Introduction
The analgesic effect of opioids has been well
demonstrated in animal behavioral studies as well as human clinical
trials (1,2). Opioids are potent analgesics, which
exert their pharmacological effects by binding and activating
specific receptors in the nervous system (3,4).
Accumulating evidence has demonstrated that opioids also exert an
immunomodulatory function (5). Under
inflammatory conditions, local inflammatory cells, including
monocytes, granulocytes, lymphocytes and macrophages, synthesize
and release opioids (6). Opioids
also bind to receptors on immune cells to regulate inflammatory
cytokines, which may constitute another mechanism of opioid-induced
analgesia (7).
Rheumatoid arthritis (RA) is a chronic, systemic
type of autoimmune inflammatory arthritis, which may lead to severe
joint pain, as well as destruction of cartilage and bone (8). A large number of cytokines, including
interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α are
active in the joints of patients with RA. These cytokines have a
fundamental role in RA pathology, causing inflammation, pain and
articular destruction (9). Previous
studies have indicated that injection of opioids into knee joints
after surgery caused prolonged post-operative analgesia in patients
(10–12), which may be associated with their
immunomodulatory function. Based on these results, the present
study hypothesized that opioids also produce analgesic effects on
RA via regulating peripheral inflammation.
In the present study, a rat model of
collagen-induced arthritis (CIA) was generated. The effects of
β-endorphin on pain and inflammation in CIA rats were assessed. The
mRNA levels of TNF-α, IL-1β and IL-6 in synovial tissue and their
protein levels in inflamed tissue of the paws were also measured in
order to determine whether the analgesic effect of β-endorphin on
RA is associated with its anti-inflammatory activity.
Materials and methods
Animals
A total of 30 male Wistar rats (weight, 100–120 g;
age, 4 weeks), were purchased from the Shanghai Laboratory Animal
Center of the Chinese Academy of Sciences (Shanghai, China). Rats
were housed in temperature-controlled animal cages at 25±1°C with a
relative humidity of 55±5%, under a standard 12-h light/dark cycle
(8:00 a.m.-8:00 p.m.), with free access to food and water. All
experiments were performed between 8:00 a.m. and 4:00 p.m. All
animals were treated in accordance with the regulations of the
State Science and Technology Commission of China for the care and
use of laboratory animals (State Science and Technology Commission
order no. 2, 1988), and the protocols were approved by the Ethics
Committee of Zhejiang Chinese Medical University (Hangzhou,
China).
Induction of arthritis with
collagen
Bovine type II collagen (CII; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was dissolved overnight in 0.1 mol/l
acetic acid (2 mg/ml) at 4°C and emulsified with incomplete
Freund's adjuvant (IFA; Sigma-Aldrich; Merck KGaA) to a final
concentration of 1 mg/ml. Rats were anesthetized with 10% chloral
hydrate (350 mg/kg, intraperitoneal) (Sinopharm Chemical Reagent
Co., Ltd., Shanghai, China) and placed on a heated small animal
operating table (Harvard Apparatus, Cambridge, USA) in a prone
position. No rats exhibited signs of peritonitis. Bovine CII (2
mg/kg) was intradermally injected into six sites at the base of the
tail and back of the rats.
β-Endorphin treatment
In the experiment, a CIA model was successfully
established in total of 22 rats. These 22 CIA rats were randomly
divided into the CIA + saline group and CIA + β-endorphin group
(n=11 per group). β-Endorphin (Bachem, Bubendorf, Switzerland) was
dissolved in saline at a concentration of 5 nmol/ml. Rats were
intraperitoneally injected with 1 ml β-endorphin once every other
day from day 18 after the injection of CII until day 28. The rats
in the CIA + saline group were intraperitoneally injected with 1 ml
saline.
Tail flick latency (TFL) test
The TFL was assessed to determine thermal
hyperalgesia using a tail-flick apparatus (Ugo Basile, Comerio,
Italy). Rats were immobilized except for free tail movement. Heat
from an infrared source was administered to the tail with a
radiation intensity of 30 mW/cm2. The TFL was defined as
the time of heat exposure until withdrawal of the tail, which was
recorded by a single blinded observer. In order to avoid tissue
damage, a cut-off time of 10 sec was implemented, with this
duration being defined as representing the maximum analgesic
effect. The average TFL was obtained from three consecutive trials
with an interval of 3 min performed on 1/3 of the tail at the
distal end.
Arthritis evaluation
Paw arthritic signs characterized by edema and
erythema were inspected daily following the CII/IFA injection. To
evaluate the incidence and severity of arthritis, lesions on all
four paws of each rat were graded by using an arthritic scoring
system (13). Lesions were graded
using a scale from 0 to 4 according to the extent of edema and
erythema of the periarticular tissues; 16 was the potential maximum
of the combined arthritic scores per animal. The severity scores
were defined as follows: 0, no evidence of erythema and swelling;
1, erythema and mild swelling confined to the mid-foot (tarsals) or
ankle joint; 2, erythema and mild swelling extending from the ankle
to the foot; 3, erythema and moderate swelling extending from the
ankle to the metatarsal joints; and 4, erythema and severe swelling
encompassing the ankle, foot and digits.
Paw volume measurement
The paw volume was measured by a water displacement
plethysmometer (Ugo Basile). The hind paws were respectively
immersed in an electrolyte solution up to the boundary between the
hairy and non-hairy skin, and the volume displacement in the
chamber was determined electronically. The percentage of the paw
swelling was calculated by the following formula: %
Swelling=(Vt-V0)/V0×100, where Vt represents the paw volume after
saline/β-endorphin injection and V0 represents the basal paw
volume.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of TNF-a, IL-1β and
IL-6 in synovial tissue
Rats were anesthetized with 10% (w/v) chloral
hydrate at a dose of 350 mg/kg (intraperitoneal) on day 28 after
the injection of CII, the synovial tissues of rat knees were
isolated and removed and subsequently frozen in liquid nitrogen and
immediately stored at −80°C. No rats exhibited signs of
peritonitis. After homogenization of synovial tissue, total RNA was
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and purified with the Prime Script® RT
reagent Kit with gDNA Eraser (cat. no. PR0474A; Takara Bio Inc.,
Tokyo, Japan). Total RNA (1 µg) was then reverse-transcribed into
complementary DNA using the abovementioned kit. Gene expression of
TNF-α, IL-1β and IL-6 was analyzed by real-time qPCR using the
CFX96™ real-time PCR detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). GAPDH served as an internal control for
detecting the expression of target genes. The primers for TNF-α,
IL-1β, IL-6 and GAPDH were designed with Primer Premier 5.0
software (Premier Biosoft International, Palo Alto, CA, USA)
(primer sequences are listed in Table
I) and were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China). The PCR mixture of 20 µl contained 10 µl SsoFast EvaGreen
supermix (Bio-Rad Laboratories, Inc.), 0.3 µl sense and anti-sense
primers (400 nM), 1 µl template complementary DNA and 8.4 µl
RNase/DNase-free water. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 30 min, followed by 40
cycles of 10 sec at 95°C and 30 sec at 61.9°C. Each sample was
measured in triplicate. The generation of specific PCR products was
confirmed by melting curve and gel analysis. The expression ratio
was calculated using the 2−∆∆Cq method (14).
 | Table I.Primer sets used for polymerase chain
reaction. |
Table I.
Primer sets used for polymerase chain
reaction.
| Gene | Sense primer | Anti-sense
primer |
|---|
| IL-1β |
5′-GACCTGCTAGTGTGTGATGTTC-3′ |
5′-GCTCATGGAGAATACCACTTG-3′ |
| IL-6 |
5′-TTCCAATGCTCTCCTAATGG-3′ |
5′-CTTAGGCATAGCACACTAG-3′ |
| TNF-α |
5′-TCACACTCAGATCATCTTC-3′ |
5′-CAGCCTCCTCTCTGCCATCAAG-3′ |
| GAPDH |
5′-TGCTGAGTATGTCGTGGAG-3′ |
5′-GTCTTCTGAGTGGCAGTGAT-3′ |
ELISA for TNF-α, IL-1β and IL-6 in paw
inflammatory tissue
Samples were pulverized in liquid nitrogen, resolved
in cell lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) containing protease inhibitor cocktail (10% v/v; Bio Basic
Inc., Markham, ON, Canada), sonicated on ice (5×5 sec), stored for
1 h at 4°C, and then centrifuged at 13,201 × g for 30 min at 4°C to
obtain the protein extract. TNF-α, IL-1β and IL-6 were determined
using a Rat TNF-α ELISA Kit (cat. no. ELR-TNFalpha-001), Rat IL-1β
ELISA Kit (cat. no. ELR-IL1beta-001) and Rat IL-6 ELISA Kit (cat.
no. ELR-IL6-001; all Raybiotech Corp., Atlanta, GA, USA),
respectively.
Statistical analysis
All values are expressed as mean ± standard error of
the mean, and differences between groups were analyzed using the
independent-samples t-test. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL,
USA).
Results
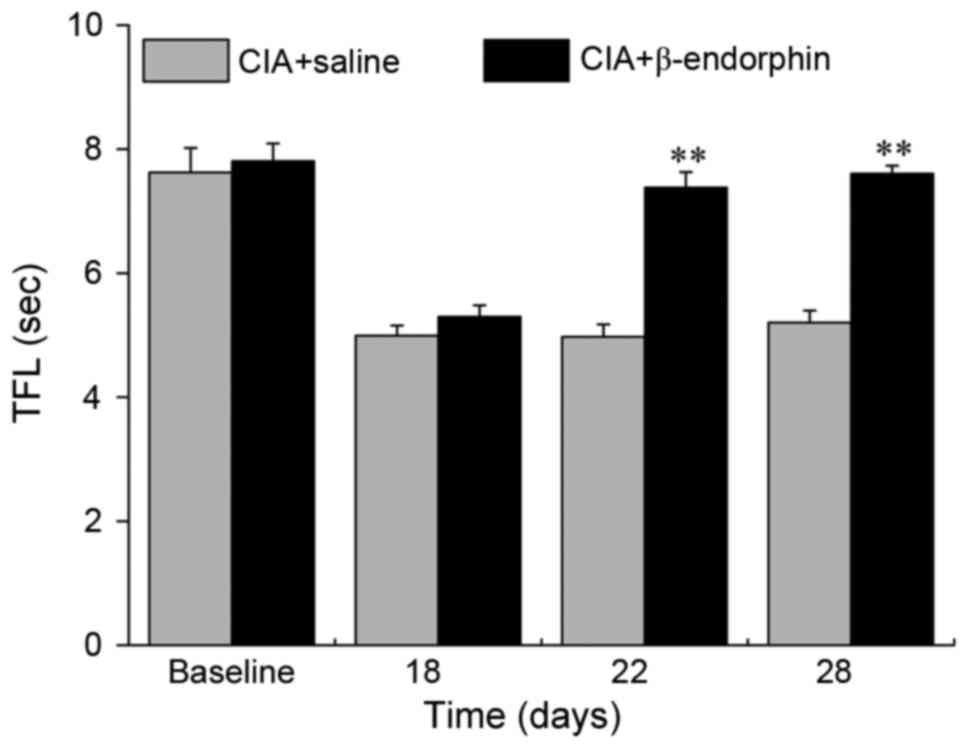
Effect of β-endorphin on TFL
The mean TFL in the CIA + saline group and CIA +
β-endorphin group is presented in Fig.
1. No significant difference in the TFL between the two groups
was identified prior to β-endorphin injection. The TFL was
significantly increased in the CIA + β-endorphin group when
compared with that in the CIA + saline group on days 22 and 28
(P<0.01).
Effect of β-endorphin on arthritis
index
As presented in Fig.
2, no significant difference in the arthritis index was present
between the CIA + saline group and the CIA + β-endorphin group on
day 18 after the injection of CII prior to β-endorphin treatment.
The arthritis index was significantly reduced in the CIA +
β-endorphin group when compared with that in the CIA + saline group
on days 22 and 28 (P<0.05 and P<0.01, respectively).
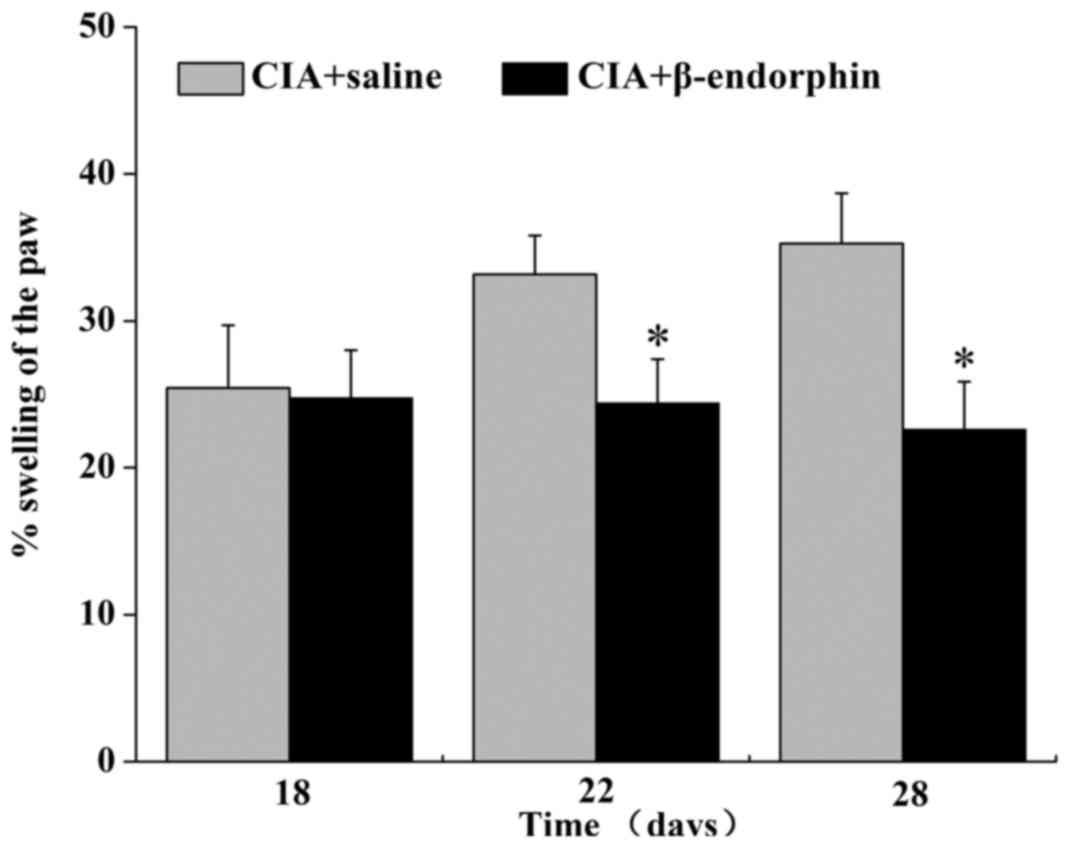
Effect of β-endorphin on paw
swelling
The results on the percentage of paw swelling are
displayed in Fig. 3. Prior to
β-endorphin treatment (day 18), no significant difference in paw
swelling was noted between the CIA + saline group and the CIA +
β-endorphin group. Of note, on days 22 and 28, paw swelling in the
CIA + β-endorphin group was significantly suppressed when compared
with that in the CIA + saline group (P<0.05).
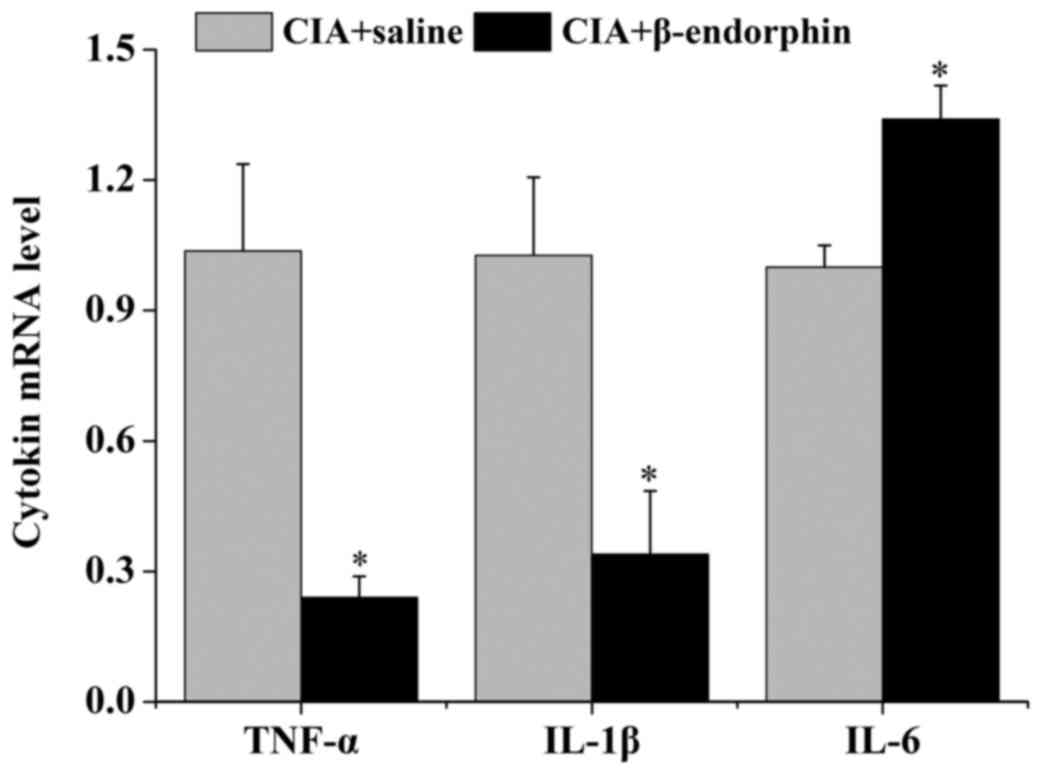
Effect of β-endorphin on TNF-α, IL-1β
and IL-6 mRNA expression in synovial tissue
As presented in Fig.
4, the mRNA levels of TNF-α and IL-1β in synovial tissue of the
CIA + β-endorphin group were significantly downregulated when
compared with those in the CIA + saline group (P<0.05) on day
28. However, β-endorphin treatment had no significant effect on the
IL-6 mRNA level of CIA rats.
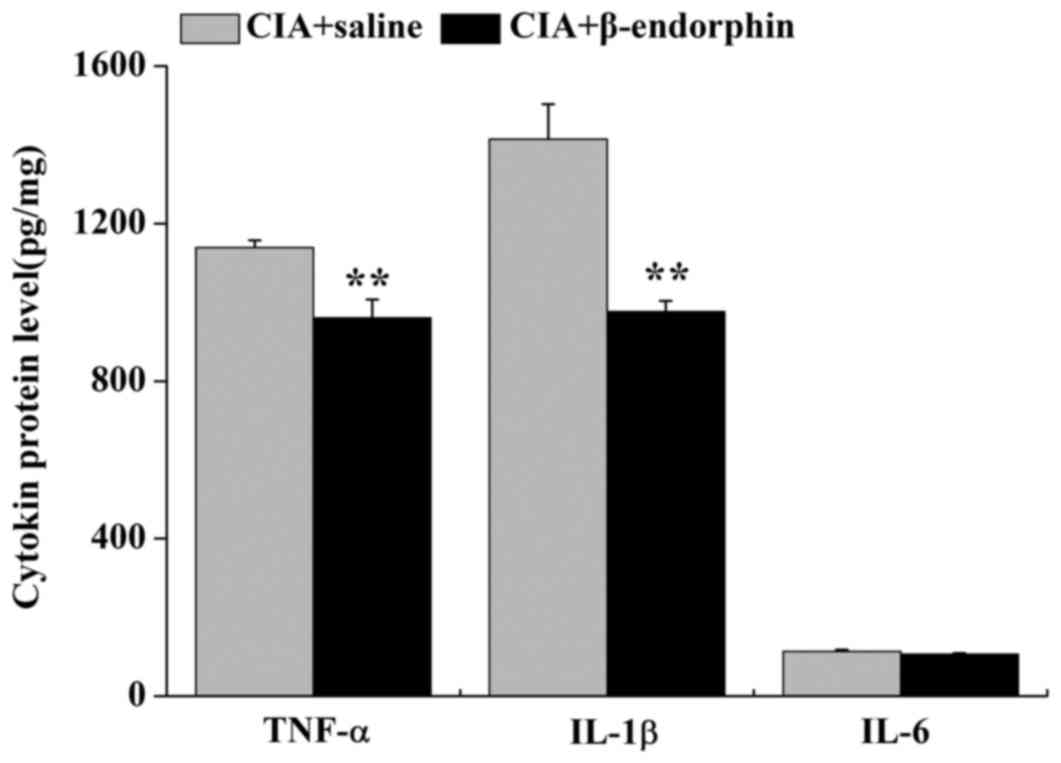
Effect of β-endorphin on the protein
levels of TNF-α, IL-1β and IL-6 in paw inflammatory tissue
As presented in Fig.
5, the protein levels of TNF-α and IL-1β in inflammatory paw
tissue of the CIA + β-endorphin group were downregulated when
compared with those in the CIA + saline group (P<0.01). No
significant difference in the protein levels of IL-6 was identified
between the CIA + saline group and the CIA + β-endorphin group.
Discussion
It is well known that opioids have a potent
analgesic effect by activating their receptors in peripheral
sensory nerve fibers and their terminals or the central nervous
system. It has been demonstrated that opioid receptors are also
distributed on immune cells (15).
In the present study, it was demonstrated that administration of
exogenous β-endorphin not only produces an analgesic effect, but
also exerts an anti-inflammatory effect in rats with CIA. It
selectively suppresses IL-1β and TNF-α in synovial tissue and paw
inflammatory tissue.
The animal model of CIA is a commonly adopted
experimental arthritis model, since its manifestations are similar
to the clinical presentation of patients with RA (16). In the present study, CIA rats
exhibited hyperalgesia, and their arthritis score and paw swelling
were increased, indicating that the collagen-induced inflammatory
pain was successfully established. The results indicated that
intraperitoneal injection of exogenous β-endorphin effectively
attenuated hyperalgesia in CIA rats. Furthermore, it also
suppressed inflammation, as indicated by lowered arthritis score
and paw swelling. Thus, it was suggested that the analgesic effect
of β-endorphin on CIA rats was not only exerted through activating
its receptors on nerve terminals, but that it may also be
associated with the intervention with the inflammatory process.
In the present study, the levels of the
pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in synovium and
paw inflammatory tissue were then determined. Numerous studies have
indicated that the physiological mechanisms of RA involve the
release of pro-inflammatory cytokines, including TNF-α, IL-1β and
IL-6 (17–20). TNF-α has a key role in the
pathogenesis of RA (17,21), which comprises its ability to induce
the production of other pro-inflammatory cytokines, including IL-1β
and IL-6. Together, these cytokines induce the production and
release of chemokines that attract leukocytes from the blood into
the inflamed tissue (22). The
present RT-qPCR and ELISA results indicated that intraperitoneal
injection of β-endorphin decreased TNF-α and IL-1β levels in the
paws and synovium, while it had no significant effect on IL-6
levels. Apart from the nervous system, opioid receptors have been
reported to be distributed on immune cells (15). The downregulation of TNF-α and IL-1β
levels by β-endorphin observed in the present study may result from
the activation of its receptor on immune cells that produced an
inhibitory effect on the inflammatory process. The reason why
β-endorphin selectively suppressed TNF-α and IL-1β but not IL-6
requires further investigation.
In summary, the present study indicated that
β-endorphin suppressed inflammation and partially downregulated
peripheral pro-inflammatory mediators in inflammatory pain. The
anti-inflammatory effect of β-endorphin may have a role in its
analgesic effect. β-Endorphin may be of great value in the control
of inflammatory pain.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81072855 and 81303039), the
Zhejiang Provincial Natural Science Foundation of China (grant nos.
Z2100979 and LY12H27015), and the key Science and Technology
Innovation Team of Zhejiang Province (grant no. 2013TD15).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ and JF designed and performed the experimental
protocols. XH, XY, YS and YW performed the animal experiments. LH
performed ELISA, SQ performed RT-qPCR, XH wrote the initial draft
of the manuscript and performed statistical analysis and image
acquisition. YJ and JF supervised the data analysis, manuscript
design and revisions.
Ethical approval and consent to
participate
The study protocols were approved by the Ethics
Committee of Zhejiang Chinese Medical University (Hangzhou,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stein C: The control of pain in peripheral
tissue by opioids. N Engl J Med. 332:1685–1690. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein C and Yassouridis A: Peripheral
morphine analgesia. Pain. 71:119–121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machelska H, Cabot PJ, Mousa SA, Zhang Q
and Stein C: Pain control in inflammation governed by selectins.
Nat Med. 4:1425–1428. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rittner HL, Machelska H and Stein C:
Leukocytes in the regulation of pain and analgesia. J Leukoc Biol.
78:1215–1222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hale KD, Ghanta VK, Gauthier DK and
Hiramoto RN: Effects of rotational stress of different duration on
NK cell activity, proinflammatory cytokines, and POMC-derived
peptides in mice. Neuroimmunomodulation. 9:34–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mousa SA, Shakibaei M, Sitte N, Schäfer M
and Stein C: Subcellular pathways of beta-endorphin synthesis,
processing, and release from immunocytes in inflammatory pain.
Endocrinology. 145:1331–1341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walker JS: Anti-inflammatory effects of
opioids. Adv Exp Med Biol. 521:148–160. 2003.PubMed/NCBI
|
|
8
|
Lee DM and Weinblatt ME: Rheumatoid
arthritis. Lancet. 358:903–911. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brennan FM and McInnes IB: Evidence that
cytokines play a role in rheumatoid arthritis. J Clin Invest.
118:3537–3545. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stein C, Comisel K, Haimerl E, Yassouridis
A, Lehrberger K, Herz A and Peter K: Analgesic effect of
intraarticular morphine after arthroscopic knee surgery. N Engl J
Med. 325:1123–1126. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joshi GP, McCarroll SM, McSwiney M,
O'Rourke P and Hurson BJ: Effects of intraarticular morphine on
analgesic requirements after anterior cruciate ligament repair. Reg
Anesth. 18:254–257. 1993.PubMed/NCBI
|
|
12
|
Whitford A, Healy M, Joshi GP, McCarroll
SM and O'Brien TM: The effect of tourniquet release time on the
analgesic efficacy of intraarticular morphine 1after arthroscopic
knee surgery. Anesth Analg. 84:791–793. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song HP, Li X, Yu R, Zeng G, Yuan ZY, Wang
W, Huang HY and Cai X: Phenotypic characterization of type II
collagen-induced arthritis in Wistar rats. Exp Ther Med.
10:1483–1488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coggeshall RE, Zhou S and Carlton SM:
Opioid receptors on peripheral sensory axons. Brain Res.
764:126–132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang M, Xiao C, Wu Q, Niu M, Yao Q, Li K,
Chen Y, Shi C, Chen D, Feng G and Xia C: Anti-inflammatory effect
of Sanshuibaihu decoction may be associated with nuclear
factor-kappa B and p38 MAPK alpha in collagen-induced arthritis in
rat. J Ethnopharmacol. 127:264–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dayer JM, Beutler B and Cerami A:
Cachectin/tumor necrosis factor stimulates collagenase and
prostaglandin E2 production by human synovial cells and dermal
fibroblasts. J Exp Med. 162:2163–2168. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Furst DE, Breedveld FC, Kalden JR, Smolen
JS, Burmester GR, Bijlsma JW, Dougados M, Emery P, Keystone EC,
Klareskog L and Mease PJ: Updated consensus statement on biological
agents, specifically tumour necrosis factor {alpha} (TNF{alpha})
blocking agents and interleukin-1 receptor antagonist (IL-1ra), for
the treatment of rheumatic diseases, 2005. Ann Rheum Dis. 64 Suppl
4:iv2–i14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arend WP, Malyak M, Guthridge CJ and Gabay
C: Interleukin-1 receptor antagonist: Role in biology. Annu Rev
Immunol. 16:27–55. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kishimoto T: Interleukin-6: From basic
science to medicine-40 years in immunology. Annu Rev Immunol.
23:1–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bertolini DR, Nedwin GE, Bringman TS,
Smith DD and Mundy GR: Stimulation of bone resorption and
inhibition of bone formation in vitro by human tumour necrosis
factors. Nature. 319:516–518. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brennan FM, Chantry D, Jackson A, Maini R
and Feldmann M: Inhibitory effect of TNF alpha antibodies on
synovial cell interleukin-1 production in rheumatoid arthritis.
Lancet. 2:244–247. 1989. View Article : Google Scholar : PubMed/NCBI
|