Introduction
Acute respiratory infections (ARIs) represent a
health issue of great importance, leading cause of mortality in
children worldwide, particularly in developing countries (1). These represent about 50% of all
diseases in children aged <5 years (2). The major viral agents of ARI include
influenza A, B, and C viruses (FLU), respiratory syncytial virus
(RSV), parainfluenza virus (PIV), adenovirus (ADV), human
metapneu-movirus (hMPV), human coronavirus (HCoV), and Rhinovirus
(HRV).
The etiology of respiratory diseases is
multifactorial and includes, among others, interactions between
genetic predisposition and environmental factors (3). Numerous studies have confirmed that
short- and long-term exposures to ambient air pollutants can be
associated with a wide range of pathologies, in particular
respiratory diseases and cancer. Although air pollution has not
been shown as the sole cause of respiratory infections, it has been
reported that several air pollutants were correlated with increased
morbidity of respiratory infections (4). In urban areas, irrespective of seasonal
frequencies, this correlation increases due to the high incidence
of polluting factors. Several epidemiological studies have
documented a positive association between exposure to particulate
air pollution and respiratory symptoms especially among children
(5–7).
It is profusely reported that during the past three
decades, large areas of Naples county in the Southern Italy
Campania region have been extensively contaminated by environmental
toxic agents, in particular for the presence of many landfills of
industrial wastes. This region was already defined as one of the
geographical areas most at risk of neoplastic and respiratory
diseases for environmental factors in the report of WHO (World
Health Organization) in 1997 (8).
Although, the epidemiological studies for the
association between air pollution and the incidence of respiratory
infection in this geographical area are relatively few, virus types
identification and their molecular characterization is fundamental
not only for surveillance, for diagnostic and therapeutic purposes,
but also for highlight the relationships between respiratory
diseases in children and concentrations of environment pollutions
(9).
In the present study we characterized the viral
spectrum and pattern of ARIs in children from children's hospital
‘Santobono Pausillipon’ which centralizes pediatric diseases of the
entire Naples province. The aim of this study was to determine the
association between respiratory viruses types, patients features
(sex, age, season of disease occurrence) and, in particular,
geographical origin.
Materials and methods
Patients selection
We retrospectively reviewed the electronic medical
records of 356 patients between 11 days and 14 years, with
suspected respiratory infection, evaluated at the Santobono
Hospital of Naples, between 1 January 2016 and 31 January 2017,
with FilmArray® Respiratory Panel (FARP) testing on
NasoPharyngeal Swab (NPS).
From patient electronic medical records the
following information was obtained: Demographics (age and sex),
month of hospitalization, and geographical origin.
We have divided the patients into three age groups
(Early childhood, 0–2 years; preschool age, 2–5 years; third
childhood: 5–11 years), in the season of hospitalization (winter:
December, January, February; spring: March, April, May; summer:
June, July, August; autumn: September, October, November) and
geographical origin (metropolitan area of Naples and extra-urban
areas).
FilmArray testing
NPSs were collected according to a standard
procedure, kept in viral transport medium, and stored at −20°C
prior to analysis.
FARP (FilmArray® Respiratory Panel
BioFire Diagnostics LLC 390; Wakara Way Salt Lake City, UT, USA) is
a test based on multiplex PCR. The FilmArray RP cartridge is
designed for the simultaneous detection and identification of
following viruses and bacteria of the upper respiratory tract:
Influenza A virus (H1N1, H1N1 2009, and H3N2), influenza B virus,
RSV, PIVs 1–4, ADV, HRV/enterovirus (the assay does not distinguish
between these two pathogens), HMPV, HCoV (229E, HKU1, OC43, and
NL63), Mycoplasma pneumoniae, Chlamydophila pneumoniae, and
Bordetella pertussis. The FilmArray instrument and pouch
system have been described in detail elsewhere (10). The research use only version of the
FilmArray RP system reported a cycle threshold for each positive
PCR assay (11).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
(SPSS Inc., Chicago, IL, USA). Viral prevalence were compared using
the Chi-square test for categorical variables, and the cartogram
was drawn using Excel software (Microsoft Corporation, Redmond, WA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic characteristics
A total of 356 nasopharyngeal swabs were collected
and analyzed. In detail, 163 patients were female (45.78%) and 193
were male (54.21%). Most of the patients (319) were <5 years old
while 36 patients were >5 years old. Patients are
heterogeneously distributed in different seasons, while regarding
geographical origin, 123 (37.9%) patients are of Naples
metropolitan area, 201 (62.03%) originated from neighboring
municipalities, and for 32 patients this information is
lacking.
In our study we considered only viral infections,
but we detected also other etiologic agents (Bordetella
pertussis, Chlamydophila pneumoniae, Mycoplasma pneumoniae),
highlighted the presence of these infections in 24 patients
(6.7%).
Respiratory infection viruses
distribution
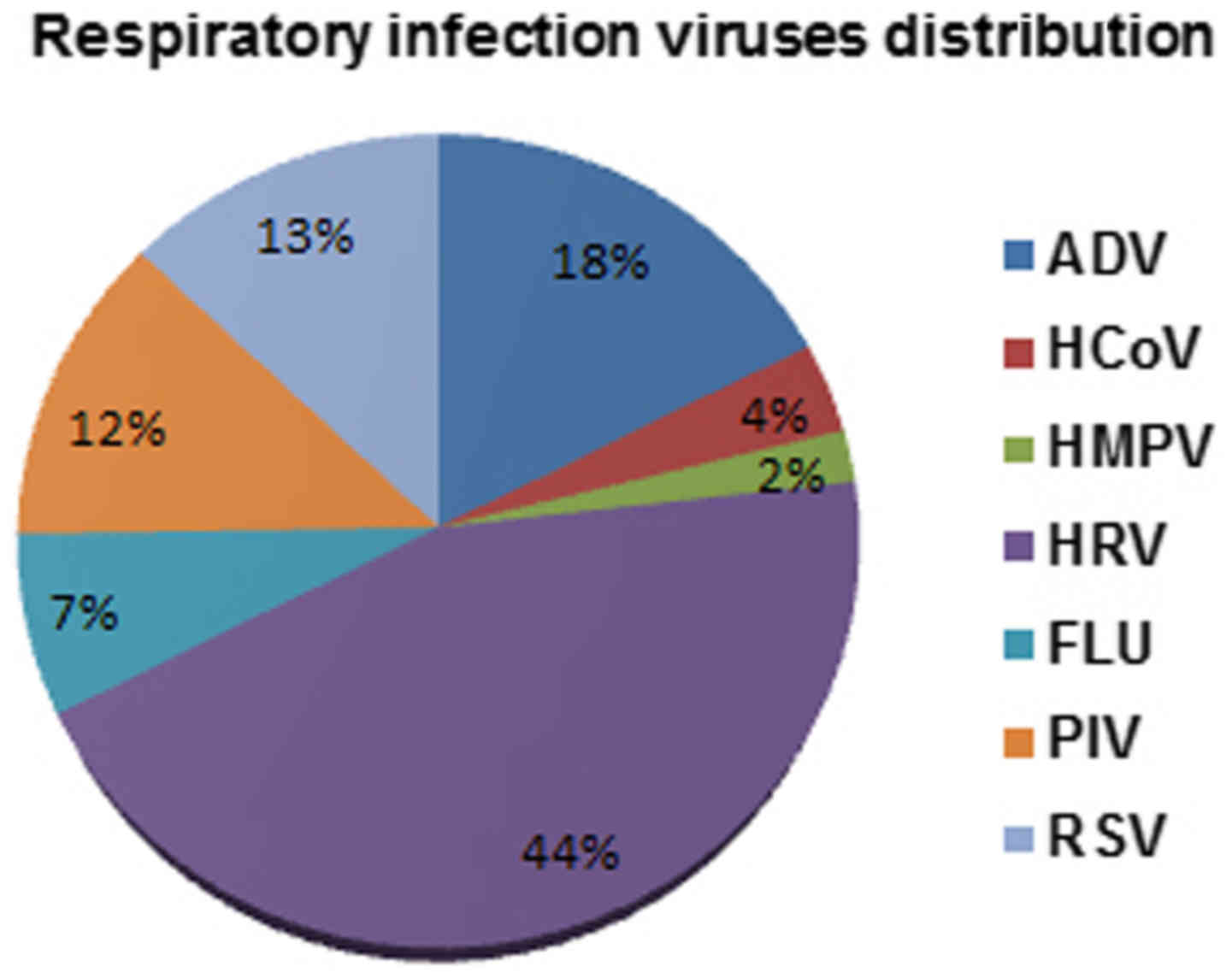
The total rate of detection of all seven viruses was
78% (278/356) of patients. In detail, HRV viruses were detected in
44% of patients, followed by ADV viruses (18%), RSV viruses (13%),
and PIV (12%). A lower incidence has been instead highlighted for
FLU (7%), HCoV (4%) and HMPV (2%) (Fig.
1).
In a significant proportion of individuals
co-infections were also highlighted. In detail, double
co-infections were detected in 69/256 (26.9%) patients, triple in
17/256 (6.6%) and quadruple infections only in 1 patient (Table I). The more frequent association was
detected between HRV and PIV viruses 19/87 (21%), followed by HRV
and ADV viruses 15/87 (17.24%), and HRV and RSV viruses 9/87
(10.3%) (Table I).
 | Table I.Co-infections with seven respiratory
viruses. |
Table I.
Co-infections with seven respiratory
viruses.
| Co-infections | N |
|---|
| HRV + PIV | 19 |
| HRV + ADV | 15 |
| HRV + RSV | 9 |
| HRV + ADV +
PIV | 4 |
| ADV + PIV | 4 |
| HRV + HMPV | 4 |
| HRV-ADV + HCoV | 3 |
| ADV + FLU | 3 |
| RSV + HCoV | 3 |
| ADV + HCoV | 2 |
| FLU + RSV | 2 |
| HRV + HCoV | 2 |
| HCoV + PIV | 2 |
| RSV + ADV +
HMPV | 2 |
| HRV + RSV +
ADV | 2 |
| RSV + HMPV | 2 |
| HRV + RSV +
PIV | 2 |
| FLU + PIV | 1 |
| HRV + FLU | 1 |
| HRV + HCoV +
PIV | 1 |
| RSV + ADV + HRV +
PIV | 1 |
| RSV + ADV +
HCoV | 1 |
| HRV + RSV +
HCoV | 1 |
| HRV + RSV +
HMPV | 1 |
Sex and age distribution
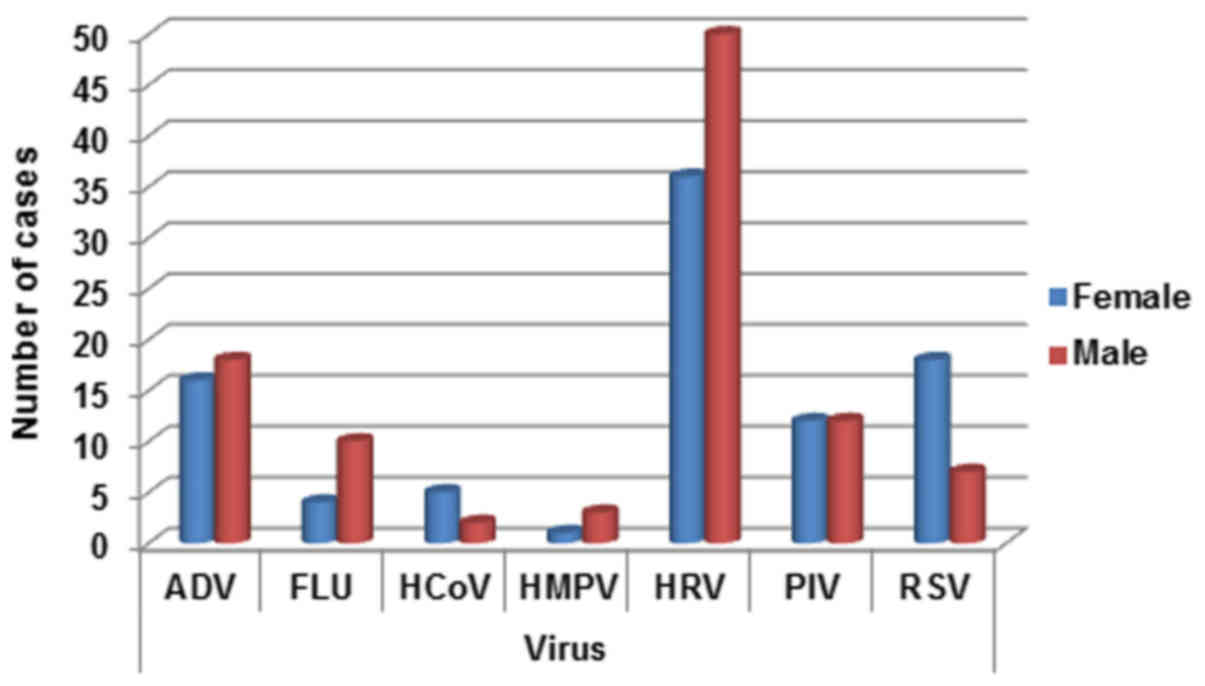
Viruses appear heterogeneously distributed between
sex, with the exception of PIV. In detail, ADV was slightly higher
in males (18/34, 52.9%), while FLU (10/14, 71.4%), HMPV (3/4, 75%)
and HRV (50/86, 58.13%) were strongly higher in males children (see
detection rate Fig. 2). On the
contrary HCoV (5/7, 71.4%) and RSV (18/25, 72%) are better
represented in female patients (see Fig.
2).
Regarding age distribution a decline in the
incidence of viral infections with age was observed for respiratory
viruses, except for FLU (Fig. 3). In
fact the detection rate for FLU viruses was lowest in 2–5 years
patients. The detection rate for ADV gradually decrease from 0–2
years patients to >5 years patients. The same trend was
highlighted for HCoV, PIV, HRV and RSV (with a consistently
increasing of detection rate in 0–2 year-old patients). HMPV was
detected prevalently in 0–2 year-old children (Fig. 3).
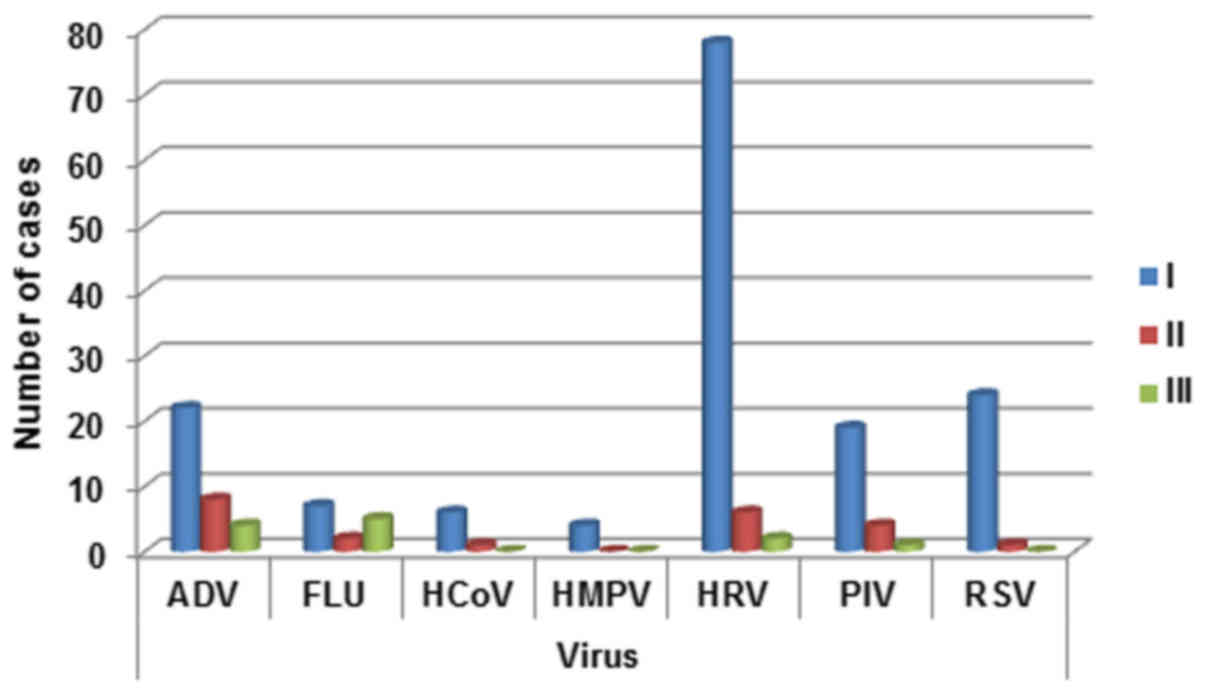 | Figure 3.Respiratory infection viruses
distribution in the three age groups (0–2 years, 2–5 years; >5).
ADV, adenovirus; FLU, influenza A, B, and C viruses; HCoV, human
coronavirus; HMPV, human metapneu-movirus; HRV, rhinovirus; PIV,
parainfluenza virus; RSV, respiratory syncytial virus. |
Seasonal distribution
The total detection rate for all respiratory viruses
in spring, summer, autumn and winter was calculated. The rates of
detection was more heterogeneous during the different seasons.
In detail, ADV infection was prevalent in summer
(20/34, 58.8%) and spring (10/34, 29.4%) seasons, FLU was prevalent
in spring (7/14, 50%) and winter (4/14, 28.5%), HMPV in winter
(2/4, 50%), HCoV in autumn/winter (3/7, 42.8%), HRV in autumn
(34/86, 39.5%) and summer (30/86, 34.8%), PIV in summer (13/24,
34.8%) and RSV in winter (24/25, 96%) (Fig. 4).
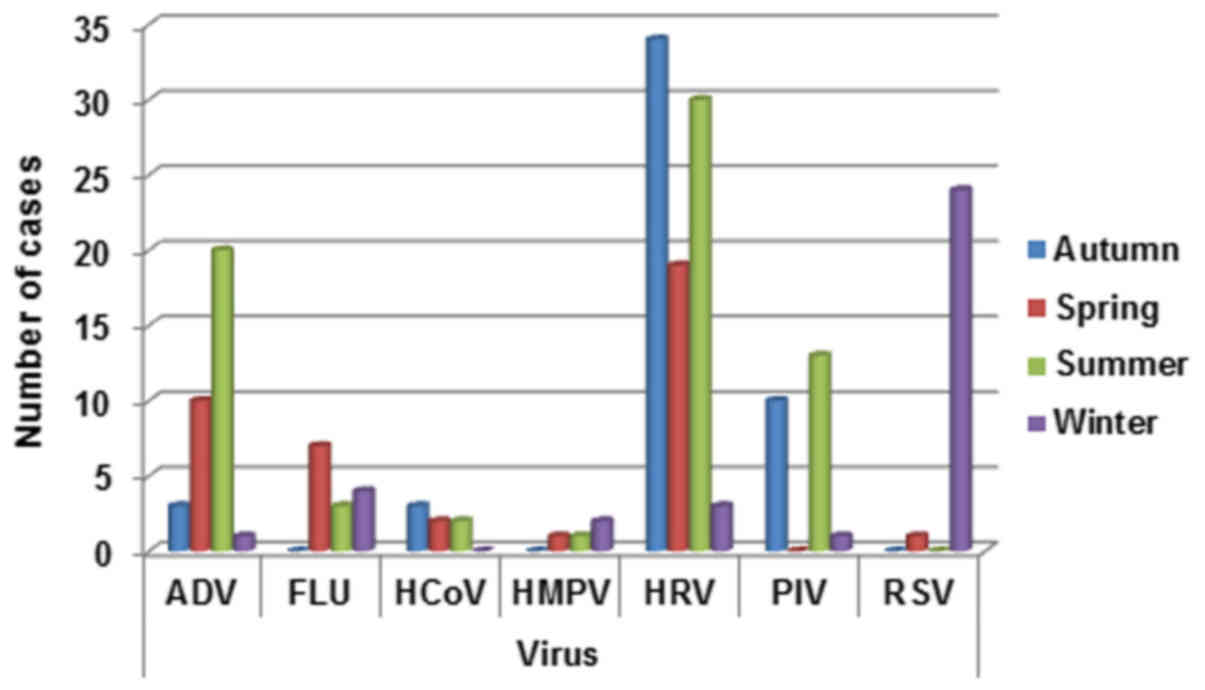 | Figure 4.Respiratory infection viruses
distribution by season (winter: December, January, February;
spring: March, April, May; summer: June, July, August; autumn:
September, October, November). ADV, adenovirus; FLU, influenza A,
B, and C viruses; HCoV, human coronavirus; HMPV, human
metapneu-movirus; HRV, rhinovirus; PIV, parainfluenza virus; RSV,
respiratory syncytial virus. |
Geographical distribution
The pediatric patients were divided into two main
groups according to origin from the metropolitan of Naples and
neighboring municipalities. The detection rates for the two areas
appear different with a prevalent distribution in extra-urban
areas. This appears more evident especially for ADV, HRV and RSV
viral infections (Fig. 5).
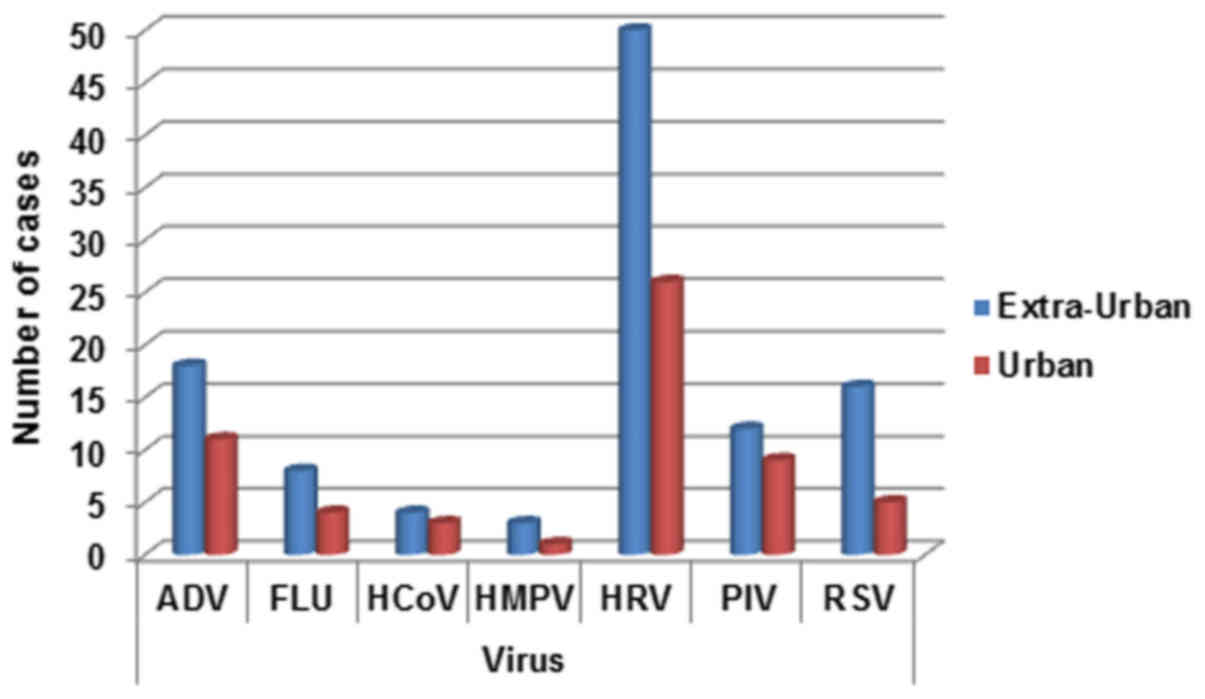 | Figure 5.Respiratory infection viruses
distribution for territorial origin (urban, Naples metropolitan
area; extra-urban, neighboring municipalities). ADV, adenovirus;
FLU, influenza A, B, and C viruses; HCoV, human coronavirus; HMPV,
human metapneu-movirus; HRV, rhinovirus; PIV, parainfluenza virus;
RSV, respiratory syncytial virus. |
Discussion
WHO reported that ARI can represent among the
leading causes of mortality in children under 5 years of age.
Many studies in the literature have described in
detail the viruses mainly associated with ARI and also their
distribution, but showing much conflicting data on populations from
different countries (1). These
variations may be due to socio-economic factors, geographical and
climatic differences and efficiency of local health care
systems.
In recent years, advances in PCR techniques have
aided in the rapid and accurate detection of common respiratory
pathogens from patient specimens. Multiplex PCR can identify and
differentiate a large panel of viral and bacterial targets
simultaneously and are more rapid and more sensitive methods than
cultures or antigen detection (12,13).
In the present report we analyzed the distribution
of seven respiratory viruses in a case series of 356 hospitalized
patients in metropolitan area of Naples and in the neighboring
municipalities. The incidence of all analyzed virus is prevalent in
early childhood. The distribution of viruses, considered
individually, appears to be very heterogeneous, with the largest
percentage of HRV in line with other studies on different
populations (14,15). HRV (including Rhinovirus and
Enterovirus) are RNA viruses related to Picornavirus family
(16). HRV is associated with the
common cold, but can also be implicated in exacerbating asthma
attacks and severe complications (15). The Enteroviruses are categorized into
four species, which include a total of 89 serotypes associated with
different clinical manifestations (16,17).
In our case series HRV appears more expressed in the
male population and its seasonality is mainly associated with
summer and autumn, in line with the data present in the literature
(16,18).
RSV and ADV are, after HRV, the most common virus in
our case series, with RSV prevalent among the female children and
ADV among the male population.
RSV is a member of the Paramyxoviridae RNA
viruses family related to human metapneumovirus and PIVs. RSV is
the most common cause of severe respiratory illness in infants with
acute bronchiolitis as a leading cause of hospitalization (19).
In our case series the winter seasonality of RSV
viruses corresponds to the other reported data (19).
ADVs are DNA viruses uncoated consists of seven
species (A-G) and classified by hemagglutination with about 55
serotypes. The species of ADV B, C and E cause acute respiratory
disease with the main risk factor the long stay indoors (18) while the ADV (species A, D, F and G)
can cause cystitis, gastroenteritis and conjunctivitis (20).
In our study the seasonal distribution of ADV is
prevalent in summer in line with the literature (21).
While there are no substantial differences in PIV
distribution, FLU seems prevalent in the male population and their
seasonality is consistent with data reported in the literature
(22). Finally, HCoV and hMPV
viruses are less frequent in our population, with the first more
present in male pediatric population and hMPV in female
children.
Coronavirus HCoV are characterized 4 serological
variants (229E, HKU1, NL63 and OC43), and are most commonly
associated with infections of the upper respiratory tract (23).
hMPV belong to the Paramyxoviridae family and
the infection in newborns and young children is commonly associated
with bronchiolitis (24).
It is also widely documented the occurrence of
co-infections in some cases. In our case series the more frequent
co-infection are between HRV and PIV viruses and HRV and ADV
viruses. These data, in some cases, contrast with other reports
(25). However, this might be
closely related to the geographical location, climate and different
social and socio-cultural conditions.
Co-infections were more common in pediatric patients
than in adults as documented by other studies (26–28).
However, multiple viral infections can be linked to
hospital stay, abuse of antibiotic and social conditions, but there
are not proves that co-infections can worsen the disease course.
Our hospital centralizes the majority of patients coming from the
metropolitan area of Naples but also from neighboring
municipalities. Our data are interesting for the geographic
distribution of patients. In fact all the investigated viruses have
a detection rate higher in surrounding municipalities than in the
metropolitan area of Naples. This is in contrast with most of the
data present in the literature, where the prevalence is just in
urban areas due to the high presence of pollutants (29–31).
However, during the past three decades, surrounding
areas of Naples have been extensively contaminated by environmental
toxic agents, in particular for the presence of many landfills of
industrial wastes. The most contaminated areas were defined as
‘Land of Fire’ (32).
The relationship between air pollution and
respiratory infections has become an increased public health
concern in recent years (33–35). In
fact, the etiology of respiratory diseases is multifactorial and
includes, among others, interactions between genetic predisposition
and environmental factors as climate change, chemical air pollution
and airborne pollens. The short-term respiratory effects of air
pollution include decreases in pulmonary function (36), increases in inflammatory biomarkers
and respiratory symptoms (37),
infections (38), and respiratory
mortality (39).
The environmental risk factors may have an impact on
children's respiratory health, above all in urban areas, especially
because children inhale more pollutants per kilogram of body weight
than adults (40).
In conclusion, the risk factors between populations
can be extremely different, suggesting the need to adequately
characterize epidemiology of ARIs to implement prevention and
control program.
References
|
1
|
Simoes EAF, Cherian T, Chow J,
Shahid-Salles SA, Laxminarayan R and John TJ: Acute respiratory
infections in childrenSource Disease Control Priorities in
Developing Countries. Jamison DT, Breman JG, Measham AR, Alleyne G,
Claeson M, Evans DB, Jha P, Mills A and Musgrove P: second edition.
Washington (DC): World Bank; 2006
|
|
2
|
Shi T, McLean K, Campbell H and Nair H:
Aetiological role of common respiratory viruses in acute lower
respiratory infections in children under five years: A systematic
review and meta-analysis. J Glob Health. 5:0104082015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Decramer M, Janssens W and Miravitlles M:
Chronic obstructive pulmonary disease. Lancet. 379:1341–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurt OK, Zhang J and Pinkerton KE:
Pulmonary health effects of air pollution. Curr Opin Pulm Med.
22:138–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierse N, Rushton L, Harris RS, Kuehni CE,
Silverman M and Grigg J: Locally generated particulate pollution
and respiratory symptoms in young children. Thorax. 61:216–220.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qian Z, Chapman RS, Hu W, Wei F, Korn LR
and Zhang JJ: Using air pollution based community clusters to
explore air pollution health effects in children. Environ Int.
30:611–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramani VK, Pattankar J and Puttahonnappa
SK: Acute respiratory infections among under-five age group
children at urban slums of Gulbarga City: A longitudinal study. J
Clin Diagn Res. 10:LC08–LC13. 2016.PubMed/NCBI
|
|
8
|
The World Health Report 1997-conquering
suffering, enriching humanity. World Health Forum. 18:248–260.
1997.PubMed/NCBI
|
|
9
|
Moreno-Valencia Y, Hernandez-Hernandez VA,
Romero-Espinoza JA, Coronel-Tellez RH, Castillejos-Lopez M,
Hernandez A, Perez-Padilla R, Alejandre-Garcia A, La Rose-Zamboni
D, Ormsby E. C and Vazquez-Perez JA: Detection and characterization
of respiratory viruses causing acute respiratory illness and asthma
exacerbation in children during three different seasons (2011–2014)
in Mexico City. Influen Other Resp Virus. 9:287–292. 2015.
View Article : Google Scholar
|
|
10
|
Poritz MA, Blaschke AJ, Byington CL,
Meyers L, Nilsson K, Jones DE, Thatcher SA, Robbins T, Lingenfelter
B, Amiott E, et al: FilmArray, an automated nested multiplex PCR
system for multi-pathogen detection: Development and application to
respiratory tract infection. PLoS One. 6:e260472011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hammond SP, Gagne LS, Stock SR, Marty FM,
Gelman RS, Marasco WA, Poritz MA and Baden LR: Respiratory virus
detection in immunocompromised patients with FilmArray respiratory
panel compared to conventional methods. J Clin Microbiol.
50:3216–3221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elnifro EM, Ashshi AM, Cooper RJ and
Klapper PE: Multiplex PCR: Optimization and application in
diagnostic virology. Clin Microbiol Rev. 13:559–570. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibib DR, Matushek SM, Beavis KG, Gawel
SH and Charnot-Katsikas A: BioFire filmarray respiratory panel for
detection of enterovirus D68. J Clin Microbiol. 54:457–459. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miller EK, Lu X, Erdman DD, Poehling KA,
Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB,
et al: Rhinovirus-associated hospitalizations in young children. J
Infect Dis. 195:773–781. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asner SA, Petrich A, Hamid JS, Mertz D,
Richardson SE and Smieja M: Clinical severity of
rhinovirus/enterovirus compared to other respiratory viruses in
children. Influenza Other Respir Viruses. 8:436–442. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anzueto A and Niederman MS: Diagnosis and
treatment of rhinovirus respiratory infections. Chest.
123:1664–1672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacques J, Moret H, Minette D, Lévêque N,
Jovenin N, Deslée G, Lebargy F, Motte J and Andréoletti L:
Epidemiological, molecular, and clinical features of enterovirus
respiratory infections in French children between 1999 and 2005. J
Clin Microbiol. 46:206–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Romero JR: Enteroviruses and
Parechoviruses: In: Manual of Clinical Microbiology. Murray PR,
Baron EJ, Jorgensen MA, Pfaller MA and Landry ML: ASM press;
Washington D.C.: pp. 1392–1404. 2007
|
|
19
|
Mohapatra SS and Boyapalle S:
Epidemiologic, experimental, and clinical links between respiratory
syncytial virus infection and asthma. Clin Microbiol Rev.
21:495–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Metzgar D, Osuna M, Kajon AE, Hawksworth
AW, Irvine M and Russell KL: Abrupt emergence of diverse species B
adenoviruses at US military recruit training centers. J Infect Dis.
196:1465–1473. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Centers for disease control and
prevention, . Centers for disease control and prevention, National
Center for Immunization and Respiratory Diseases (NCIRD), Division
of Viral Diseases (DVD) Web site. https://www.cdc.gov/ncird/overview/websites.html#dvdNovember
14–2016
|
|
22
|
Centers for Disease Control and Prevention
(CDC): Prevention and control of seasonal influenza with vaccines;
Recommendations of the Advisory Committee on Immunization
Practices-United States, 2013–2014. MMWR Recomm Rep. 62:1–4314.
2013.
|
|
23
|
Kuypers J, Martin ET, Heugel J, Wright N,
Morrow R and Englund JA: Clinical disease in children associated
with newly described coronavirus subtypes. Pediatrics. 119:e70–e76.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kahn JS: Epidemiology of human
metapneumovirus. Clin Microbiol Rev. 19:546–557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang D, He Z, Xu L, Zhu X, Wu J, Wen W,
Zheng Y, Deng Y, Chen J, Hu Y, et al: Epidemiology characteristics
of respiratory viruses found in children and adults with
respiratory tract infections in southern China. Int J Infect Dis.
25:159–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bellau-Pujol S, Vabret A, Legrand L, Dina
J, Gouarin S, Petitjean-Lecherbonnier J, Pozzetto B, Ginevra C and
Freymuth F: Development of three multiplex RT-PCR assays for the
detection of 12 respiratory RNA viruses. J Virol Methods.
126:53–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bezerra PG, Britto MC, Correia JB, Duarte
Mdo C, Fonceca AM, Rose K, Hopkins MJ, Cuevas LE and McNamara PS:
Viral and atypical bacterial detection in acute respiratory
infection in children under five years. PLoS One. 6:e189282011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Auburn H, Zuckerman M, Broughton S,
Greenough A and Smith M: Detection of nine respiratory RNA viruses
using three multiplex RT-PCR assays incorporating a novel RNA
internal control transcript. J Virol Methods. 176:9–13. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwartz J: Air pollution and children's
health. Pediatrics. 113 4 Suppl:S1037–S1043. 2004.
|
|
30
|
Trasande L and Thurston GD: The role of
air pollution in asthma and other pediatric morbidities. J Allergy
Clin Immunol. 115:689–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bono R, Romanazzi V, Bellisario V,
Tassinari R, Trucco G, Urbino A, Cassardo C, Siniscalco C,
Marchetti P and Marcon A: Air pollution, aeroallergens and
admissions to pediatric emergency room for respiratory reasons in
Turin, northwestern Italy. BMC Public Health. 16:7222016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pizzi C, Arpino G, Acampora G, Aiello N,
DE Rosa A, Diaferia I, DI Nunzio A, Fragna G, Franco A, Russo M, et
al: Cancer prevalence in the city of Naples: Contribution of the GP
database analyses to the cancer registries network. Mol Clin Oncol.
1:726–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lipfert FW: Long-term associations of
morbidity with air pollution: A Catalogue and Synthesis. Jul
5–2017.(Epub ahead of print).
|
|
34
|
Ding PH, Wang GS, Guo YL, Chang SC and Wan
GH: Urban air pollution and meteorological factors affect emergency
department visits of elderly patients with chronic obstructive
pulmonary disease in Taiwan. Environ Pollut. 224:751–758. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshizaki K, Brito JM, Silva LF,
Lino-Dos-Santos-Franco A, Frias DPE, Silva RC, Amato-Lourenço LF,
Saldiva PH, de Fátima Lopes Calvo Tibério I, Mauad T and Macchione
M: The effects of particulate matter on inflammation of respiratory
system: Differences between male and female. Sci Total Environ.
586:284–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lagorio S, Forastiere F, Pistelli R,
Iavarone I, Michelozzi P, Fano V, Marconi A, Ziemacki G and Ostro
BD: Air pollution and lung function among susceptible adult
subjects: A panel study. Environ Health. 5:112006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bono R, Tassinari R, Bellisario V, Gilli
G, Pazzi M, Pirro V, Mengozzi G, Bugiani M and Piccioni P: Urban
air and tobacco smoke as conditions that increase the risk of
oxidative stress and respiratory response in youth. Environ Res.
137:141–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dominici F, Peng RD, Bell ML, Pham L,
McDermott A, Zeger SL and Samet JM: Fine particulate air pollution
and hospital admission for cardiovascular and respiratory diseases.
JAMA. 295:1127–1134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dominici F, McDermott A, Daniels M, Zeger
SL and Samet JM: Revised analyses of the national morbidity,
mortality, and air pollution study: Mortality among residents of 90
cities. J Toxicol Environ Health A. 68:1071–1092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bono R, Bellisario V, Romanazzi V, Pirro
V, Piccioni P, Pazzi M, Bugiani M and Vincenti M: Oxidative stress
in adolescent passive smokers living in urban and rural
environments. Int J Hyg Environ Health. 217:287–293. 2014.
View Article : Google Scholar : PubMed/NCBI
|