Introduction
Osteoarthritis is a degenerative disease
characterized by joint pain, tenderness, stiffness, joint swelling,
restricted movement and joint deformities (1). In recent years, an increasing number of
patients are being diagnosed with osteoarthritis, which has a
notable impact on human health and quality of life (2,3). The
causes of osteoarthritis are complex, and the pathogenesis related
to this disease is not well understood (4). Osteoarthritis is divided into primary
and secondary osteoarthritis according to the presence of local and
systemic risk factors (5).
Osteoarthritis is frequently diagnosed as rheumatoid arthritis or
ankylosing spondylitis in clinical differential diagnosis (1,6).
Previous studies have indicated that agents targeting rheumatoid
arthritis are relatively ineffective at present (7,8).
Therefore, there is an urgent requirement for more efficient
treatments for osteoarthritis with minimal side effects.
Platelet-rich plasma (PRP) is an autologous blood
sample that contains highly concentrated platelets and multiple
cell growth factors. PRP promotes synovial cell proliferation and
differentiation and may recover cartilage morphology (9). PRP also possesses multifunctional
outcomes for the treatment of osteoarthritis, including
osteonecrosis of the femoral head, cartilage injury and rheumatoid
arthritis (9). Previous research has
suggested that PRP exhibits benefits for injurious articular
cartilage repair through the removal of harmful inflammation
factors in patients with joint diseases (5). It has previously been reported that PRP
was beneficial for rheumatoid arthritis without side effects
through inhibition of inflammatory factor levels in synovial fluid
(10). In addition,
treatment-emergent adverse effects of PRP were not systematically
reported in clinical investigation (10). The therapeutic outcomes of PRP
isolated from autologous peripheral blood mononuclear cells,
including blood products rich in cytokines, growth factors and
other bio-active molecules, has been reported to be an efficient
and innovative treatment protocol (11). Furthermore, a study by Sadabad et
al (12) investigated the
efficiency of PRP vs. hyaluronic acid for the treatment of knee
osteoarthritis. A study Khoshbin et al (13) evaluated the available Level I and
Level II literature on PRP as a therapeutic intervention in the
management of symptomatic knee osteoarthritis in a systematic
review. These reports demonstrated that intravenous injection of
PRP was able to repair tendons and damaged articular bone, and
primarily contribute to inflammatory elimination, which may have an
important role in the morphology, collagen microarchitecture and
subsequent mechanical properties of the injected vein.
Previous reports have indicated that inflammatory
cytokines have an essential role in the initiation and development
of osteoarthritis, targeting the synovium in joint diseases
(14,15). A study by Battaglia et al
(16) reported the efficacy of
ultrasound-guided intra-articular injections of PRP vs. hyaluronic
acid for hip osteoarthritis, which demonstrated that PRP was more
effective at reducing inflammation and relieving pain. Furthermore,
a study by Laudy et al (17)
demonstrated that PRP injections in patients with knee
osteoarthritis resulted in decreased pain, improved function and
global assessment, and changes regarding joint imaging. A study by
Meheux et al (18) suggested
that PRP injection significantly improved validated
patient-reported outcomes in patients with symptomatic knee
osteoarthritis at 6 and 12 months post-injection and indicated
similarities and differences in outcomes based on the PRP
formulations used in the analyzed studies. These clinical reports
suggest that PRP exhibits a potential efficacy in treatment of
osteoarthritis.
In the present study, the efficacy and outcomes of
PRP were evaluated in younger patients, aged between 18 and 30
years, with knee osteoarthritis. Inflammatory factors were analyzed
following treatment with PRP or a placebo. Treatment-emergent
adverse events in patients with knee osteoarthritis after PRP were
also investigated in the present study. Therapeutic efficacy of PRP
for knee osteoarthritis was evaluated by clinical arthritis scores.
The present findings suggested that PRP has a therapeutic effect on
knee osteoarthritis progression and highlighted its potential as an
anti-inflammatory treatment agent for knee osteoarthritis.
Materials and methods
Ethics statement
The present phase-III study (XAJT00699978) was
carried out in strict accordance with the recommendations in the
Guide for Honghui Hospital of Xi'an Jiaotong University College of
Medicine (Xi'an, China) between February 2009 and October 2014.
Ethical approval was granted by the Defense Research Committee on
the Ethics of Experiments (Honghui Hospital, Xi'an Jiaotong
University College of Medicine, Xi'an, China). All patients were
required to review trial protocols and amendments, and subsequently
provided their informed consent.
Patients
A total of 366 patients with knee osteoarthritis,
aged 18–30 years and with a Karnofsky performance status (19) ≥80% were enrolled between February
2009 and October 2014 in the present study. Patients were randomly
divided into two groups and once-weekly, double-blind trials were
conducted in Xi'an Jiangtong University College of Medicine. A
detailed description of the inclusion/exclusion criteria,
allocation method and other details can be found in previously
published studies (20,21). A total of 8 ml blood was harvested
from the cubital vein and centrifuged for 5 min at 1,500 × g.
Patients with knee osteoarthritis received PRP (2, 4, 8, 10, 12 or
14 ml) treatment through intralesional injections and a placebo was
used as a control. All patients were hospitalized throughout the
duration of the study.
Study design
The present double-blind study was carried out in
three phases: Baseline stage, double-blind treatment phase (4-week
dose-titration treatment) and 4-week post-treatment stage for
patients who volunteered to complete the ongoing extension study.
Patients were randomized to once-weekly, double-blind treatment
with PRP (2, 4, 8, 10, 12 or 14 ml) or placebo (10 ml normal
saline). The optimal dosage of PRP was determined to be 10 ml.
Enzyme-linked immunosorbent assay
(ELISA)
The plasma concentration levels of hepatocyte growth
factor (HGF; ab100687), intercellular adhesion molecule 1 (ICAM-1;
ab83760), osteopontin (OPN; ab91655), platelet-derived endothelial
cell growth factor (PD-ECGF; ab193691), vascular endothelial growth
factor (VEGF; ab119576), platelet-derived growth factor (PDGF;
ab21234), insulin-like growth factor 1 (IGF-1; ab108873),
transforming growth factor-β (TGF-β; ab92486), interferon-γ (IFN-γ;
ab177743), interleukin (IL)-6 (ab46402), IL-17A (ab83688), tumor
necrosis factor-α (TNF-α; ab181421), IL-1β and receptor activator
of nuclear factor κB ligand (RANKL; ab100749) in patients with knee
osteoarthritis were analyzed using ELISA kits (Abcam, Cambridge,
UK). All procedures were carried out according to the
manufacturer's instructions.
Magnetic resonance imaging (MRI)
scanning
MRI was performed for all subjects to assess the
therapeutic effects of PRP for knee osteoarthritis. A 3.0-T MRI
scanner (Hitachi, Ltd., Tokyo, Japan) was used to evaluate the
damaged area, joint inflammation and synovial proliferation as a
marker for disease status. All data were transferred to the
post-processing workstation. The data for the knee was recorded and
used to calculate the degree of the lesion. Clinical osteonecrosis
of the femoral head scores were evaluated using a scale of 0–2, as
previously described (22). The
degree of knee osteoarthritis in the joints was scored on a scale
of 0–5, as previously described (23).
Efficacy and safety assessments
Efficacy assessments, including the median percent
reduction scores and response rate, were analyzed in patients with
knee osteoarthritis from baseline and during the 4-week treatment
period. The median percent reduction scores were measured using the
Karnofsky score and the analysis was conducted according to
previous clinical studies (24,25).
Furthermore, assessments of the most frequent treatment-emergent
adverse events were evaluated in all randomized patients who
received the study drug and had at least one post-dose safety
assessment. Dose-response analysis was conducted after the last PRP
injection (26).
Statistical analysis
All data were presented as the mean ± standard error
of the mean. Differences between mean values were assessed using
the Student's t-test for unpaired data. Comparisons of data between
multiple groups were performed with analysis of variance followed
by the Student-Newman-Keuls test. Continuous variables were
reported as the mean with a 95% confidence interval (CI). Treatment
effect was presented as the median reduction in knee osteoarthritis
over the treatment period. Non-parametric Hodges-Lehmann estimates
of median drug treatment effects and 95% CI were provided. Response
rates and treatment-emergent adverse events were analyzed using the
χ2 test by SPSS 20.0 (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 366 patients with knee osteoarthritis
were recruited and included in the present analysis. The mean age
of patients was 24 years. All patients received either the agent
(PRP) or placebo. Patients were randomized into two groups and
treated with PRP or placebo. At baseline, mean age, body mass index
and time since knee osteoarthritis diagnosis were similar between
the two groups. The characteristics of patients with knee
osteoarthritis are summarized in Table
I. Notably, there was a higher percentage of male patients than
female patients with knee osteoarthritis. Overall, 350 patients
with knee osteoarthritis completed the maintenance period of the
phase III studies.
 | Table I.Characteristics of patients with knee
osteoarthritis. |
Table I.
Characteristics of patients with knee
osteoarthritis.
| Parameter | PRP group | Placebo group |
|---|
| Total patients
(%) | 310 (84.5%) | 56 (15.5%) |
| Sex (F/M) | 150/160 | 20/36 |
| Performance status
(Karnofsky) |
|
|
| 100 | 121 | 13 |
| 90 | 112 | 25 |
| 80 | 77 | 18 |
| Prior
treatment |
|
|
|
Surgery | 85 | 20 |
|
Antibody therapy | 103 | 18 |
|
Others | 122 | 18 |
Duration of treatment, dose-limiting
toxicity and maximum tolerated dose (MTD)
The median overall duration of PRP treatment was 8
weeks. Patients in the PRP group were treated with 2, 4, 8, 12 or
14 ml of PRP. As shown in Table II,
12 ml PRP once a week was identified as the MTD. Patients who
received at least one dose of study therapy with post-baseline
safety evaluation were included in the safety population. Following
the last dose of PRP, it was observed that the common
treatment-emergent adverse events were hypertension, diarrhea,
vomiting, lethargy, rash, proteinuria, fatigue, constipation,
weight decrease, appetite decrease, epistaxis, hypertriglyceridemia
and peripheral edema (Table II).
The most frequent treatment-emergent adverse events with a Common
Toxicity Criteria grade ≥3 were hypertension and proteinuria (≥5%
each; Table III). Accordingly,
treatment of PRP also presented a dose-dependent effect, and the
optimum therapeutic dose PRP was identified as 10 ml. The data for
the 12 (n=28) and 14 ml (n=18) doses demonstrated a high number of
adverse events, so these doses were considered inadvisable and few
patients were treated at these dose levels.
 | Table II.Treatment-related adverse events. |
Table II.
Treatment-related adverse events.
| Adverse event | Total (n=54) | PRP 2–4 ml
(n=12) | PRP 8–10 ml
(n=24) | PRP 12–14 ml
(n=18) |
|---|
| Hypertension | 11 | 2 | 5 | 4 |
| Diarrhea | 4 | 1 | 2 | 1 |
| Proteinuria | 10 | 2 | 5 | 3 |
| Vomiting | 1 | 0 | 1 | 0 |
| Lethargy | 3 | 0 | 1 | 2 |
| Rash | 7 | 2 | 4 | 1 |
| Fatigue | 3 | 0 | 2 | 1 |
| Constipation | 3 | 1 | 0 | 2 |
| Weight
decreased | 2 | 0 | 1 | 1 |
| Decreased
appetite | 1 | 0 | 0 | 1 |
| Epistaxis | 4 | 2 | 1 | 1 |
|
Hypertriglyceridemia | 2 | 1 | 1 | 0 |
| Edema
peripheral | 3 | 1 | 1 | 1 |
 | Table III.Treatment-related hypertension and
proteinuria by Common Toxicity Criteria grade. |
Table III.
Treatment-related hypertension and
proteinuria by Common Toxicity Criteria grade.
| Adverse event | Total (n=54) | PRP 2–4 ml
(n=12) | PRP 8–10 ml
(n=24) | PRP 12–14 ml
(n=18) |
|---|
| Hypertension | 11 | 2 | 5 | 4 |
| Grade
1 | 4 | 0 | 2 | 2 |
| Grade
2 | 4 | 1 | 2 | 1 |
| Grade
3 | 3 | 1 | 1 | 1 |
| Proteinuria | 10 | 2 | 5 | 3 |
| Grade
1 | 2 | 0 | 1 | 1 |
| Grade
2 | 3 | 0 | 2 | 1 |
| Grade
3 | 5 | 2 | 2 | 1 |
Pharmacokinetic analysis
In the presence of PRP, it was observed that the
majority of patients with knee osteoarthritis kept steady-state
plasma concentrations after a last dose compared with placebo
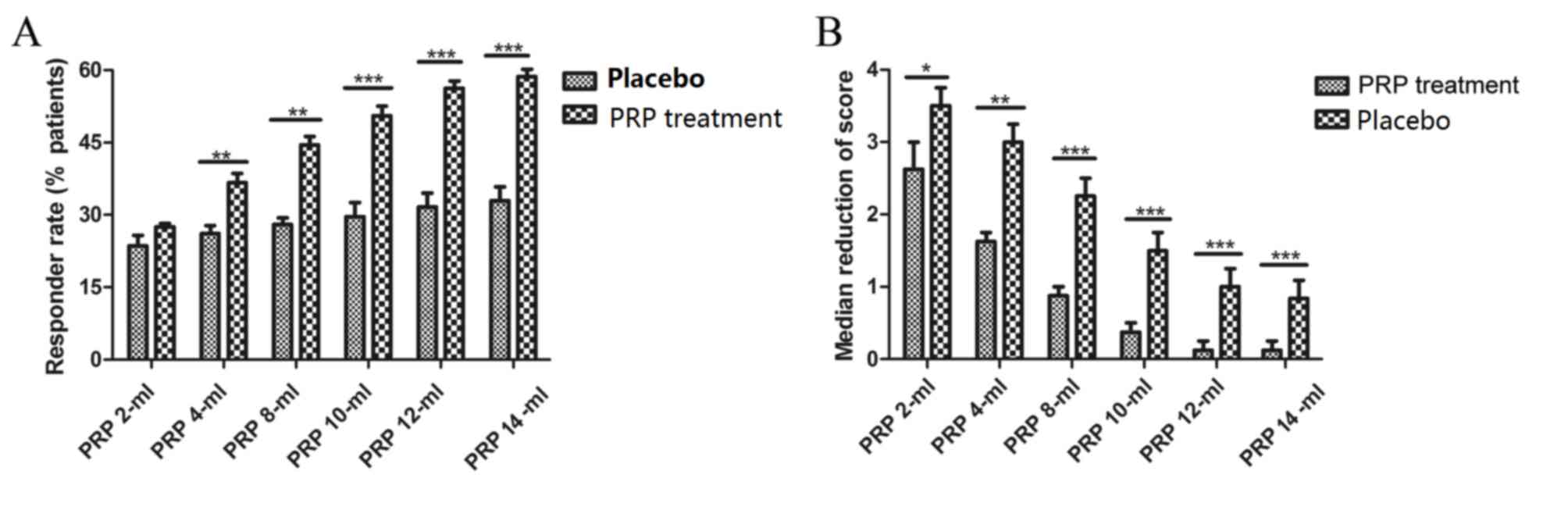
(Fig. 1A). Also, the dose response
rates were observed via changes in Karnofsky performance score. An
improvement in clinical score was observed regardless of the
presence or absence of PRP (Fig.
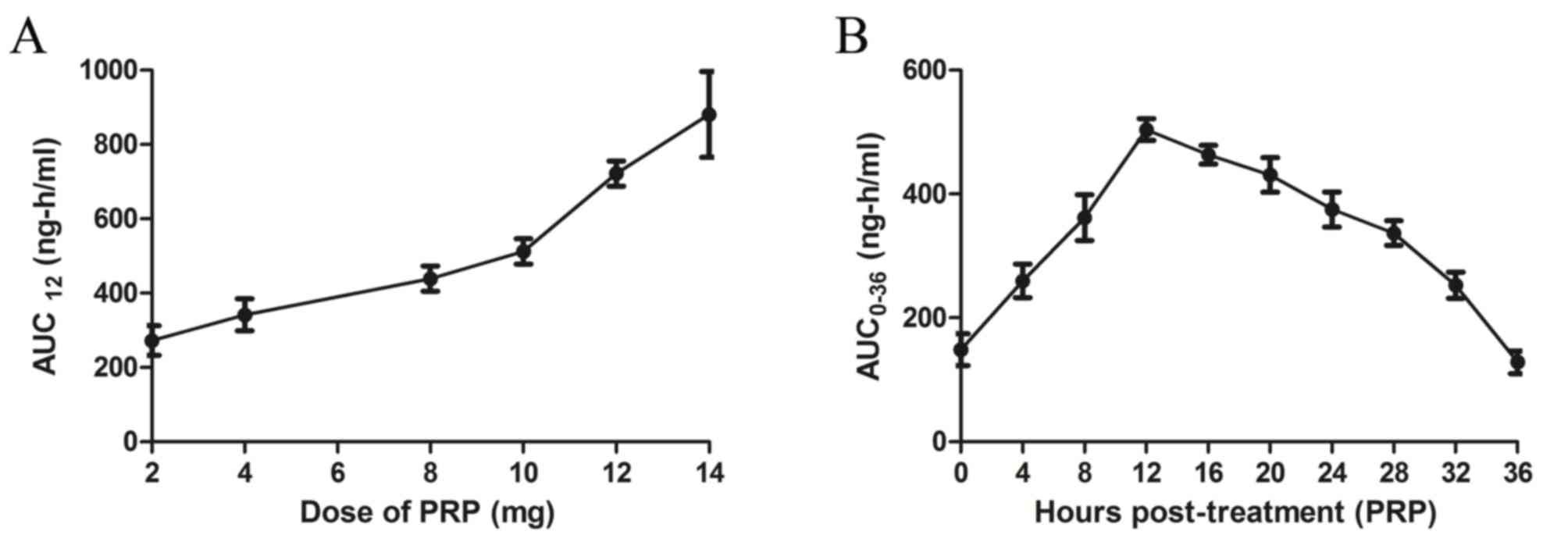
1B). In addition, plasma concentrations of PRP increased in a
dose-dependent manner in patients receiving PRP treatment (Fig. 2A). In the population pharmacokinetic
analysis, PRP plasma concentration peaked at 12 h post-treatment
(Fig. 2B).
Inflammatory factors
Plasma concentrations of inflammatory factors were
analyzed in patients treated with PRP or placebo after the last
dose of treatment. As demonstrated in Fig. 3A, plasma concentrations of IL-17A
(P<0.001), IL-1β (P<0.01), TNF-α (P<0.01), RANKL
(P<0.01), IL-6 (P<0.01) and IFN-γ (P<0.001) were
significantly downregulated after PRP treatment compared with the
placebo treatment in an 8-week observation. As demonstrated in
Fig. 3B, plasma concentrations of
HGF (P<0.001), ICAM-1 (P<0.01), OPN (P<0.01), PD-ECGF
(P<0.001), VEGF (P<0.001), PDGF (P<0.01), IGF-1
(P<0.001) and TGF-β (P<0.001) were significantly upregulated
after PRP treatment compared with the placebo treatment.
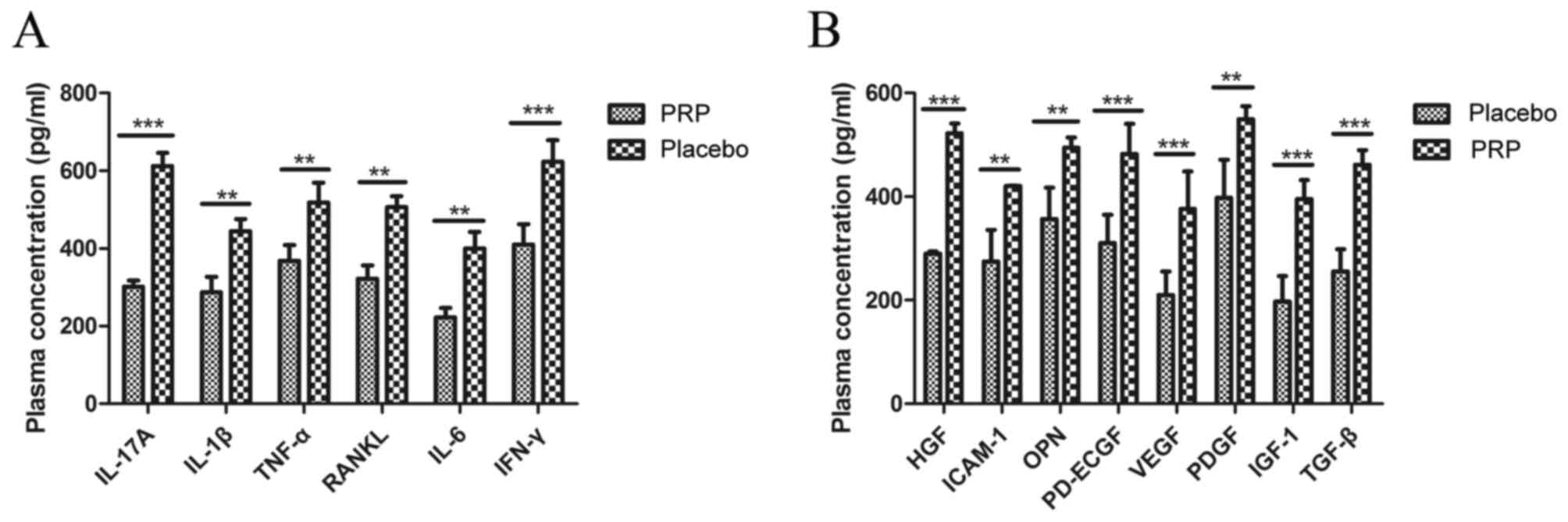 | Figure 3.Inflammatory and pro-angiogenic
factors in patients with knee osteoarthritis after treatment with
PRP (10 ml) or placebo. (A) Plasma concentrations of IL-17A, IL-1β,
TNF-α, RANKL, IL-6 and IFN-γ after an 8-week observation. (B)
Plasma concentration of HGF, ICAM-1, OPN, PD-ECGF, VEGF, PDGF,
IGF-1 and TGF-β after an 8-week observation. Data are presented as
the mean + standard error of the mean. **P<0.01 and
***P<0.001 vs. placebo. PRP, platelet-rich plasma; IL,
interleukin; TNF-α, tumor necrosis factor-α; RANKL, receptor
activator of nuclear factor κB ligand; IFN-γ, interferon-γ; HGF,
hepatocyte growth factor; ICAM-1, intercellular adhesion molecule
1; OPN, osteopontin; PD-EGCF, platelet-derived endothelial cell
growth factor; VEGF, vascular endothelial growth factor; PDGF,
platelet-derived growth factor, IGF-1, insulin-like growth factor
1; TGF-β, transforming growth factor β. |
Clinical arthritis scores
The response rates to PRP for patients with knee
osteoarthritis were evaluated by clinical arthritis scores in the
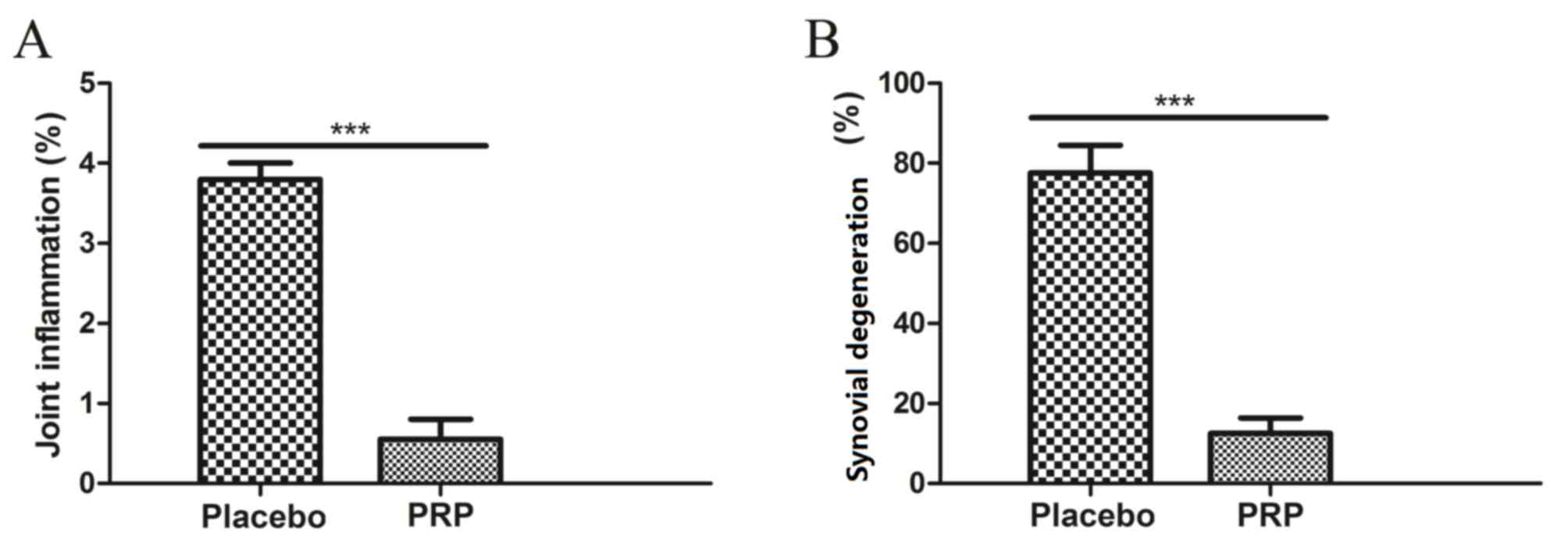
presence and absence of PRP. As demonstrated in Fig. 4A, PRP alleviated osteoarthritis and
repaired damaged tissue determined by MRI parameters as compared
with the placebo. As shown in Fig.
4B, PRP presented beneficial effects in preventing joint
inflammation and synovial proliferation compared with the
placebo.
Neovascularization and size of damaged
area
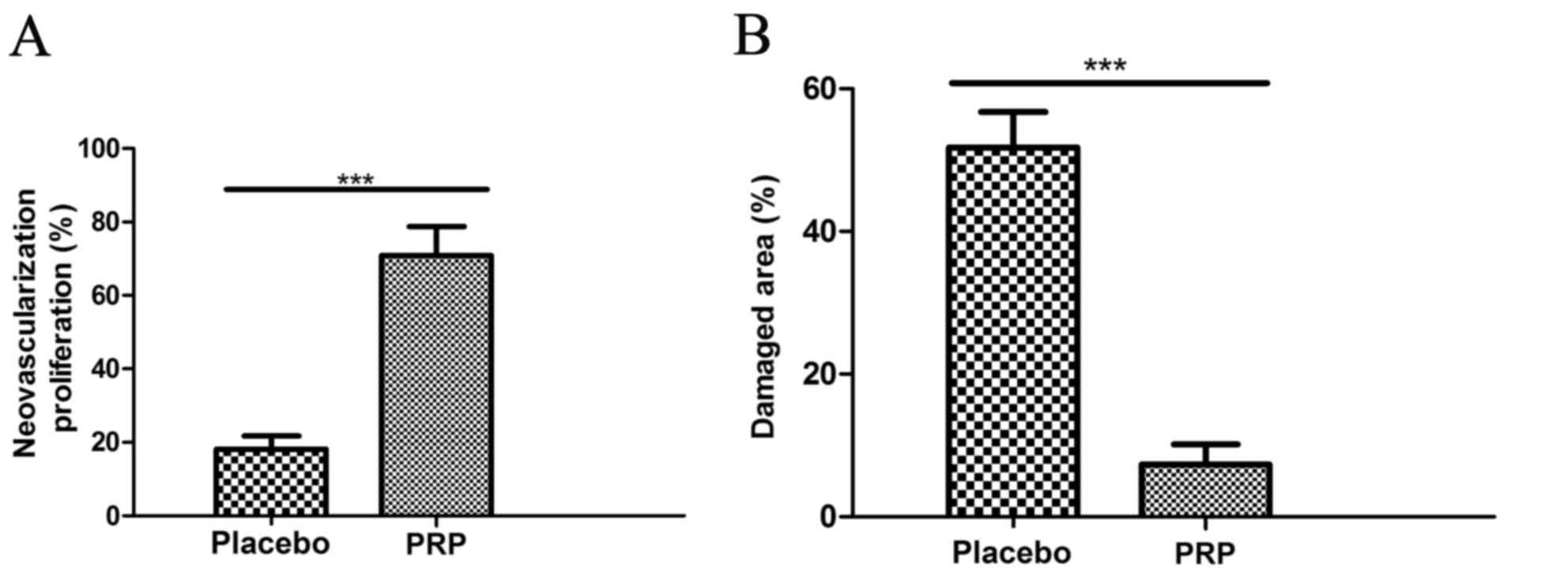
It was observed that neovascularization was
significantly promoted (Fig. 5A) and
the damaged area significantly decreased (Fig. 5B) by PRP treatment compared with the
placebo treatment (both P<0.001). These clinical outcomes
indicated that PRP at MTD 12 ml dose improved the clinical features
of knee osteoarthritis.
Discussion
The purpose of the present study was to demonstrate
the efficacy and safety of PRP in patients with knee
osteoarthritis, and in turn provide a rationale for PRP dosing
recommendations. It was observed that PRP not only alleviated
inflammation through humoral and cellular immune responses, but
also had beneficial effects on MRI parameters., which was
consistent with a previous study (20). As overall PRP therapeutic effects in
the articular environment derive from an interaction with the
pre-existing environment and other cells, and some surgical
protocols involve the application of both platelets and cells,
previous studies have investigated the effect of PRP on arthritis
of different origins (25–27). Furthermore, the present investigation
evaluated the MDT and treatment-emergent adverse events of PRP in
the treatment of patients with knee osteoarthritis. The clinical
data indicated that the most common treatment-emergent adverse
events were hypertension and proteinuria (≥10%), which was
consistent with adverse events in the overall phase II study
(27). The present results also
indicated that PRP-treated patients with knee osteonecrosis had
markedly improved synovial hyperplasia, inflammatory cell influx,
destruction of the cartilage and angiogenesis analyzed by
histological staining, as compared with the placebo-treated
patients. These preclinical data suggest that PRP may be an
effective agent for treatment of osteonecrosis of the femoral
head.
Osteoarthritis is a serious chronic degenerative
disease that affects patient health and quality of life (24). Although osteoarthritis may affect all
human joints, knee osteoarthritis is the most common type among
adolescents and adults (25). In
recent years, non-surgical treatments for knee osteoarthritis have
become more widely used, such as PRP, corticosteroid injection and
hyaluronic acid (25,28). A previous study described various
methods for knee osteoarthritis treatment, including decompression
with bone morphogenic proteins, growth factors, bone grafting and
stem cells (29). In addition, a
systematic review and meta-analysis compared the efficacy of PRP
and hyaluronic acid for treatment of knee osteoarthritis and it was
demonstrated that PRP injection was more effective than hyaluronic
acid in a 2-year meta-analysis (12). These results were supported by the
clinical outcomes of the present study in younger patients with
knee osteoarthritis.
PRP is a prominent biomedical blood product that
presents efficient outcomes for the treatment of patients with knee
osteoarthritis, cartilage disorders and rheumatoid arthritis
(30). As PRP has been approved as
an agent for knee osteoarthritis therapy, it is important for
clinicians to understand the potential pharmacokinetic interactions
in order to maximize the therapeutic benefits of PRP and reduce the
risk of treatment-emergent adverse events. The present study
revealed that repeated administration of PRP (10 ml per week)
relieved the pathogenesis of knee osteoarthritis. A previous report
indicated that inflammatory cytokines form a complex regulatory
signal network in osteonecrosis of the femoral head that is
mediated by various intracellular kinase signaling pathways to
regulate recruitment, stimulation and activation of autoimmune
cells (31). Although the causes of
knee osteoarthritis are not fully understood, laboratory and
clinical evidence has suggested that inflammatory cytokines may
contribute to its pathogenesis (32,33).
Theoretically, blocking inflammatory factor pathways may interrupt
the inflammatory process and limit joint damage (34,35). In
the present study, clinical results indicated that inflammation
factors were regulated following PRP treatment, which has not been
previously reported. Furthermore, the stimulatory effects of PRP
treatment have been demonstrated to promote proliferation and
chondrogenic differentiation, which may produce beneficial
molecules for the maintenance of articular cartilage perform
(23,36). The results of the present study
suggest that PRP treatment improves inflammatory cell influx and
angiogenesis.
Although a previous study has reported the direct
effects of various drugs and PRP on knee osteoarthritis, it is
essential to investigate the overall role of PRP in affecting the
entire joint cytokine homeostasis (37). PRP has a long half-life and therefore
is beneficial for treatment of knee osteoarthritis as PRP may be
expected to degrade slowly (32).
The results of the present study suggested that pharmacokinetic
interactions of PRP are important determinants in optimizing
therapy for knee osteoarthritis. Therefore, it is necessary for
clinicians to monitor clinical responses and tolerability when
patients are treated with PRP. In conclusion, the present findings
indicate that PRP treatment for patients with knee osteoarthritis
had beneficial effects in regulating inflammatory factors, and
alleviating joint inflammation, cartilage destruction and bone
damage.
References
|
1
|
Onuora S: Osteoarthritis: Molecular
imaging detects activated macrophages. Nat Rev Rheumatol.
12:3132016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma YW, Jiang DL, Zhang D, Wang XB and Yu
XT: Radial extracorporeal shock wave therapy in a person with
advanced osteonecrosis of the femoral head. Am J Phys Med Rehabil.
95:e133–e139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee GW, Park KS, Kim DY, Lee YM,
Eshnazarov KE and Yoon TR: Results of total hip arthroplasty after
core decompression with tantalum rod for osteonecrosis of the
femoral head. Clin Orthop Surg. 8:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ,
Eckstein F, Grago J, Boudreau RM, Englund M and Guermazi A: Partial
meniscectomy is associated with increased risk of incident
radiographic osteoarthritis and worsening cartilage damage in the
following year. Eur Radiol. 27:404–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang H, He S, Zhang X, Luo S, Zhang B,
Duan X, Zhang Z, Wang W, Wang Y and Sun Y: A network pharmacology
approach to uncover the pharmacological mechanism of XuanHuSuo
powder on osteoarthritis. Evid Based Complement Alternat Med.
2016:32469462016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poquet N, Williams M and Bennell KL:
Exercise for Osteoarthritis of the Hip. Phys Ther. 96:1689–1694.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maricar N, Callaghan MJ, Parkes MJ, Felson
DT and O'Neill TW: Clinical assessment of effusion in knee
osteoarthritis-A systematic review. Semin Arthritis Rheum.
45:556–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beumer L, Wong J, Warden SJ, Kemp JL,
Foster P and Crossley KM: Effects of exercise and manual therapy on
pain associated with hip osteoarthritis: A systematic review and
meta-analysis. Br J Sports Med. 50:458–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smyth NA, Haleem AM, Ross KA, Hannon CP,
Murawski CD, Do HT and Kennedy JG: Platelet-rich plasma may improve
osteochondral donor site healing in a rabbit model. Cartilage.
7:104–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu CJ, Sun JB, Bi ZG, Wang XM and Yang CL:
Evaluation of platelet-rich plasma and fibrin matrix to assist in
healing and repair of rotator cuff injuries: A systematic review
and meta-analysis. Clin Rehabil. 31:158–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vannini F, Di Matteo B and Filardo G:
Platelet-rich plasma to treat ankle cartilage pathology - from
translational potential to clinical evidence: A systematic review.
J Exp Orthop. 2:22015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sadabad HN, Behzadifar M, Arasteh F,
Behzadifar M and Dehghan HR: Efficacy of platelet-rich plasma
versus hyaluronic acid for treatment of knee osteoarthritis: A
systematic review and meta-analysis. Electron Physician.
8:2115–2122. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khoshbin A, Leroux T, Wasserstein D, Marks
P, Theodoropoulos J, Ogilvie-Harris D, Gandhi R, Takhar K, Lum G
and Chahal J: The efficacy of platelet-rich plasma in the treatment
of symptomatic knee osteoarthritis: A systematic review with
quantitative synthesis. Arthroscopy. 29:2037–2048. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hodge JA, Kawabata TT, Krishnaswami S,
Clark JD, Telliez JB, Dowty ME, Menon S, Lamba M and Zwillich S:
The mechanism of action of tofacitinib - an oral Janus kinase
inhibitor for the treatment of rheumatoid arthritis. Clin Exp
Rheumatol. 34:318–328. 2016.PubMed/NCBI
|
|
15
|
van der Goes MC, Jacobs JW and Bijlsma JW:
Rediscovering the therapeutic use of glucocorticoids in rheumatoid
arthritis. Curr Opin Rheumatol. 28:289–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Battaglia M, Guaraldi F, Vannini F, Rossi
G, Timoncini A, Buda R and Giannini S: Efficacy of
ultrasound-guided intra-articular injections of platelet-rich
plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics.
36:e1501–e1508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laudy AB, Bakker EW, Rekers M and Moen MH:
Efficacy of platelet-rich plasma injections in osteoarthritis of
the knee: A systematic review and meta-analysis. Br J Sports Med.
49:657–672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meheux CJ, McCulloch PC, Lintner DM,
Varner KE and Harris JD: Efficacy of intra-articular platelet-rich
plasma injections in knee osteoarthritis: A systematic review.
Arthroscopy. 32:495–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nahler G: Karnofsky performance status.
Dictionary of Pharmaceutical Medicine. 1–102. 2009. View Article : Google Scholar
|
|
20
|
Raeissadat SA, Rayegani SM, Hassanabadi H,
Fathi M, Ghorbani E, Babaee M and Azma K: Knee osteoarthritis
injection choices: Platelet-Rich Plasma (PRP) versus hyaluronic
acid (A one-year randomized clinical trial). Clin Med Insights
Arthritis Musculoskelet Disord. 8:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rodriguez-Merchan EC: Intraarticular
Injections of Platelet-rich Plasma (PRP) in the management of knee
osteoarthritis. Arch Bone Jt Surg. 1:5–8. 2013.PubMed/NCBI
|
|
22
|
Zalavras CG and Lieberman JR:
Osteonecrosis of the femoral head: Evaluation and treatment. J Am
Acad Orthop Surg. 22:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bai F, Tian H, Niu Z, Liu M, Ren G, Yu Y,
Sun T1, Li S and Li D: Chimeric anti-IL-17 full-length monoclonal
antibody is a novel potential candidate for the treatment of
rheumatoid arthritis. Int J Mol Med. 33:711–721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gobbi A, Lad D and Karnatzikos G: The
effects of repeated intra-articular PRP injections on clinical
outcomes of early osteoarthritis of the knee. Knee Surg Sports
Traumatol Arthrosc. 23:2170–2177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Filardo G, Kon E, DI Matteo B, DI Marino
A, Sessa A, Merli ML and Marcacci M: Leukocyte-poor PRP application
for the treatment of knee osteoarthritis. Joints. 1:112–120.
2014.PubMed/NCBI
|
|
26
|
Trotti A, Byhardt R, Stetz J, Gwede C,
Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T,
et al: Common toxicity criteria: Version 2.0. an improved reference
for grading the acute effects of cancer treatment: Impact on
radiotherapy. Int J Radiat Oncol Biol Phys. 47:13–47. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Battaglia M, Guaraldi F, Vannini F, Buscio
T, Buda R, Galletti S and Giannini S: Platelet-rich plasma (PRP)
intra-articular ultrasound-guided injections as a possible
treatment for hip osteoarthritis: A pilot study. Clin Exp
Rheumatol. 29:7542011.PubMed/NCBI
|
|
28
|
Jang SJ, Kim JD and Cha SS: Platelet-rich
plasma (PRP) injections as an effective treatment for early
osteoarthritis. Eur J Orthop Surg Traumatol. 23:573–580. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pierce TP, Jauregui JJ, Elmallah RK,
Lavernia CJ, Mont MA and Nace J: A current review of core
decompression in the treatment of osteonecrosis of the femoral
head. Curr Rev Musculoskelet Med. 8:228–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kilincoglu V, Yeter A, Servet E, Kangal M
and Yildirim M: Short term results comparison of intraarticular
platelet-rich plasma (prp) and hyaluronic acid (ha) applications in
early stage of knee osteoarthritis. Int J Clin Exp Med.
8:18807–18812. 2015.PubMed/NCBI
|
|
31
|
Lebouvier A, Poignard A, Cavet M, Amiaud
J, Leotot J, Hernigou P, Rahmouni A, Bierling P, Layrolle P, Rouard
H and Chevallier N: Development of a simple procedure for the
treatment of femoral head osteonecrosis with intra-osseous
injection of bone marrow mesenchymal stromal cells: Study of their
biodistribution in the early time points after injection. Stem Cell
Res Ther. 6:682015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiu WC, Lai YP and Chou MY: Humanization
and characterization of an anti-human TNF-α murine monoclonal
antibody. PLoS One. 6:e163732011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weisman MH: TNF and anti-TNF treatment in
rheumatoid arthritis (RA). What we know and what we still need to
know. Ryumachi. 37:142–143. 1997.PubMed/NCBI
|
|
34
|
Elliot MJ, Maini RN, Feldmann M, Long-Fox
A, Charles P, Katasikis P, Brennan FM, Bijl H, Ghrayeb J and Woody
JN: Treatment of rheumatoid arthritis with chimeric monoclonal
antibodies to tumor necrosis factor alpha. Arthritis Rheum. 58 2
Suppl:S92–S101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Segal B, Rhodus NL and Patel K: Tumor
necrosis factor (TNF) inhibitor therapy for rheumatoid arthritis.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 106:778–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suzuki M, Tetsuka T, Yoshida S, Watanabe
N, Kobayashi M, Matsui N and Okamoto T: The role of p38
mitogen-activated protein kinase in IL-6 and IL-8 production from
the TNF-alpha- or IL-1beta-stimulated rheumatoid synovial
fibroblasts. FEBS Lett. 465:23–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Görmeli G, Görmeli CA, Ataoglu B, Çolak C,
Aslantürk O and Ertem K: Multiple PRP injections are more effective
than single injections and hyaluronic acid in knees with early
osteoarthritis: A randomized, double-blind, placebo-controlled
trial. Knee Surg Sports Traumatol Arthrosc. 25:958–965. 2017.
View Article : Google Scholar : PubMed/NCBI
|