimal bactericidal concentrations
of eudesmin, amoxicillin, clarithromycin, and metronidazole against
various H. pylori strains.
|
| Minimal
bactericidal concentration of H. pylori, µM |
|---|
|
|
|
|---|
| Type of
antibiotic | Strain | 26695 | v633 | v1254 | v1354 |
|---|
| Eudesmin |
| 10 | 5 | 2.5 | 10 |
| Amoxicillin |
| 0.5 | 16 | 0.25 | 2 |
| Clarithromycin |
| 0.061 | 125 | 62.5 | 125 |
| Metronidazole |
| 15.625 | 500 | 62.5 | 250 |
 | Table II.Minimal bactericidal concentrations
of eudesmin against different gram-negative and gram-positive
bacteria. |
Table II.
Minimal bactericidal concentrations
of eudesmin against different gram-negative and gram-positive
bacteria.
|
| Minimal
bactericidal concentration, µM |
|---|
|
|
|
|---|
| Strain of
bacteria | Helicobacter
pylori | Pseudomonas
aeruginosa | Salmonella
enterica serovar Typhimurium | Escherichia
coli | Streptococcus
aureus |
|---|
| Eudesmin | 10 | >320 | >320 | >320 | >320 |
Effect of eudesmin on H
pylori-infected human AGS cells in
vitro
A cell viability assay was performed in order to
study the cytotoxicity of eudesmin. The IC50 of eudesmin
was 395 µM in AGS cells. These data suggest that eudesmin exerts
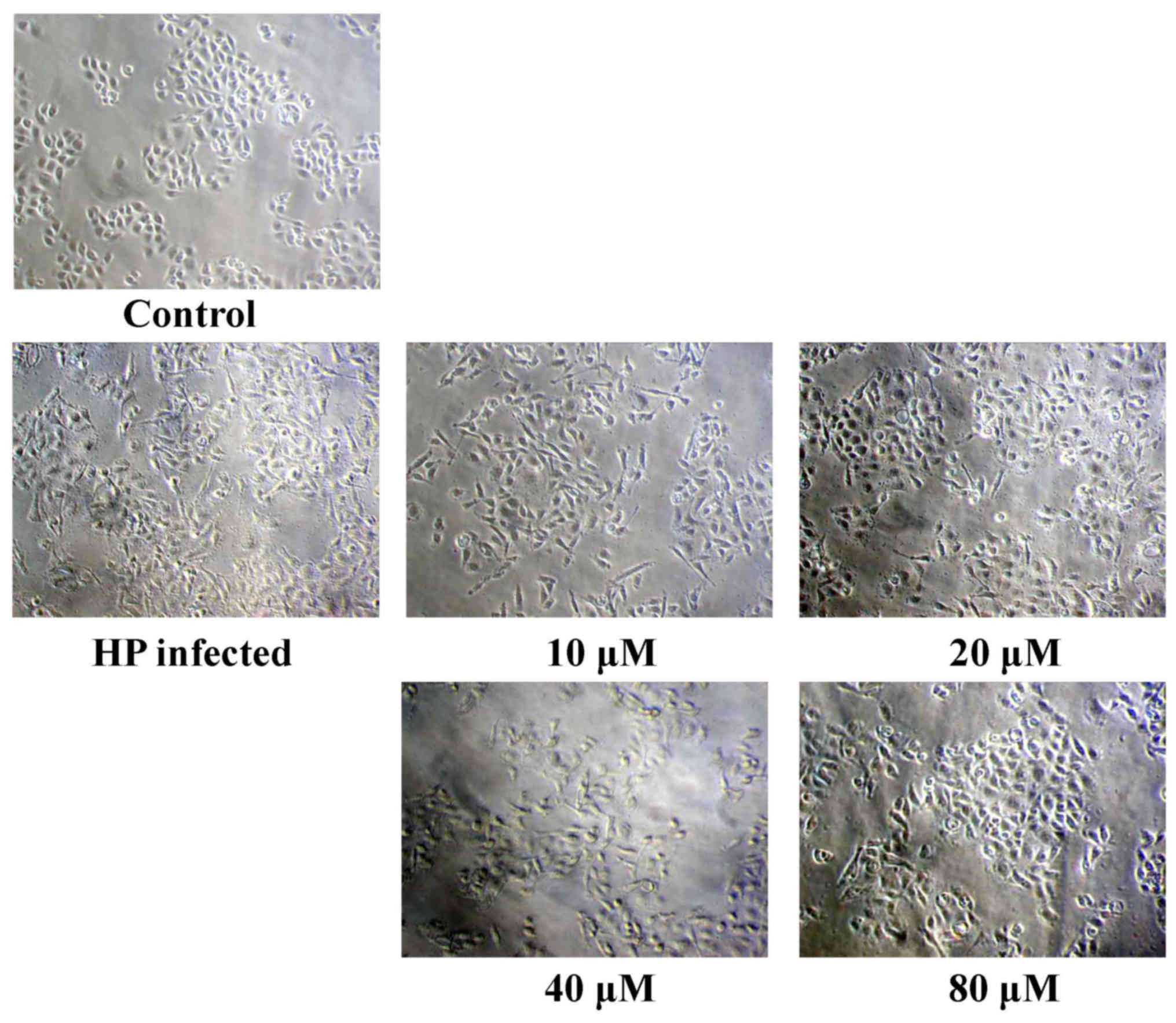
weak cytotoxic activity. The results of the present study
demonstrated that eudesmin reversed H. pylori-induced AGS
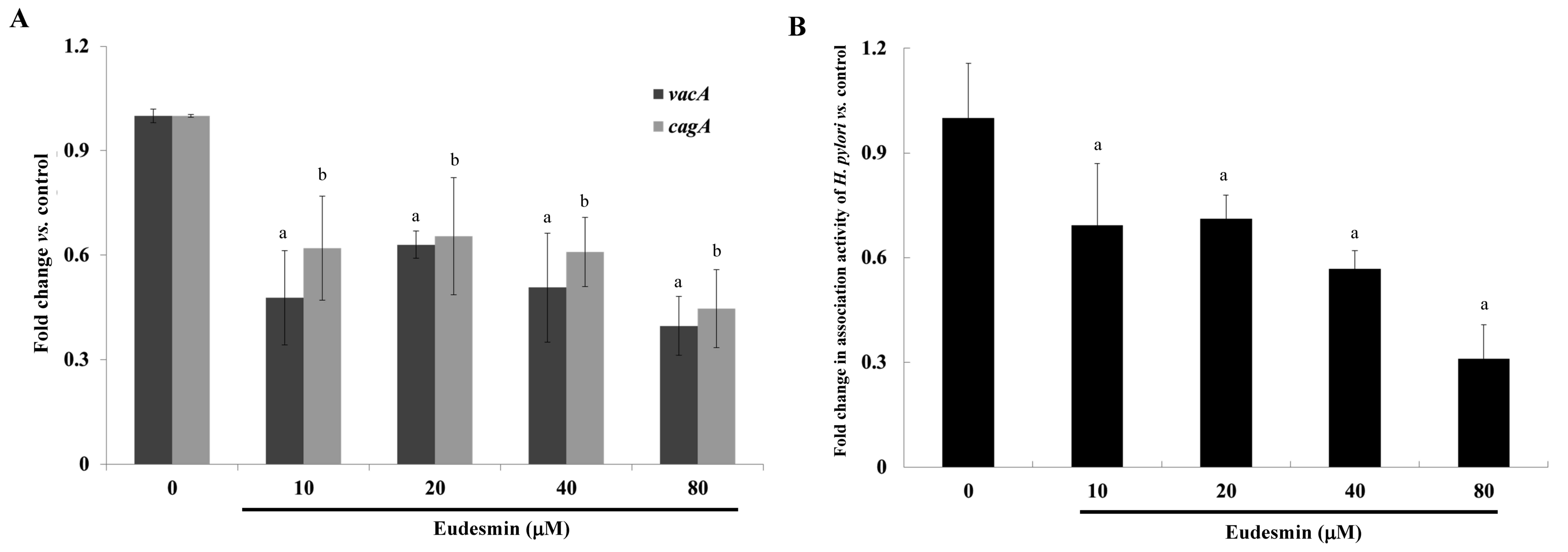
cell morphological changes, particularly at 80 µM (Fig. 4). Eudesmin significantly decreased
vacA and cagA gene expression of H.
pylori-infected AGS cells compared with those of infected mice
that did not receive treatment (P<0.05; Fig. 5A). In addition, at concentrations ≥10
µM, eudesmin significantly decreased the ability of H.
pylori to associate with AGS cells (P<0.05; Fig. 5B). Infection with H. pylori
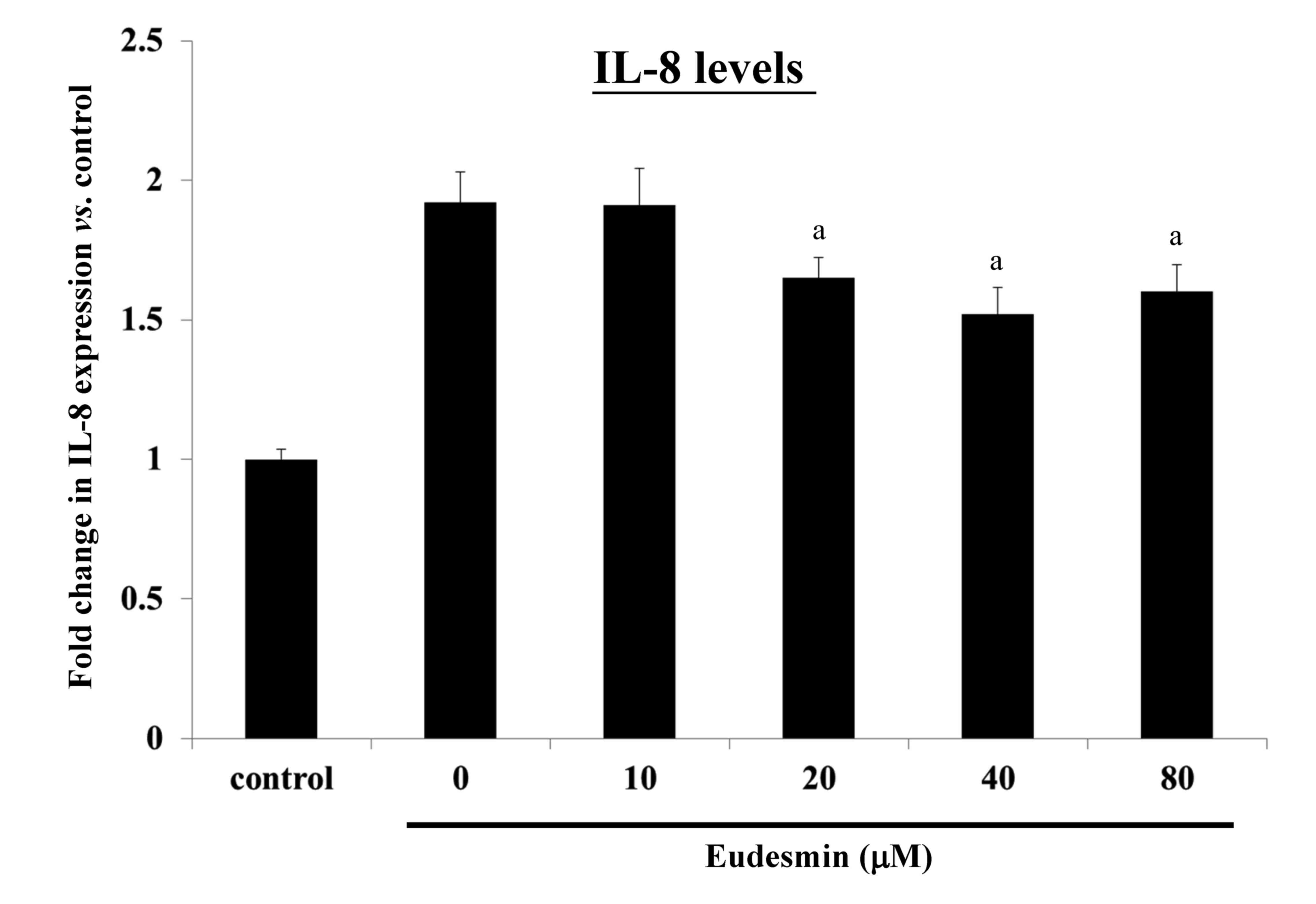
can lead to an inflammatory response, causing increased IL-8
expression. It was observed that eudesmin significantly reduced
IL-8 expression, and the inflammatory reaction, at concentrations
≥20 µM (P<0.05; Fig. 6).
Effect of eudesmin on autophagy and
apoptosis in H. pylori-infected AGS cells in vitro
The present study investigated the effect of
eudesmin on programmed cell death, through autophagy or apoptosis,
in H. pylori-infected AGS cells. Eudesmin treatment between
20 and 80 µM notably decreased autophagy-associated LC-3B protein
levels (Fig. 7). Treatment with
20–80 µM eudesmin inhibited the expression of apoptosis-associated
caspase-8, Bid, Bax, cytochrome c, caspase-9 and −3 protein
(Fig. 8). These results suggest that
eudesmin inhibits autophagy and apoptosis in H.
pylori-infected AGS cells.
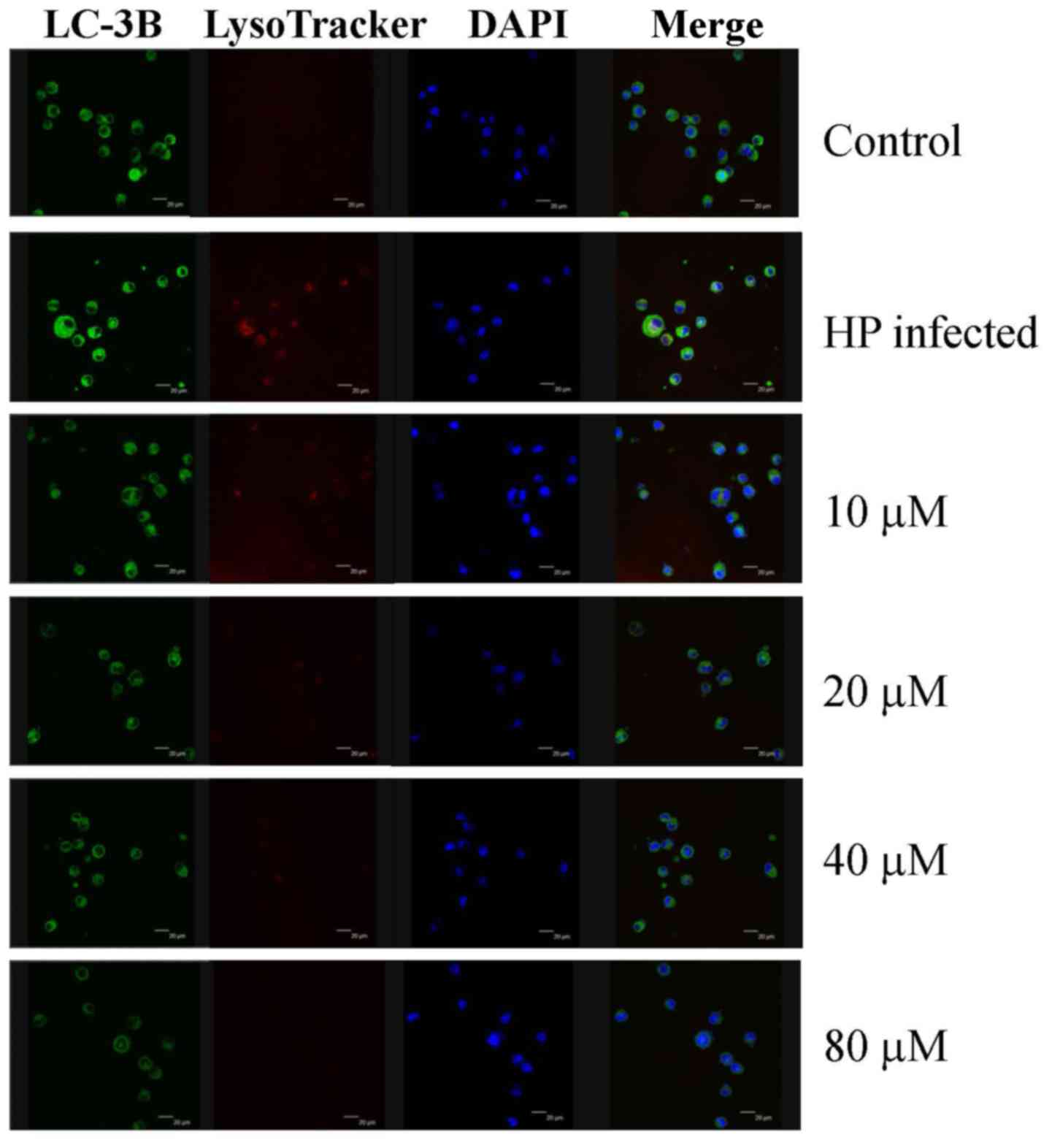 | Figure 7.Effect of eudesmin on LC-3B
expression in H. pylori-infected AGS cells. H. pylori
26695-infected AGS cells were treated with 0, 10, 20, 40 and 80 µM
of eudesmin for 12 h. Cells were fixed, then stained with LC3-B
antibody, and LysoTracker and DAPI dyes. LC-3B protein expression
was detected by immunostaining and visualized under a confocal
microscope. LC-3B, microtubule-associated protein 1A/1B-light chain
3, isoform B; DAPI, 4′,6-diamidino-2-phenylindole; HP,
Helicobacter pylori. |
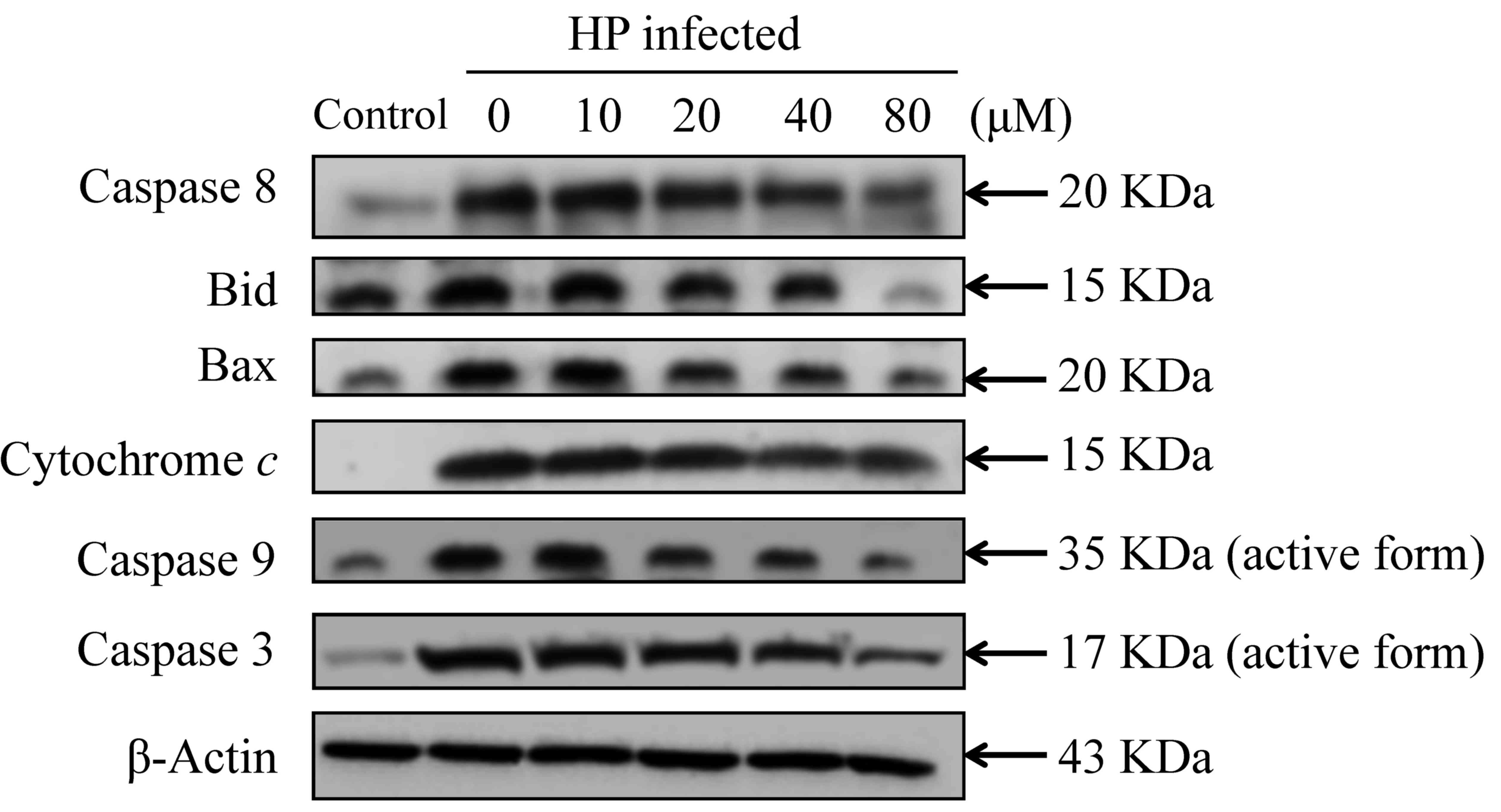 | Figure 8.Effect of eudesmin on
apoptosis-associated protein levels in H. pylori-infected
AGS cells. H. pylori 26695 infected AGS cells were treated
with 0, 10, 20, 40 and 80 µM of eudesmin for 6 h, then subjected to
western blotting. Western blotting of caspase-8, Bid, Bax,
cytochrome c, caspase-9, and −3 protein levels was
performed. The β-actin was used as an internal control for
equivalent protein loading. HP, Helicobacter pylori. |
Effect of eudesmin on a mice model of
H. pylori infection
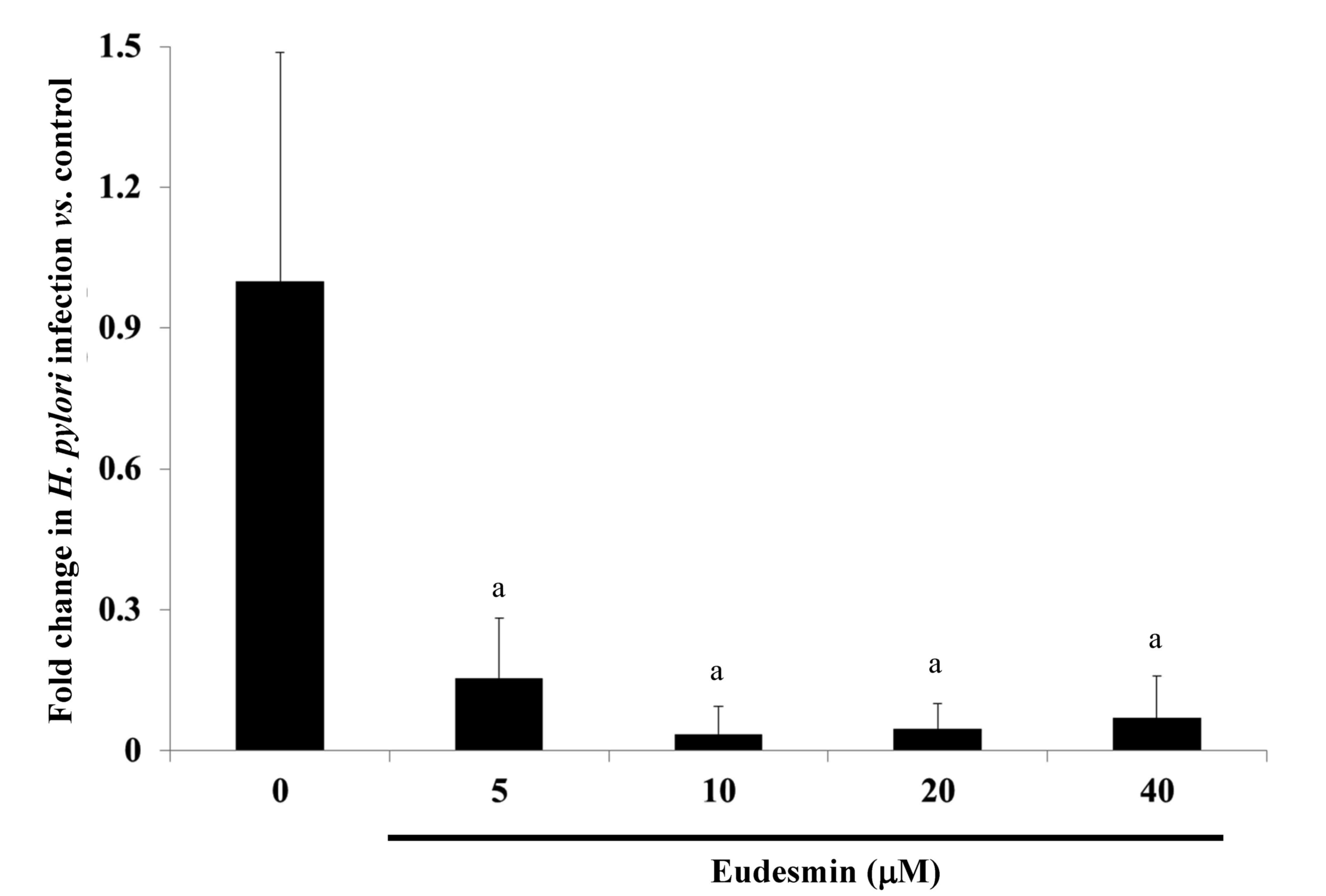
To study the effects of eudesmin in vivo,
eudesmin (5, 10, 20 and 40 µM) was used to treat H.
pylori-infected mice for 3 days. Results from the present study
identified that the lowest dose (5 µM) of eudesmin was sufficient
to significantly decrease H. pylori-load in the stomach
tissue of infected mice (P<0.05; Fig.
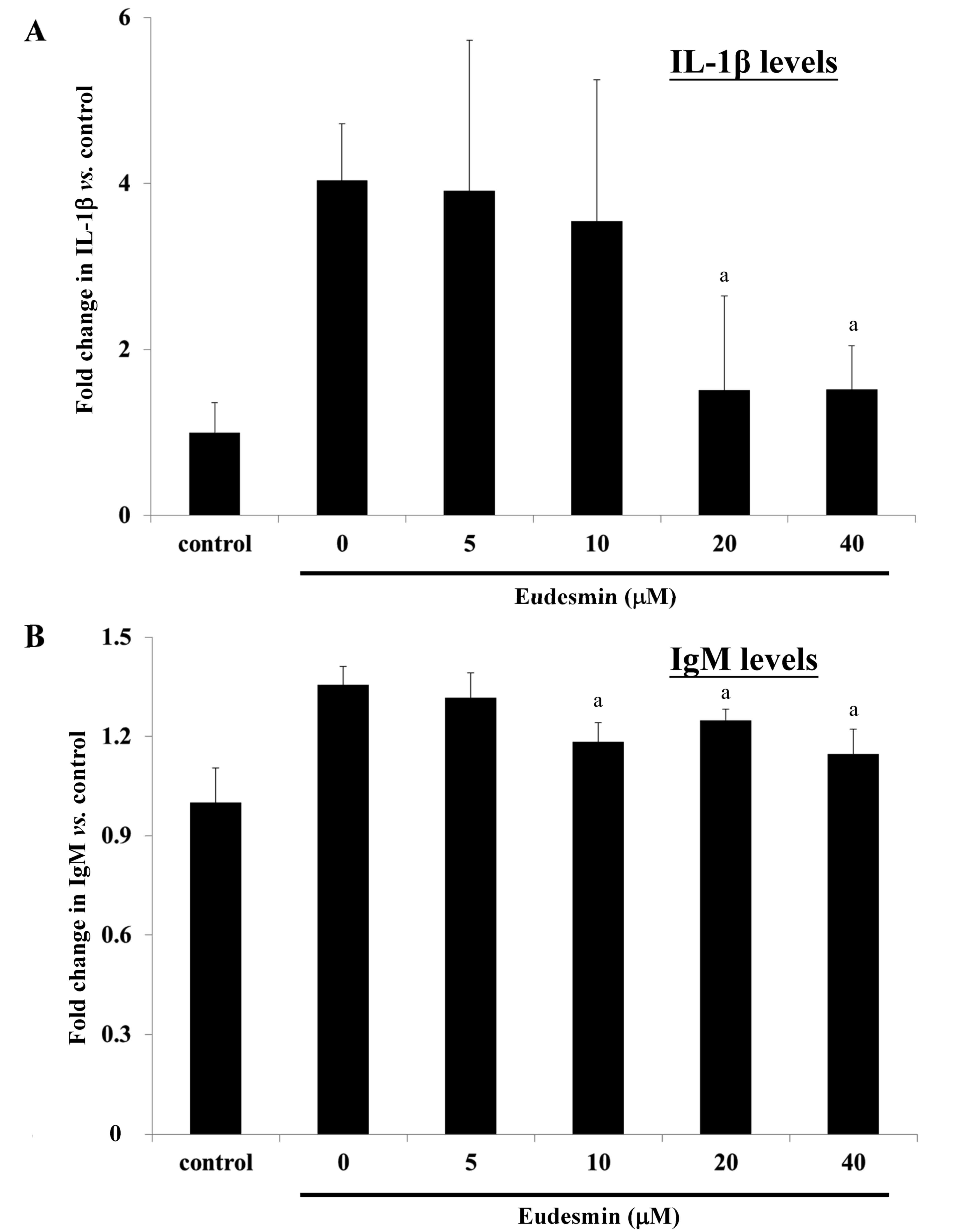
9). In addition, serum levels of IL-1β (Fig. 10A) and IgM (Fig. 10B) in H. pylori-infected mice
were significantly suppressed at concentrations of 20 and 40 µM
eudesmin, respectively (P<0.05). These data suggest that
eudesmin reduces inflammatory and immune responses to H.
pylori infection. To summarize the aforementioned results from
the in vivo and in vitro studies (Fig. 11), eudesmin may reduce the virulence
of H. pylori and also suppress H. pylori induced
inflammation, autophagy and apoptosis.
Discussion
It has previously been demonstrated that eudesmin
can be isolated from a number of different plants, including
Apiaceae, Rutaceae, Ochnaceae and
Magnoliaceae (17,18). The present study, to the best of our
knowledge, is the first to describe the isolation of eudesmin from
Fatsia polycarpa Hayata. Numerous previous studies have
identified the biological functions of eudesmin, including that its
cytotoxic, anti-bacterial, anti-fungal and inhibitory effects on
TNF-α production (13–16). However, the effects of eudesmin on
H. pylori infection have not been tested previously. The
present study, to the best of our knowledge, is the first to
investigate the effects of eudesmin on H. pylori-infected
AGS cells in vitro and to study the possible mechanisms
involved in eudesmin's anti-bacterial activity.
In the present study, the lowest MBC of eudesmin was
2.5 µM against the antibiotic resistant strain v1254 of H.
pylori. However, the IC50 of eudesmin was 395 mM in
AGS cells and the MBC of eudesmin against other common
Gram-negative and Gram-positive bacteria was >320 µM. These
results suggest that eudesmin is specifically bactericidal against
H. pylori but has low cytotoxicity. At a concentration of
250 µM, eudesmin could abolish the morphological structure of H.
pylori under visualized under a scanning electron microscope.
The mechanisms underlying the destruction of the morphology and
bactericidal activity against H. pylori by eudesmin should
be further investigated. Following the treatment of H.
pylori 26695 with eudesmin, expression of vacA, but not
cagA, was suppressed. The protease VacA is a well-known
virulence factor of H. pylori, which causes infected cells
to undergo apoptosis (23,24). In addition, VacA serves a role in
autophagy in infected cells (24).
Interestingly, in H. pylori-infected human AGS cells,
eudesmin interfered with vacA and cagA gene
expression. CagA protein is delivered into the host cell by a type
IV secretion system of H. pylori, where it induces the
expression of proinflammatory cytokines (25). Therefore, data from the present study
indicates that eudesmin can cause damage to H. pylori
bacteria and interfere with its expression of certain virulence
factors while infection occurs.
It has previously been reported that H.
pylori can induce autophagy in gastric epithelial cells and
professional phagocytes (10,11).
Deen et al (26) reported
that H. pylori infection can induce canonical autophagy in
macrophages. In addition, H. pylori has been reported to
induce gastric epithelial cell apoptosis through the activation of
the cell-surface death receptor pathway and the mitochondrial
pathway (9,10). These pathways activate caspase-3 to
initiate apoptosis. Furthermore, it has been observed that the VacA
protein of H. pylori is involved in inducing apoptosis and
autophagy in gastric epithelial cells during infection (12). Since eudesmin interferes with the
expression of VacA, the positive outcome of eudesmin treatment in
H. pylori infection models may be due to the fact that the
virulence of H. pylori is attenuated. In addition, the
present study identified that eudesmin decreased the expression of
proteins involved in apoptosis and autophagy of H.
pylori-infected AGS cells, such as LC-3B, caspase-3, caspase-9,
caspase-8, Bax, and Bid, suggesting that it suppresses H.
pylori-induced apoptosis and autophagy. Increased apoptosis is
associated with the development of gastric carcinoma. Thus,
eudesmin may prevent the development gastric carcinoma in H.
pylori-infected individuals.
H. pylori infection may result in gastritis
(1,2). The pathogenesis of gastritis involves
the host cell's inflammatory response. Inflammation is the primary
host response against microbial infections (4,5).
Multiple pathways are involved that mediate the activation of
caspases, which subsequently induce the secretion of
pro-inflammatory cytokines, such as IL-1β and IL-8 (8). IL-1β is an important pro-inflammatory
cytokine that is a powerful inhibitor of gastric acid secretion
(27). It has been demonstrated that
the expression of IL-1β in gastric mucosa is upregulated following
H. pylori infection (8) and
IL-1β may serve a central role in the initiation of the
inflammatory response to infection. Previous studies have reported
that H. pylori induces the expression of caspase-1 and IL-1β
in macrophages and dendritic cells (4,8). The
present study determined the effects of eudesmin on H.
pylori-mediated inflammation in the AGS human gastric
adenocarcinoma epithelial cell line. Eudesmin treatment efficiently
reduced IL-8 expression by AGS cells in response to H.
pylori infection. It was also observed that eudesmin decreased
IL-1β expression in a mouse model of H. pylori
infection.
The results of the present study indicate a proposed
mechanism by which eudesmin suppresses H. pylori-triggered
inflammation, autophagy and apoptosis. The results of the present
study suggest that H. pylori infection induces inflammation
in AGS cells, which results in an upregulation of IL-8 and IL-1β
in vitro and in vivo. In conclusion, the present
study demonstrates that the administration of eudesmin efficiently
eradicates H. pylori and attenuates H. pylori-induced
epithelial cell death through autophagy and apoptosis. The high
efficacy of the eudesmin treatment observed makes eudesmin a
promising novel non-antibiotic therapy of H. pylori
infection.
Acknowledgements
The present study was supported by the Department of
Chinese Medicine and Pharmacy, Ministry of Health and Welfare
(CCMP99-RD-208), Ministry of Science and Technology, Taiwan
NSC-101-2621-B-039-003, China Medical University (CMU103-S-05) and
Yen Tjing Ling Medical Foundation (CI-103-21).
References
|
1
|
de Bernard M and Josenhans C: Pathogenesis
of Helicobacter pylori infection. Helicobacter. 19 Suppl 1:S11–S18.
2014. View Article : Google Scholar
|
|
2
|
Kountouras J, Zavos C, Gavalas E and
Tzilves D: Challenge in the pathogenesis of autoimmune
pancreatitis: Potential role of Helicobacter pylori infection via
molecular mimicry. Gastroenterology. 133:368–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes
VE, Patel J, Dirden-Kramer B, Boldogh I, Ernst PB and Crowe SE:
Helicobacter pylori infection induces oxidative stress and
programmed cell death in human gastric epithelial cells. Infect
Immun. 75:4030–4039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clevers HC and Bevins CL: Paneth cells:
Maestros of the small intestinal crypts. Annu Rev Physiol.
75:289–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peek RM Jr, Fiske C and Wilson KT: Role of
innate immunity in Helicobacter pylori-induced gastric malignancy.
Physiol Rev. 90:831–858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Federico A, Gravina AG, Miranda A,
Loguercio C and Romano M: Eradication of Helicobacter pylori
infection: Which regimen first? World J Gastroenterol. 20:665–672.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nam SY, Park BJ, Ryu KH and Nam JH: Effect
of Helicobacter pylori infection and its eradication on the fate of
gastric polyps. Eur J Gastroenterol Hepatol. 28:449–454.
2016.PubMed/NCBI
|
|
8
|
Yang JC, Yang HC, Shun CT, Wang TH, Chien
CT and Kao JY: Catechins and sialic acid attenuate Helicobacter
pylori-triggered epithelial caspase-1 activity and eradicate
Helicobacter pylori infection. Evid Based Complement Alternat Med.
2013:2485852013.PubMed/NCBI
|
|
9
|
Castano-Rodriguez N, Kaakoush NO, Goh KL,
Fock KM and Mitchell HM: Autophagy in Helicobacter pylori infection
and related gastric cancer. Helicobacter. 20:353–369. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Labbé K and Saleh M: Cell death in the
host response to infection. Cell Death Differ. 15:1339–1349. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu WH, Liu TC and Mong MC: Antibacterial
effects and action modes of asiatic acid. Biomedicine (Taipei).
5:162015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raju D, Hussey S, Ang M, Terebiznik MR,
Sibony M, Galindo-Mata E, Gupta V, Blanke SR, Delgado A,
Romero-Gallo J, et al: Vacuolating cytotoxin and variants in
Atg16L1 that disrupt autophagy promote Helicobacter pylori
infection in humans. Gastroenterology. 142:1160–1171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Song Z, Liao DG, Zhang TY, Liu F,
Zhuang K, Luo K, Yang L, He J and Lei JP: Anticonvulsant and
sedative effects of eudesmin isolated from Acorus tatarinowii on
mice and rats. Phytother Res. 29:996–1003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho JY, Yoo ES, Baik KU and Park MH:
Eudesmin inhibits tumor necrosis factor-alpha production and T cell
proliferation. Arch Pharm Res. 22:348–353. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raimundo JM, Trindade AP, Velozo LS,
Kaplan MA, Sudo RT and Zapata-Sudo G: The lignan eudesmin extracted
from Piper truncatum induced vascular relaxation via activation of
endothelial histamine H1 receptors. Eur J Pharmacol. 606:150–154.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang YJ, Park JI, Lee HJ, Seo SM, Lee OK,
Choi DH, Paik KH and Lee MK: Effects of (+)-eudesmin from the stem
bark of magnolia kobus DC. var. borealis Sarg. on neurite outgrowth
in PC12 cells. Arch Pharm Res. 29:1114–1118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang CM, Chen HT, Li TC, Weng JH, Jhan YL,
Lin SX and Chou CH: The role of pentacyclic triterpenoids in the
allelopathic effects of Alstonia scholaris. J Chem Ecol. 40:90–98.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Batista AND, Batista JM, Lopez SN, Furlan
M, Cavalheiro AJ, Silva DHS, Bolzani VDS, Nunomura M and Yoshida M:
Aromatic compounds from three Brazilian Lauraceae species. Quim
Nova. 33:321–323. 2010. View Article : Google Scholar
|
|
19
|
Poon SK, Chang CS, Su J, Lai CH, Yang CC,
Chen GH and Wang WC: Primary resistance to antibiotics and its
clinical impact on the efficacy of Helicobacter pylori
lansoprazole-based triple therapies. Aliment Pharmacol Ther.
16:291–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lai CH, Kuo CH, Chen PY, Poon SK, Chang CS
and Wang WC: Association of antibiotic resistance and higher
internalization activity in resistant Helicobacter pylori isolates.
J Antimicrob Chemother. 57:466–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Kelly LK, Ayub K, Graham DY and Go
MF: Genotypes of Helicobacter pylori obtained from gastric ulcer
patients taking or not taking NSAIDs. Am J Gastroenterol.
94:1502–1507. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
da Costa DM, Pereira Edos S and Rabenhorst
SH: What exists beyond cagA and vacA? Helicobacter pylori genes in
gastric diseases. World J Gastroenterol. 21:10563–10572. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Torres J and Backert S: Pathogenesis of
Helicobacter pylori infection. Helicobacter. 13 Suppl 1:S13–S17.
2008. View Article : Google Scholar
|
|
25
|
Alzahrani S, Lina TT, Gonzalez J, Pinchuk
IV, Beswick EJ and Reyes VE: Effect of Helicobacter pylori on
gastric epithelial cells. World J Gastroenterol. 20:12767–12780.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deen NS, Huang SJ, Gong L, Kwok T and
Devenish RJ: The impact of autophagic processes on the
intracellular fate of Helicobacter pylori: More tricks from an
enigmatic pathogen? Autophagy. 9:639–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
El-Omar EM, Carrington M, Chow WH, McColl
KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N,
et al: Interleukin-1 polymorphisms associated with increased risk
of gastric cancer. Nature. 404:398–402. 2000. View Article : Google Scholar : PubMed/NCBI
|