Introduction
The use of contact lenses has increased due to the
various visual benefits they provide, including the convenience of
not wearing glasses, the possibility of a fully corrected visual
field and the total correction of refractive errors in cases of
anisometropic amblyopia (1). Despite
recent advances in lens composition materials that allow increased
corneal oxygenation, the use of contact lenses with low oxygen
transmissibility (low Dk/L) remains common in Mexico, since these
lenses are markedly less expensive (2). Additionally, patients who wear lenses
continuously without removing them overnight commonly seek
consultation for various complaints, such as red eye, discomfort,
tearing and reduced visual clarity (3).
There is evidence that contact lenses with low
oxygen transmissibility limit the oxygen flow to the cornea
(4,5), thus increasing the production of lactic
acid and decreasing the local pH (6,7). It has
also been suggested that the continuous use of the contact lens
induces an inflammatory response (8–10). The
study by Thakur and Willcox (11)
evaluated the alterations of proinflammatory cytokines levels in
tear fluid induced by continuous contact lens use for 6 days in
patients who developed contact lens-associated acute red eye or
contact lens peripheral ulcer, and observed an elevation in
interleukin (IL)-1β and interleukin 8 (IL-8) levels in these
patients. By contrast, in NACLWs who wore soft lenses continually
for 8 h (overnight) for the first time, a decrease in the
concentrations of IL-8, leukotriene B4 and IL-6 was observed
(12).
Although prolonged contact lens use is highly
atypical in developed countries, the significance of the present
study originates from the fact that the population investigated had
various characteristics that made wearing contact lenses
continuously for long periods a common habit, including a low
socioeconomic status, poor patient education and self-neglect
(13). To the best of our knowledge,
there are currently no studies in the literature analyzing the
alterations in the tear fluid composition induced subsequent to a
month of continuous use of contact lenses. Therefore, the present
study aimed to evaluate the effects of continuous contact lens use
on the tear fluid composition after 1 day, 1 week and 1 month in
NACLWs.
Patients and methods
Study design, patients and tear sample
collection
The present prospective, nonrandomized clinical
trial involved NACLWs who had continuously worn contact lenses
(Soft Lens 59, Bausch & Lomb, Rochester, New York, US) for the
first time for the duration of 1 day, 1 week and 1 month and 21
non-contact lens users as a control group (age range, 18–23 years;
mean age, 21.3 years; 11 males/10 females). NACLWs were assigned to
one of 3 groups. In group 1 (n=21; age range 18–23 years; mean age
21.2 years; 10 males/11 females), patients continuously wore
contact lenses for 1 day. In group 2 (n=21; age range 19–22 years;
mean age 21.3 years; 10 males/11 females), patients continuously
wore contact lenses for 1 week. In group 3 (n=21; age range 17–22
years; mean age 21.4 years; 11 males/10 females), patients
continuously wore contact lenses for 1 month.
All patients were evaluated by an ophthalmologist in
the Department of Anterior Segment of the Didactic Medical Unit in
the Autonomous University of Aguascalientes (Aguascalientes,
Mexico) during the period January 2015 to December 2015.
Patients underwent slit-lamp examination to exclude
any anterior segment condition, including conjunctivitis,
blepharitis, keratitis and/or corneal ectasia. Dry eye was
ruled-out in all patients using the Schirmer's test (HUB
Pharmaceutical, LLC. Rancho Cucamonga, CA, USA), tear breakup time
by Fluorescein Sodium Ophthalmic Strips Bio-Glo (HUB
Pharmaceutical, LLC) and Lissamine™ green strips (HUB
Pharmaceutical, LLC). All patients with systemic diseases
(diabetes, hypertension, hepatic and/or autoimmune disease) were
excluded.
Tear samples were collected at the end of the period
contact lenses were worn for during a three-hour period (between
9:00 a.m. and 12:00 p.m.) from the lower tear meniscus without
touching the eye or eyelids using a hand-directed 1 ml
BRAND® pipette bulb (cat no. BR747775; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). In total, a sample of 100 µl was
obtained per collection. Immediately after collection, the samples
were centrifuged at 14,000 × g for 1 min at 4°C to remove cellular
debris (Biorad Z216 MK; Siemensstraße, Wehingen, Germany) and
stored at −20°C for later processing.
Each patient provided written informed consent. All
experiments were conducted according to the Declaration of Helsinki
standards, and approved by the Medical Ethics Committee of the
Autonomous University of Aguascalientes Research Division.
pH determination
The tear fluid pH was determined by a colorimetric
method (14) using bromothymol blue
(3′,3′-Dibromothymolsulfonphthalein; cat no. 114413; Sigma-Aldrich;
Merck KGaA) as an indicator, which has ionic activity in the pH
range between 6.0 and 8.0. Color alterations occur as the pH
increases or decreases, ranging from yellow at pH 6.0 to blue at pH
8.0 (Fig. 1).
Measurement of IL-8, IL-1β and
interferon (IFN)-γ
Specific ELISA kits were employed to determine the
tear levels of various proteins, including IFN-γ (cat. no.
900-k27), IL-1β (cat. no. 900-k47.) and IL-8 (cat. no. 900-k18; all
obtained from Peprotech, Inc., Rocky Hill, NJ, USA). Briefly,
96-well plates were coated with the primary antibody at a
concentration of 0.5 µg/ml (1:200) prior to being incubated
overnight at room temperature. Following aspiration and washing (4
times) of wells 300 µl blocking buffer (1% bovine serum albumin in
PBS) was then added and the plate was incubated for 1 h. Following
aspiration and washing, the standard solution or 10 µl of tear
sample was added to wells in duplicate and incubated for 2 h. Then
the plate was washed 4 times and the detection secondary antibody
(biotinylated) at 0.5 µg/ml was added and incubated during 2 h at
room temperature. After that, avidin peroxidase (1:2,000) from the
same ELISA kit was added and incubated for 30 min at room
temperature, followed by the addition of 100 µl 2,2-azine-bis
(3-ethylbenzothiazoline-6-sulfonic acid). The absorbance was
subsequently measured at 415 nm with the wavelength correction set
at 650 nm in an iMark™ microplate absorbance reader
spectrophotometer (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Finally, the cytokine levels were calculated by linear regression
analysis.
Determination of enzymes, total
protein (TP), electrolytes and osmolarity
To determine the concentrations of TP, sodium
(Na+), chloride (Cl−), potassium
(K+) and calcium (Ca2+), as well as the
enzyme activity of aspartate aminotransferase (AST), alanine
aminotransferase (ALT), alkaline phosphatase (AP) and lactate
dehydrogenase (LDH) in the tear fluid, dry chemistry techniques
were used. The analyses were performed using the automatized VITROS
5600 Immunodiagnostic system (Ortho Clinical Diagnostics, Raritan,
NJ, USA). The following analytes were used: 309 Na+ Sodium, 307
Cl-Chloride, 308 K+ Potassium, 318 Ca+ Calcium 320, AST Aspartate
Aminotransferase, 322 ALT Alanine Aminotransferase, 321 ALKP
Alkaline Phosphatase, 323 LDH Lactate Dehydrogenase, according to
the manufacturer's protocols. These equipment and reagents were
approved by the Food and Drug Administration (Silver Spring, MD,
USA). Briefly, the method for electrolytes quantitation was based
on direct potentiometry (Na+, K+, Cl-) or colorimetry (Ca++). For
Na+, K+ and Cl- a drop of patient sample (10 µl) and a drop of
VITROS Reference Fluid (10 µl) were collocated on separate halves
of the slide resulting in migration of both fluids toward the
center of the paper bridge. A stable liquid junction was formed
that connected the reference electrode to the sample electrode.
Each electrode produced an electrochemical potential in response to
the activity of the ion. The potential difference between the two
electrodes was proportional to the Na+, K+ and Cl-concentration in
the sample. For calcium, a drop of patient sample was deposited on
the slide and was evenly distributed by spreading the layer to the
underlying layers. The bound calcium was dissociated from binding
proteins, allowing the calcium to penetrate through the spreading
layer into the underlying reagent layer. There, the calcium formed
a complex with Arsenazo III dye, causing a shift in the absorption
maximum. Following incubation, the reflection density of the
colored complex was measured spectrophotometrically with an
automatized VITROS 5600 Immunodiagnostic system (Ortho Clinical
Diagnostics, Raritan, NJ, USA). The amount of colored complex
formed was proportional to the calcium concentration in the sample.
Quantitation of ALT, AST, AP and LDH was performed by
spectrophotometry (automatized VITROS 5600 Immunodiagnostic system;
Ortho Clinical Diagnostics, Raritan, NJ, USA) in a test of multiple
points where the rate of change in reflection density was
proportional to enzyme activity at 340 nm (ALT and LDH), 670 nm
(AST) or 400 nm (AP).
The osmolarity was calculated using the following
formula: Osmolarity (mOsm/l) = 2 × (Na+ +
K+).
Statistical analysis
Statistical analysis and graphs were performed using
the GraphPad Prism version 6.0 software (GraphPad Software, Inc.,
San Diego, CA, USA). Descriptive analysis data are reported as the
mean ± standard deviation. For inferential analysis, repeated
measures analysis of variance and Dunnett's as post-hoc test were
conducted. P<0.05 was considered to indicate a statistically
significant difference.
Results
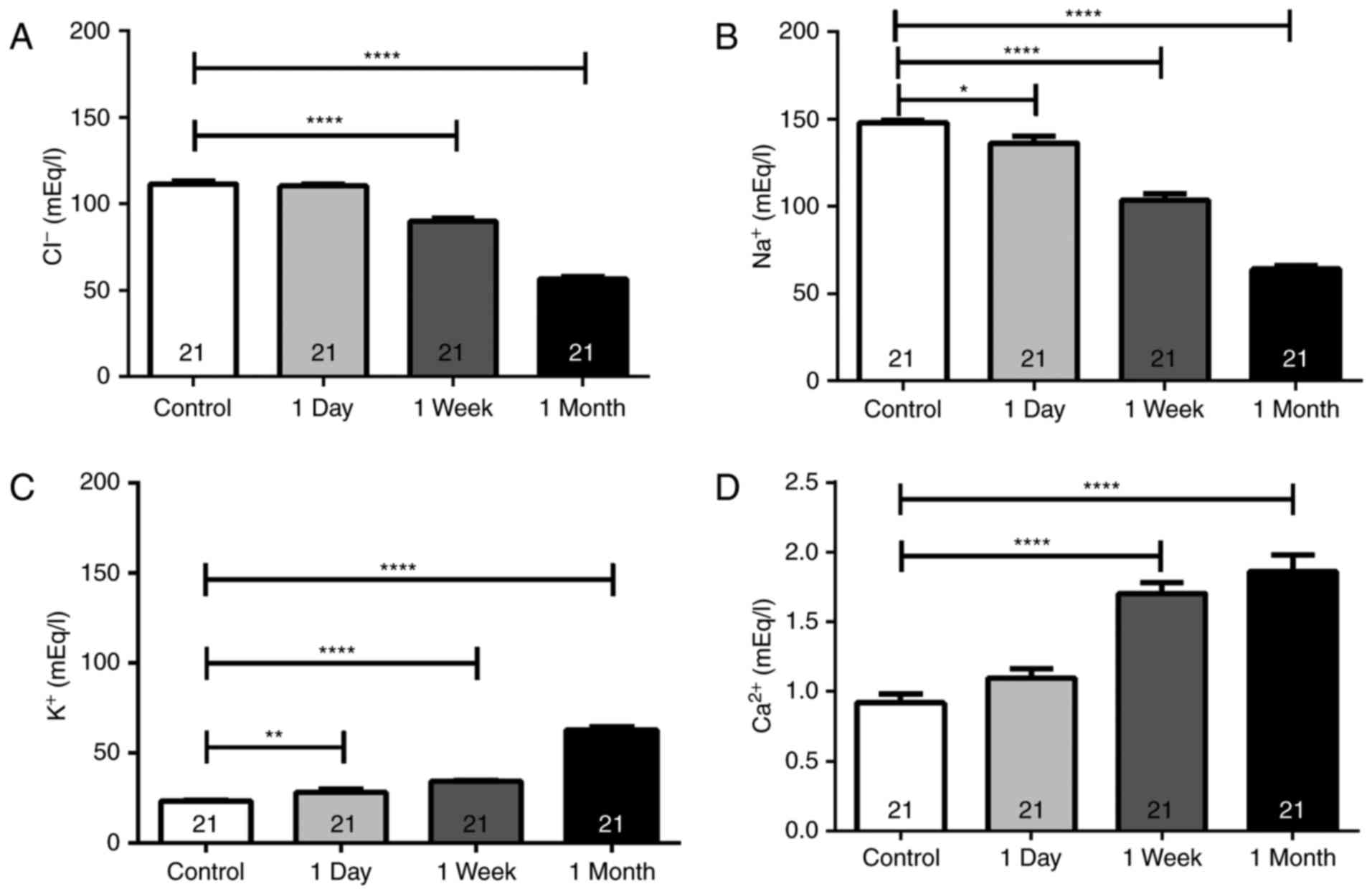
Electrolyte levels
The tear concentration of Cl−,
Na+, K+ and Ca2+ in NACLWs was
analyzed in the present study (Table
I; Fig. 2). A significant
decrease was observed in the Cl− tear levels following
the first week of contact lens use, which was further decreased
after 1 month when compared with the controls (Table I; Fig.
2). Similarly, the Na+ concentration in the tear
samples significantly decreased after 1 day of use with further
decrements observed after 1 week and 1 month when compared with the
control Na+ concentration (Table I). By contrast, the K+
concentrations significantly increased after 1 day, further
increasing after 1 week and 1 month when compared with the controls
(Table I; Fig. 2). Similarly, the Ca2+ tear
concentrations significantly increased after 1 week and 1 month of
continuous lens use compared with those in the controls (Table I; Fig.
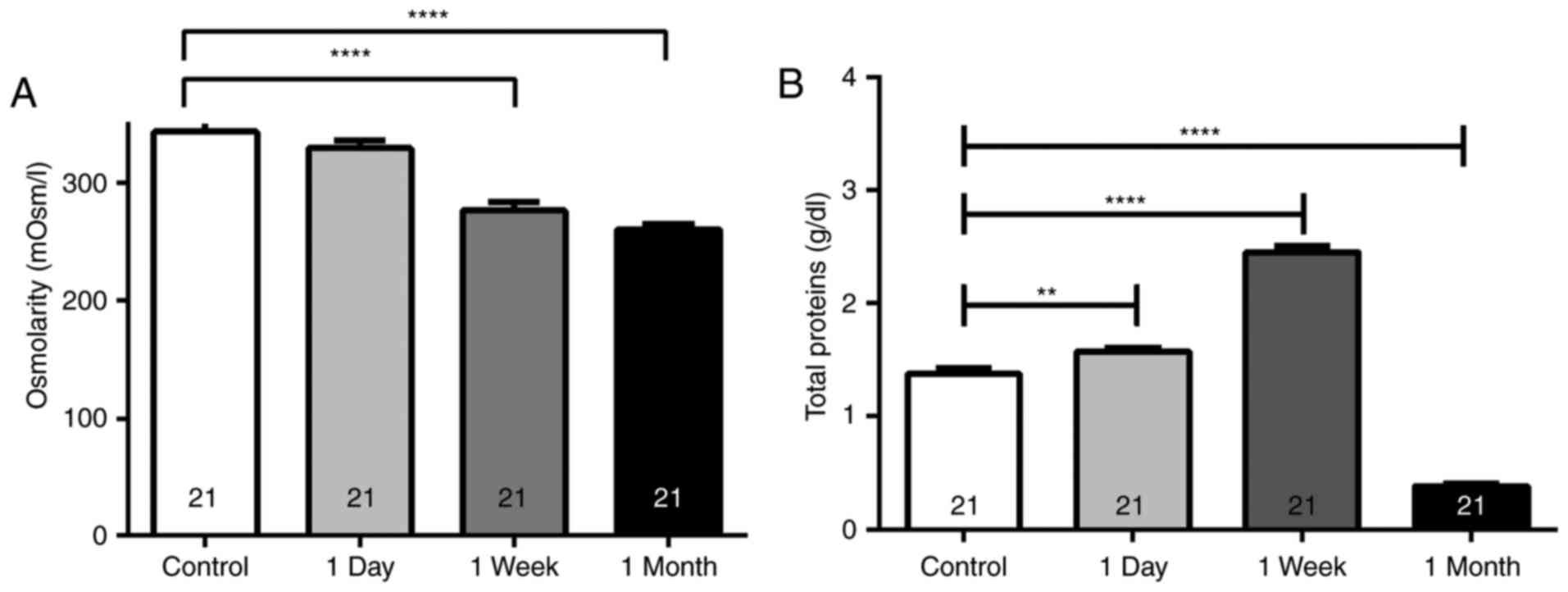
2). Furthermore, the osmolarity was significantly decreased
after 1 week (276.5±33.1 mOsm/l) and 1 month (260.6±20.7 mOsm/l) of
continuous contact lens use compared with that in the control group
(343.5±13.2 mOsm/l; P<0.001; Fig.
3A).
 | Table I.Electrolyte levels in tear fluid. |
Table I.
Electrolyte levels in tear fluid.
| Electrolyte | Control [mEq/l] | 1 day [mEq/l] | 1 week [mEq/l] | 1 month [mEq/l] |
|---|
| Cl− | 111.3±1.9 | 110.2±1.0 | 89.71±1.9 |
56.62±1.3c |
| Na+ | 147.9±1.5 |
136.2±3.8a |
103.5±3.5c |
63.96±1.7c |
| K+ | 23.06±0.64 |
28.02±1.5b |
34.00±0.74c |
62.74±1.5c |
| Ca2+ | 0.91±0.06 | 1.09±0.07 | 1.71±0.08 | 1.86±0.11 |
TP levels
The present study assessed whether there were any
variations in the TP tear concentration in patients wearing contact
lenses continuously (Fig. 3B). The
results detected a significant increase in the TP levels (1.56±0.04
g/dl; P<0.01) after 1 day, with the highest increase observed
after 1 week (2.45±0.05 g/dl; P<0.0001) when compared with the
controls (1.38±0.05 g/dl; Fig. 3B).
However, after 1 month of contact lens use, the TP levels
(0.38±0.02 g/dl) decreased even beyond those observed in the
control group (P<0.0001; Fig.
3B).
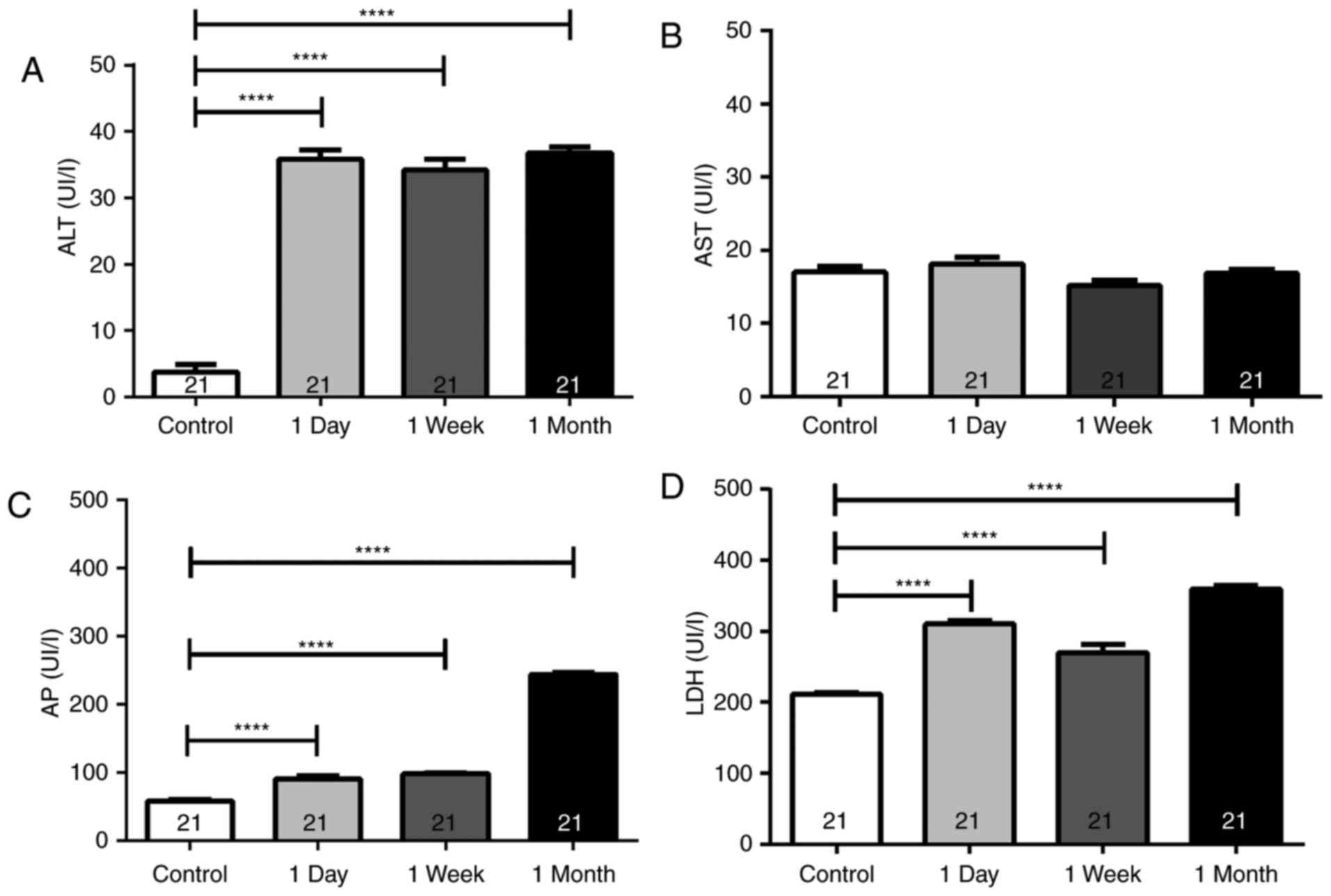
Tissue-damage enzymes
The present study results observed a marked increase
in the ALT tear concentrations after the first day (35.9±6.3 IU/l),
which remained high after 1 week (34.2±7.4 IU/l) and 1 month
(36.8±4.3 IU/l) of continuous lens use, when compared with the
control group (3.7±5.6 IU/l; P<0.0001; Fig. 4A). However, no significant
differences were observed in AST tear levels after 1 day, 1 week
and 1 month when compared with the controls (Fig. 4B; P>0.05). By contrast, the AP
levels were evidently increased after 1 day of continuous use
(90.7±18.7 IU/l), remaining similarly high after 1 week (98.62±6.7
IU/l) and further increasing after 1 month (243.8±16.5 IU/l), as
compared with the AP concentration in the controls (58.5±10.6 IU/l;
P<0.0001; Fig. 4C). Furthermore,
the LDH tear levels were analyzed, and an increase was detected
after 1 day (310.1±21.2 IU/l) that continued similarly high after 1
week (270.3±51.6 IU/l) and peaked after 1 month (359.6±21.1 IU/l)
when compared to controls ([LDH]=210.9±12.7 IU/l; P<0.0001;
Fig. 4D).
Pro-inflammatory mediators IL-8, IL-1β
and IFN-γ
A significant increase was observed in the IL-1β
level after 1 day of continuous contact lens use (0.38±0.09 ng/ml)
when compared with the controls (0.07±0.04 ng/ml; P<0.01;
Fig. 5A). However, after 1 week
(0.12±0.07 ng/ml) and 1 month (0.07±0.04 ng/ml), the IL-1β tear
levels returned to the normal values (Fig. 5A). By contrast, IL-8 concentration
began to increase significantly after 1 week (0.07±0.02 ng/ml;
P<0.05), reaching the highest level after 1 month (0.31±0.05
ng/ml; P<0.0001), as compared with the controls (0.007±0.003
ng/ml; Fig. 5B). Furthermore, IFN-γ
levels were significantly increased after 1 week of continuous
contact lens use (2.40±0.37 ng/ml) and sustained after 1 month
(2.55±0.43 ng/ml) when compared with the control group (0.16±0.09
ng/ml; P<0.0001; Fig. 5C).
Changes in tear pH
The tear pH was significantly decreased after 1 day
(6.53±0.04), and 1 week (6.22±0.032) of continuous use when
compared with that in the controls (7.44±0.034; P<0.0001;
Fig. 5D). The tear pH after 1 month
of contact lens use (7.40±0.035) was returned to levels like those
obtained in the control group (Fig.
5D).
Discussion
Although continuously wearing contact lenses for
prolonged periods of time (such as for 1 month) is highly
unadvisable, this is practiced by several patients (15). This habit is a result of various
factors, including low income, poor patient education and
self-neglect (16). The present
study investigated several biochemical and immunological
alterations associated with the continuous use of contact lenses in
NACLWs. Certain of these changes persisted throughout the evaluated
month, although other parameters were altered over the first week
and then returned to the basal levels, indicating a possible
adaptation of the local tissue to the use of contact lenses. An
example of this adaptation was the change in tear fluid pH, which
decreased after 1 day and 1 week of continuous contact lens use and
returned to the basal levels after 1 month. However, other
parameters, including IL-8, IFN-γ and LDH, remained altered at all
the evaluated time points. This may be associated with a sustained
damaging effect of contact lenses in the micro-environment of the
cornea and neighboring tissues.
The increase of IL-1β levels after 1 day of
continuous use and its subsequent return to basal levels at 1 week,
along with the increase of IL-8 and IFN-γ after 1 week and 1 month,
observed in the present study may indicate an uninterrupted
inflammatory state. This may be generated by the friction between
the lens and the corneal surface plus the hypoxia induced by the
low Dk/L lenses. In fact, it has been suggested that friction in
the presence of corneal hypoxia induces a blood flow increase, as
well as limbal and bulbar redness, and triggers an inflammatory
response (6,17,18).
The IL-8 elevation in the tear fluid detected after
1 week and 1 month of continuous contact lens wearing in the
present study may be associated with the preceding elevation of
IL-1β (observed after 1 day), since it has been previously
demonstrated that IL-1β promotes the release of IL-8 in the human
corneal epithelial cells (19).
However, the sustained elevation of IFN-γ in the contact lens
wearers may be damaging since this cytokine antagonizes IL-13, a
molecule involved in the differentiation of goblet cells and mucin
production (20). Additionally,
IFN-γ also promotes apoptosis and squamous metaplasia of the
epithelia of the cornea and bulbar conjunctiva (20). The findings of the current study
regarding the IFN-γ levels warrant further investigation since, to
the best of our knowledge, there is no substantial research on the
IFN-γ tear levels in contact lens wearers with continuous use for
prolonged periods of time. Kehinde et al (21) reported no clear trends in the IFN-γ
profile during 30 days of continuous use of contact lenses.
The elevation of tissue damage enzymes (LDH, AP, AST
and ALT) in tears may also be associated with the combined effect
of mechanical friction (caused by the lid rubbing against the
contact lens, which results in direct tissue damage) and the
hypoxia induced by the low oxygen transmissibility lenses.
Metabolic enzymes, such as LDH, AP, AST and ALT, have been detected
in the tear fluid of healthy patients at levels similar to those
observed in the serum (22–24). However, only a limited number of
studies have reported findings regarding the effect of contact
lenses on tissue damage-associated enzyme levels. For instance,
Tözsér and Berta (25) demonstrated
higher LDH levels in the tear fluid samples from patients with
mechanical conjunctivitis when compared with those from viral
conjunctivitis or bullous keratitis patients. However, there are no
other studies on the effect of contact lens use on AST, ALT or AP
tear levels. Since the LDH isoform present in tears is different
than the one found in the serum (26), the elevated levels detected in the
tear samples of the NACLW population in the present study may be
the result of a detrimental effect of contact lenses on the
anterior eye and contiguous tissues.
According to the present study findings, it is
suggested that low oxygen transmissibility (due to the lens
composition and characteristics) and the friction of the lens with
the anterior ocular surface are the causes of the observed
alterations in the cytokines and enzymes levels in tear fluid.
In conclusion, the current study demonstrated an
increase in the inflammatory cytokine levels and tissue damage
enzymes, along with variations in the pH, osmolarity and
electrolytes, in Mexican patients that continuously wore low Dk/L
contact lenses for 1 month. Therefore, it is important to strongly
advise against the continuous use of contact lenses with low oxygen
transmissibility, or to select a lens material with a higher Dk/L
and to frequently remove the contact lenses. Although the present
study inferred that the tear fluid biomarkers will return to the
basal levels after discontinuing the use of the contact lenses, a
subsequent study with serial biomarker measurement following this
discontinuation would be required to verify this.
References
|
1
|
Sulley A, Young G, Lorenz KO and Hunt C:
Clinical evaluation of fitting toric soft contact lenses to current
non-users. Ophthalmic Physiol Opt. 33:94–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morgan PB, Woods CA, Tranoudis IG, Helland
M, Efron N, Orihuela Carrillo G, Grupcheva CN, Jones D, Tan KO,
Pesinova A, et al: International contact lens prescribing in 2012.
Contact Lens Spectrum. 28:31–38. 2013.
|
|
3
|
López Alemán A and Revés Serés C: Uso
prolongado de lentes de contactoEdicions Ulleye. Xàtiva Spain: pp.
21–27. 2003
|
|
4
|
Fatt I, Weissman BA and Ruben CM: Areal
differences in oxygen supply to a cornea wearing an optically
powered hydrogel contact lens. CLAO J. 19:226–234. 1993.PubMed/NCBI
|
|
5
|
Fatt I and Weissman BA: Influence of
polarographic cathode diameter on measured oxygen transmissibility
of hydrogel contact lenses with optical power. Optom Vis Sci.
69:931–935. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stapleton F, Ramachandran L, Sweeney DF,
Rao G and Holden BA: Altered conjunctival response after contact
lens-related corneal inflammation. Cornea. 22:443–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klyce SD: Stromal lactate accumulation can
account for corneal oedema osmotically following epithelial hypoxia
in the rabbit. J Physiol. 321:49–64. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cubitt CL, Tang Q, Monteiro CA, Lausch RN
and Oakes JE: IL-8 gene expression in cultures of human corneal
epithelial cells and keratocytes. Invest Ophthalmol Vis Sci.
11:3199–3206. 1993.
|
|
9
|
Cubitt CL, Lausch RN and Oakes JE:
Differences in interleukin-6 gene expression between cultured human
corneal epithelial cells and keratocytes. Invest Ophthalmol Vis
Sci. 36:330–336. 1995.PubMed/NCBI
|
|
10
|
González-Pérez J, Villa-Collar C, Moreiras
T, Gesto I, González-Méijome JM, Rodríguez-Ares MT and Parafita M:
Tear film inflammatory mediators during continuous wear of contact
lenses and corneal refractive therapy. Br J Ophthalmol.
96:1092–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thakur A and Willcox MD: Cytokine and
lipid inflammatory mediator profile of human tears during contact
lens associated inflammatory diseases. Exp Eye Res. 67:9–19. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thakur A and Willcox MD: Contact lens wear
alters the production of certain inflammatory mediators in tears.
Exp Eye Res. 70:255–259. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loh KY and Agarwal P: Contact lens related
corneal ulcer. Malays Fam Physician. 5:6–8. 2010.PubMed/NCBI
|
|
14
|
Norn MS: Hydrogen ion concentration of
tear fluid. Estimated on the basis of a bromothymol-blue-dyed
lacrimal streak. Acta Ophthalmol (Copenh). 46:189–200. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nesburn AB: Ophthalmology-epitomes of
progress: Continuous wear contact lenses. West J Med. 128:432–433.
1978.PubMed/NCBI
|
|
16
|
Morales Mac-Hale C: Compliance with
contact lenses in Latin America: An educational, not a cultural
challenge. Cienc Tecnol Salud Vis Ocul. 13:113–125. 2015.
|
|
17
|
Efron N: Contact lens-induced changes in
the anterior eye as observed in vivo with the confocal microscope.
Prog Retin Eye Res. 26:398–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elgin Yüksel C, İskeleli G, Talaz S and
Akyol S: Comparative analysis of tear film levels of inflammatory
mediators in contact lens users. Curr Eye Res. 4:441–447. 2016.
|
|
19
|
Cavet ME, Harrington KL, Vollmer TR, Ward
KW and Zhang JZ: Anti-inflammatory and anti-oxidative effects of
the green tea polyphenol epigallocatechin gallate in human corneal
epithelial cells. Mol Vis. 17:533–542. 2011.PubMed/NCBI
|
|
20
|
Pflugfelder SC, Corrales RM and de Paiva
CS: T helper cytokines in dry eye disease. Exp Eye Res.
117:118–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kehinde LE, Elder KS and Fullard RJ:
Effects of daily vs. 30 day continuous contact lens wear on tear
cytokine levels. Invest Ophthalmol Vis Sci. 50:56562009.
|
|
22
|
Ananthi S, Santhosh RS, Nila MV, Prajna
NV, Lalitha P and Dharmalingam K: Comparative proteomics of human
male and female tears by two-dimensional electrophoresis. Exp Eye
Res. 92:454–463. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sariri R and Ghafoori H: Tear proteins in
health, disease and contact lens wear. Biochemistry (Mosc).
73:381–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C,
Tanavde V, Li XR and Beuerman RW: In-depth analysis of the human
tear proteome. J Proteomics. 75:3877–3885. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tözsér J and Berta A: Lactate
dehydrogenase activity in pathological human tears obtained with
glass capillaries correlates with the albumin content. Int
Ophthalmol. 22:289–292. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Haeringen NJ and Glasius E: Enzymes of
energy-producing metabolism in human tear fluid. Exp Eye Res.
18:407–409. 1974. View Article : Google Scholar : PubMed/NCBI
|