Introduction
Acute lung injury (ALI), also known as acute
respiratory distress syndrome (ARDS), is a common critical disease
caused by ALI (1,2). Its clinical features are the increased
permeability of alveolar capillary barrier and air exchange
dysfunction, and its typical clinicopathologic features include the
injury of pulmonary capillary endothelial cells, extensive
pulmonary edema and microatelectasis of alveolar epithelial cells,
microthrombosis and microcirculation disorder (3). Severe infections, trauma, shock,
poisoning and inhalation of toxic gases are the most common causes
of ALI (4). At present, patients
with ARDS mainly receive supportive therapy, especially the
maintenance of ventilation and oxygenation, normal cardiac function
and nutritional support, and application of nitric oxide and
corticosteroids, so as to prevent further complications (5,6).
Therefore, there is a lack of effective treatment means for
ADRS.
Sulforaphane, also known as ‘raphanin’, is the
extract of cruciferous vegetables (such as broccoli, Brussels
sprouts and cabbage). As an agonist of nuclear factor erythroid
2-related factor 2 (Nrf2), sulforaphane has been proved to be able
to activate the Nrf2 expression in the heart and the nervous
system, and protect multiple organs, such as liver, lung and kidney
(7,8). Moreover, it has also been shown that
sulforaphane can protect Nrf2-positive peritoneal macrophages in
mice (9); however, there are no
studies on it in the ADRS model induced by inflammatory factors. In
this study, the protective mechanism of sulforaphane against ADRS
was mainly studied, so as to provide a certain theoretical basis
for the clinical treatment of ADRS.
Materials and methods
Grouping and treatment of experimental
animals
A total of 30 specific pathogen-free (SPF) rabbits
(license no. BD20174321) were purchased from the Laboratory Animal
Center (Jiangsu, China). They were randomly divided into: Control
(n=10), model (n=10) and experimental groups (n=10). Rabbits in the
model group and experimental group were treated with femoral venous
injection of 0.1 ml/kg oleic acid to establish the oleic
acid-induced ARDS model, while those in control group were injected
with the same volume of normal saline. Whereas, rabbits in the
experimental group received femoral venous injection of 5 mg/ml/kg
sulforaphane (Aokai, Guangzhou, China) dissolved by normal saline,
while those in the model group were injected with the same volume
of normal saline. Within 12 h after treatment, the reactions and
deaths of rabbits in each group were observed and recorded.
Materials and detection of the
indexes
At 12 h after treatment of rabbits in each group,
they were anesthetized with chloral hydrate, and the body weight of
rabbits was recorded. Then blood was drawn from the femoral artery
until the death of rabbits. The blood was placed at 4°C for 1 h for
stratification, followed by centrifugation at 2,380 × g for 10 min.
The upper-layer serum was taken, and rabbit lung tissues were
excised and weighed, and the lung index (LI) was calculated: LI =
total lung mass (g)/body mass (kg). After weighing, lung tissues
were immediately divided into two parts; one part was stored in
liquid nitrogen for reverse transcription-polymerase chain reaction
(RT-PCR), while the other part was fixed in 4% paraformaldehyde for
hematoxylin and eosin (H&E) morphological examination and
immunohistochemistry (IHC). The study was approved by the Ethics
Committee of Cangzhou Central Hospital (Cangzhou, China).
The alveolar damage coefficient [index of
quantitative assessment (IQA)] was calculated following the steps
below: Six H&E staining sections of lung tissues were taken
from rabbits in each group, and 10 images were taken in the same
field of view, and the number of damaged alveoli (containing more
than 2 erythrocytes or neutrophils in alveoli) was calculated. The
ratio of the number of damaged alveoli to the total number of
alveoli was used as the IQA to evaluate the degree of lung
injury.
RT-PCR
Total ribonucleic acid (RNA) was extracted by using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from lung tissues,
and purified by using the extraction kit (Qiagen, Valencia, CA,
USA) according to instructions provided by the manufacturer. The
content of Nrf2 gene was detected by using the qRT-PCR kit,
followed by quantification using the fluorescence quantitative
detection system (Applied Biosystems, Foster City, CA, USA), with
β-actin as the internal reference. Primer sequences of gene
amplification are as follows: Nrf2 forward,
5′-CCCACACAAGGTTCGGCATCAC-3′ and reverse,
5′-TGGCGATTCCTCTGGCGTCT-3′; β-actin forward,
5′-CGCGCCATCAAGGAGAAGCTG-3′ and reverse,
5′-ATTGCCAATGGGTGATACCTG-3′.
Enzyme-linked immunosorbent assay
(ELISA) detection
Commercially-available ELISA kits were used to
detect the serum Nrf2 in rabbits in each group. According to
instructions provided by the manufacturer, Nrf2 was labeled by
using the double-antibody sandwich method, and the optical density
(OD) values were detected by using a microplate reader at dual
wavelengths of 450 and 600 nm, and the sample concentration was
calculated.
H&E staining and IHC
The nuclei and cytoplasm of lung tissues were
stained with H&E (Google Biological Co., Ltd., Wuhan, China),
and sections were dehydrated with gradient ethanol and then sealed
with neutral gum. The Nrf2 protein (1:500; Cell Signaling
Technology, Danvers, MA, USA) expressed in the nuclei was
specifically labeled by using the two-step method and IHC assay
kits (Zhongshan Golden Bridge Biotechnology Co., Ltd., Guangzhou,
China). H&E and IHC sections were observed under an inverted
microscope (DM-5000B; Leica Store Wetzlar, Wetzlar, Germany). At
least 3 regions were photographed in each section, and brown
yellow-stained cells in IHC staining were positive cells; the
proportion of Nrf2-positive cells in each field of view in the
total cells ≤5% indicated negative, while that >5% indicated
positive.
Arterial blood gas analysis
Blood was drawn from the femoral artery under
anesthesia, and the blood gas indexes (PaO2,
PaCO2 and SaO2) were determined by using a
blood-gas analyzer (Hunan Sanhe Apparatus, Changsha, China).
Statistical analysis
Experimental results were analyzed by using GraphPad
Prism statistical software 5.01 (GraphPad Software, Inc., La Jolla,
CA, USA). Measurement data are presented as mean ± SD, and one-way
analysis of variance (ANOVA) was used for the comparison of
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical manifestations and mortality
rate of the rabbit models
Within 12 h after modeling, rabbits in both model
and experimental groups spit pink frothy sputum, including 5
rabbits (50%) in the model group, and only 2 rabbits (20%) in the
experimental group. There were 6 deaths (60%) in the model group
and 3 deaths (30%) in the experimental group (Table I).
 | Table I.The percentage of pink frothy sputum
and death occuring in three groups at 12 h from modeling. |
Table I.
The percentage of pink frothy sputum
and death occuring in three groups at 12 h from modeling.
|
|
| Pink frothy
sputum | Death |
|---|
|
|
|
|
|
|---|
| Groups | No. | No. | % | No. | % |
|---|
| Control | 10 | 0 | 0 | 0 | 0 |
| Model | 10 | 5 | 50 | 6 | 60 |
| Experimental | 10 | 2 | 20 | 3 | 30 |
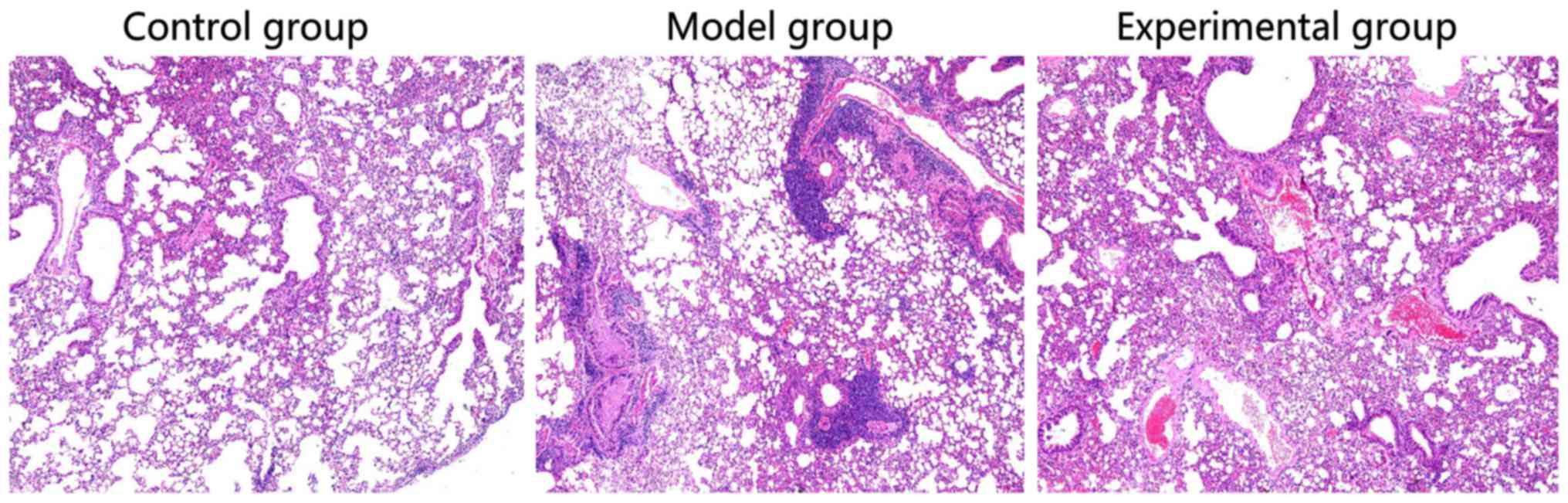
Pathological examination of lung
tissues of rabbits
Compared with the rabbit lung tissues in control
group, obvious alveolar edema, alveolar interstitial serous
exudate, aggregation of a large number of inflammatory cells, and
partial pulmonary septal thickening could be seen in rabbits in
model group. In experimental group, there was no aggregation of a
number of inflammatory cells, but only a small amount of serous
exudate (Fig. 1).
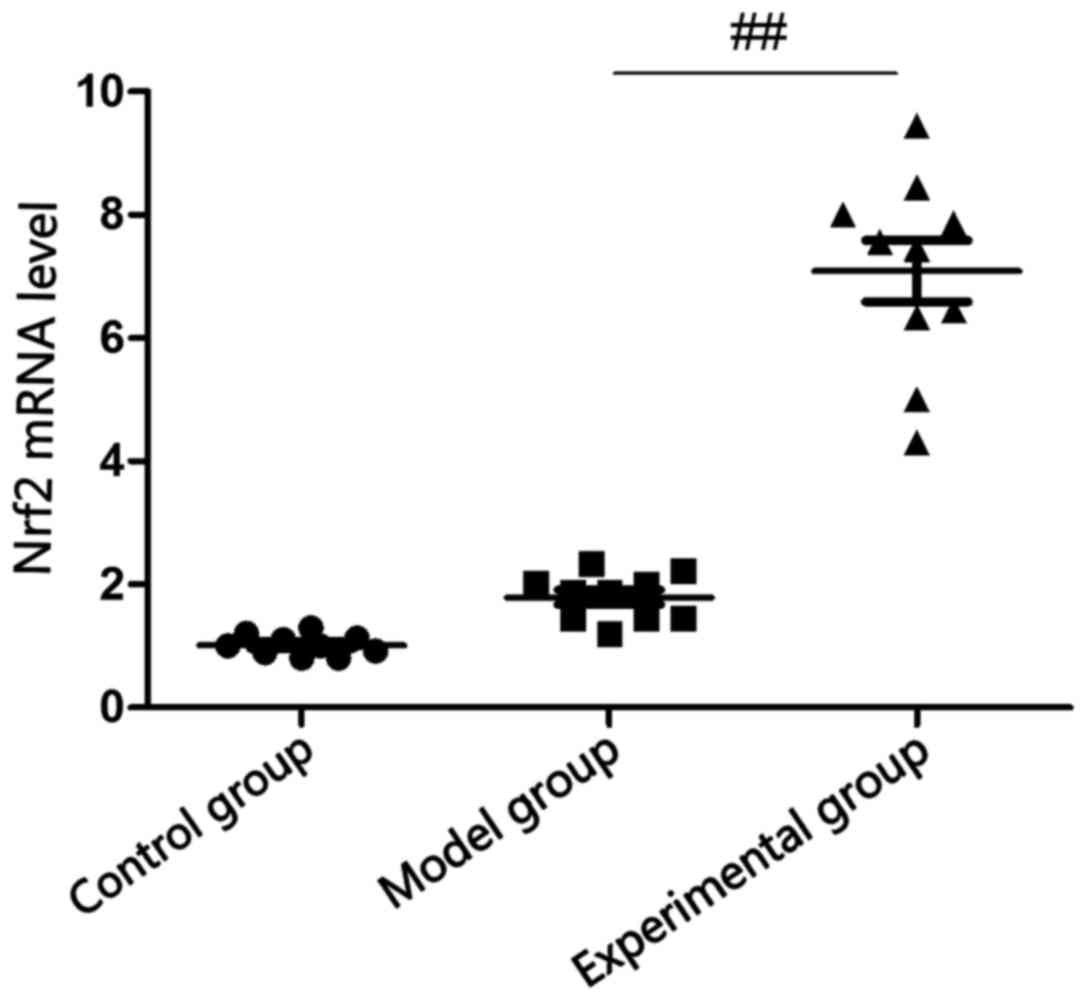
Detection of Nrf2 mRNA level in rabbit
lung tissues via RT-PCR
There was no statistically significant difference in
the comparison of Nrf2 mRNA level in rabbit lung tissues between
model group and control group (p>0.05), and Nrf2 mRNA level in
rabbit lung tissues in experimental group was significantly
increased compared with that in model group (p<0.05; Fig. 2).
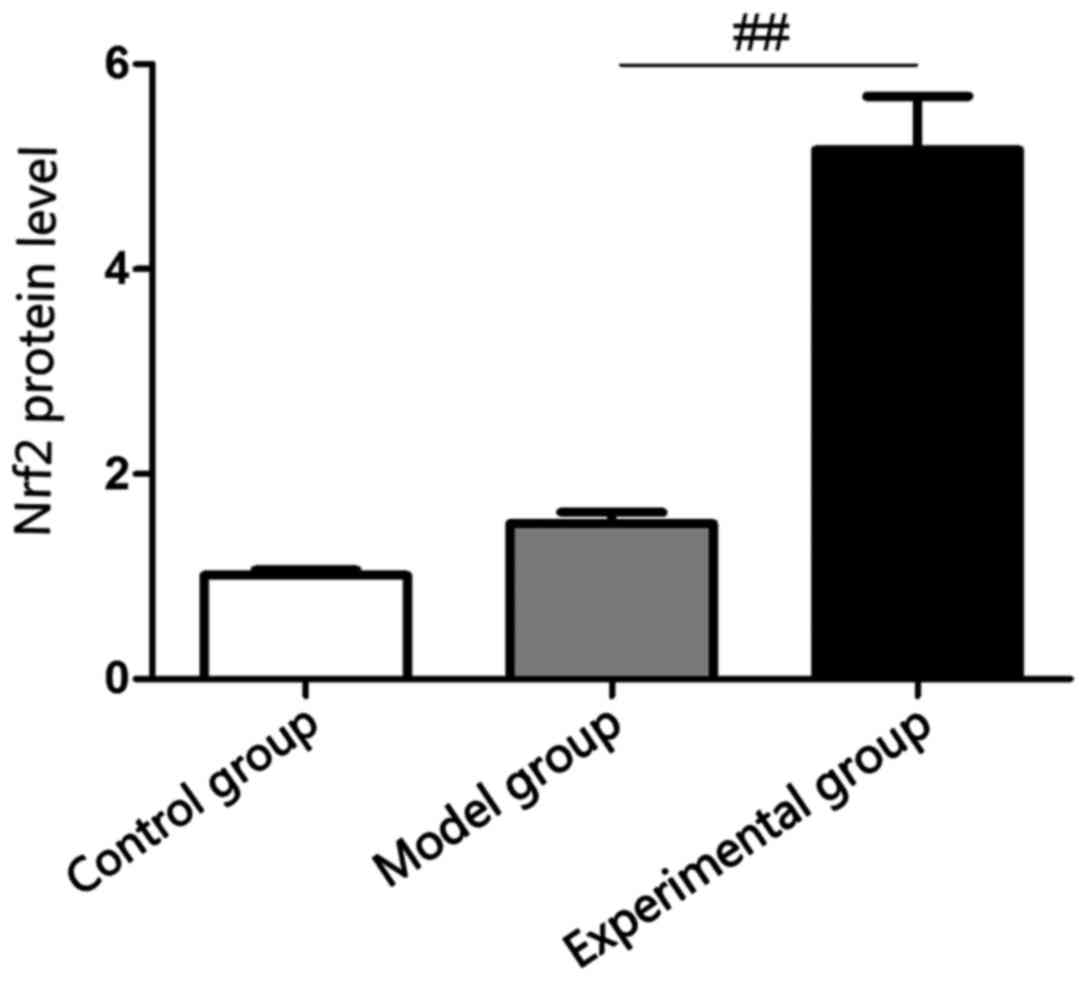
Detection of serum Nrf2 content in
rabbits via ELISA
ELISA showed that there was no statistically
significant difference in the comparison of Nrf2 protein level in
rabbit lung tissues between model and control groups (p>0.05),
and Nrf2 protein level in rabbit lung tissues in experimental group
was significantly increased compared with that in model group
(p<0.05), which were consistent with results of RT-PCR (Fig. 3).
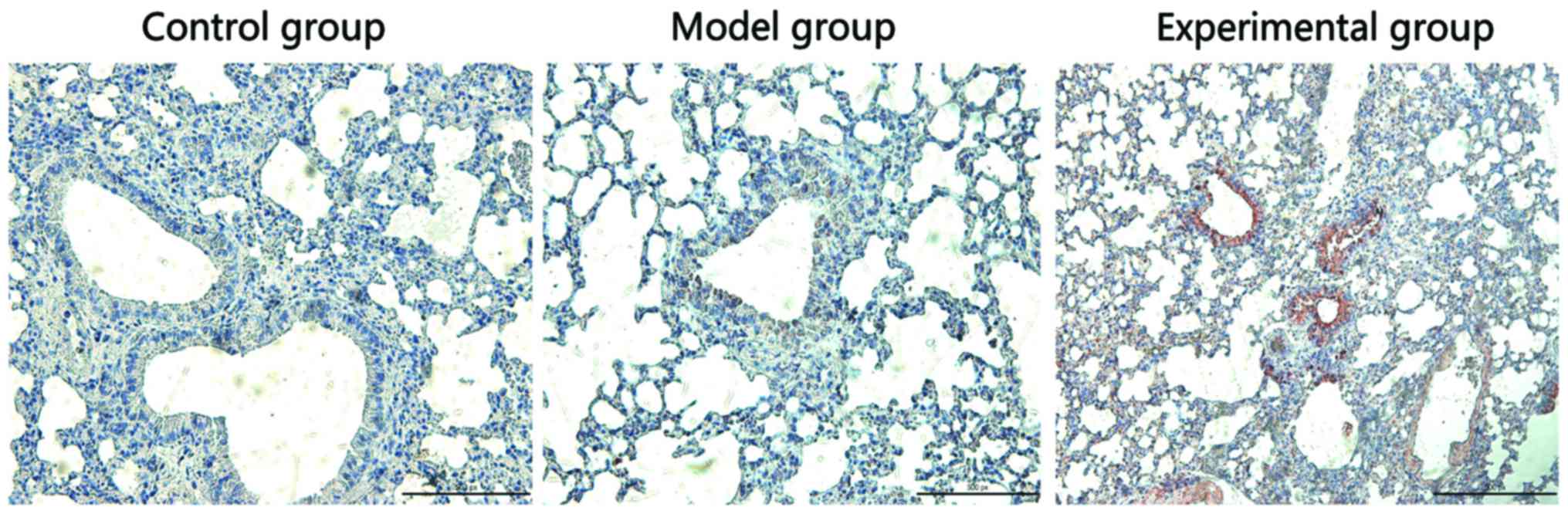
Detection of Nrf2 protein expression
in rabbit lung tissues via IHC
Nrf2 protein was mainly located in the cytoplasm. As
shown in Fig. 4, the Nrf2 expression
was negative in rabbit lung tissues in control group, which was
negative in model group, but strongly positive in the experimental
group.
Comparison of lung injury indexes
between two groups of rabbits
LI was selected to show the degree of pulmonary
edema (Table II).
 | Table II.Comparisons of lung injury indexes (LI
and IQA) between two groups of rabbits (mean ± SD). |
Table II.
Comparisons of lung injury indexes (LI
and IQA) between two groups of rabbits (mean ± SD).
| Groups | LI | IQA |
|---|
| Control | 4.32±0.12 | 12.36±2.07 |
| Model |
7.18±0.73a |
42.71±4.12b |
| Experimental |
5.76±0.42c |
22.73±6.65d |
Comparison of arterial blood gas
indexes between two groups of rabbits
Compared with those in control group, the blood gas
indexes (PaO2, PaCO2 and SaO2) in
the model and experimental groups were obviously decreased
(p<0.05); but the blood gas indexes in the experimental group
were significantly increased compared with those in the model group
(p<0.05; Table III).
 | Table III.Comparisons of PaO2,
PaCO2 and SaO2 between three groups 12 h
after modeling. |
Table III.
Comparisons of PaO2,
PaCO2 and SaO2 between three groups 12 h
after modeling.
| Index | Control | Model | Experimental |
|---|
| PaO2
(KPa) | 9.13±0.21 |
4.84±0.52a |
6.02±0.86b |
| PaC02
(KPa) | 5.42±0.27 |
2.16±0.43a |
3.94±1.05b |
| SaO2
(%) | 96.34±1.32 |
61.85±6.07a |
84.91±1.87c |
Discussion
ARDS is a non-cardiogenic pulmonary edema secondary
to alveolar damage after inflammatory process. However, the
pathogenesis of this disease remains to be elucidated (10). It was believed initially that the
primary cause of ALI is the cell activation caused by pathogenic
factors and body fluid, leading to inflammatory response syndrome
and pathological processes, such as alveolar collapse, imbalance of
ventilation/perfusion ratio and decreased lung compliance (11). The imbalance of inflammatory response
and anti-inflammatory response is of great significance in the
development of ARDS. According to its pathological features, ARDS
can be divided into early and late stages: Acute lung tissue
inflammation and injury (12).
The occurrence of inflammation begins from the
inflammatory cell exudate and immune cell-mediated breakdown of
alveolar epithelial interstitial barrier, making plasma and
proteins, flood the pulmonary interstitium and air space in turn
(13). Currently, ARDS is recognized
as a neutrophil-driven disease; moreover, it has been increasingly
recognized that innate cells (including macrophages and platelets)
and adaptive immune system are involved in the incidence of ARDS
(14,15). Experiments have found that
neutrophils and macrophages are recruited into inflammatory
lesions. The resulting inflammatory exudate interacts with the
surfactant, finally causing dysfunction (16). H&E assays in this study clearly
showed the aggregation of a number of inflammatory cells in rabbit
lung tissues, which was consistent with the above conclusion.
Nrf2 is a key regulator of antioxidant gene
activation. Reactive oxygen species (ROS) produced under
pathological conditions interact with the protein to damage the
body through a variety of pathways. Nrf2 can regulate the
expression of thioredoxin peroxidases, enhance the ability of cells
to scavenge ROS, maintain the redox equilibrium state of cells, and
reduce the oxidative damage (17).
Currently, the study on Nrf2 has shown that it is a promising
therapeutic target for ADRS, and many studies have demonstrated the
importance of Nrf2 activation in protecting ALI/ARDS (18). The study of Yu et al found
that sulforaphane increases the Nrf2 gene expression in lung
tissues of mice with lipopolysaccharide (LPS)-induced ALI (19). Nrf2 is also involved in the
infection-induced ALI. Studies have shown that pneumonia is caused
by Staphylococcus aureus, and develops into ALI in extreme
cases due to increased alveolar permeability, neutrophil
infiltration and production of cytokines. Compared with those in
Nrf2−/− mice, ROS produced by mitochondria and
mitochondrial autophagy-degradation-induced pulmonary epithelial
cell apoptosis are significantly inhibited in Nrf2+/+
transgenic mice vaccinated with Staphylococcus aureus
(20). In this study, sulforaphane
significantly increased the Nrf2 expression in rabbit lung tissues
and serum, and protect from lung injury in rabbits.
In conclusion, results of this study indicate that
sulfadoxine exerts a significant anti-inflammatory effect on ARDS
in rabbits through upregulating the Nrf2 expression. In addition,
results of this study provide a certain theoretical basis for the
application of sulforaphane and clinical prophylactic treatment of
ALI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS wrote the manuscript and helped with ELISA. ZN
and SW analyzed H&E staining and IHC. SS contributed to
arterial blood gas analysis. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Cangzhou Central Hospital (Cangzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thille AW, Esteban A, Fernández-Segoviano
P, Rodriguez JM, Aramburu JA, Vargas-Errázuriz P, Martín-Pellicer
A, Lorente JA and Frutos-Vivar F: Chronology of histological
lesions in acute respiratory distress syndrome with diffuse
alveolar damage: A prospective cohort study of clinical autopsies.
Lancet Respir Med. 1:395–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin TR, Pistorese BP, Chi EY, Goodman
RB and Matthay MA: Effects of leukotriene B4 in the human lung.
Recruitment of neutrophils into the alveolar spaces without a
change in protein permeability. J Clin Invest. 84:1609–1619. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frank JA, Wray CM, McAuley DF, Schwendener
R and Matthay MA: Alveolar macrophages contribute to alveolar
barrier dysfunction in ventilator-induced lung injury. Am J Physiol
Lung Cell Mol Physiol. 291:L1191–L1198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comstock TL and Decory HH: Advances in
corticosteroid therapy for ocular inflammation: Loteprednol
etabonate. Int J Inflamm. 2012:7896232012. View Article : Google Scholar
|
|
6
|
Emr BM, Roy S, Kollisch-Singule M, Gatto
LA, Barravecchia M, Lin X, Young JL, Wang G, Liu J, Satalin J, et
al: Electroporation-mediated gene delivery of Na+,
K+-ATPase, and ENaC subunits to the lung attenuates
acute respiratory distress syndrome in a two-hit porcine model.
Shock. 43:16–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ather JL, Alcorn JF, Brown AL, Guala AS,
Suratt BT, Janssen-Heininger YM and Poynter ME: Distinct functions
of airway epithelial nuclear factor-kappaB activity regulate
nitrogen dioxide-induced acute lung injury. Am J Respir Cell Mol
Biol. 43:443–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Taneja R, Wang W, Yao LJ,
Veldhuizen R, Gill SE, Fortin D, Inculet R, Malthaner R and Mehta
S: Human alveolar epithelial cells attenuate pulmonary
microvascular endothelial cell permeability under septic
conditions. PLoS One. 2:e553112013. View Article : Google Scholar
|
|
9
|
Fard N, Saffari A, Emami G, Hofer S,
Kauczor HU and Mehrabi A: Acute respiratory distress syndrome
induction by pulmonary ischemia-reperfusion injury in large animal
models. J Surg Res. 189:274–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan YM, Li YD, Song XL, Liu M, Diao F,
Wang Y, Sun Y, Wang ZH and Lu J: Therapeutic effects of inhaling
aerosolized surfactant alone or with dexamethasone generated by a
novel noninvasive apparatus on acute lung injury in rats. J Trauma
Acute Care Surg. 73:1114–1120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt AE and Adamski J: Education
Committee of the Academy of Clinical Laboratory Physicians and
Scientists: Pathology consultation on transfusion-related acute
lung injury (TRALI). Am J Clin Pathol. 138:498–503. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chi X, Zhang R, Shen N, Jin Y, Alina A,
Yang S and Lin S: Sulforaphane reduces apoptosis and oncosis along
with protecting liver injury-induced ischemic reperfusion by
activating the Nrf2/ARE pathway. Hepatol Int. 9:321–329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin W, Wu RT, Wu T, Khor TO, Wang H and
Kong AN: Sulforaphane suppressed LPS-induced inflammation in mouse
peritoneal macrophages through Nrf2 dependent pathway. Biochem
Pharmacol. 76:967–973. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bernard GR, Artigas A, Brigham KL, Carlet
J, Falke K, Hudson L, Lamy M, Legall JR, Morris A and Spragg R: The
American-European Consensus Conference on ARDS. Definitions,
mechanisms, relevant outcomes, and clinical trial coordination. Am
J Respir Crit Care Med. 149:818–824. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdulnour RE, Howrylak JA, Carlo T, Sham
HP, Henkels KM, Miller TE, Dolinay T, Baron RM, Choi MK, Cambronero
JG, et al: Phospholipase D regulates inflammatory responses during
acute lung injury. Am J Respir Crit Care Med. 193:A77732016.
|
|
16
|
Fernandez-Bustamante A and Repine JE:
Chronic inflammatory diseases and the acute respiratory distress
syndrome (ARDS). Curr Pharm Des. 20:1400–1408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Myzak MC, Karplus PA, Chung FL and
Dashwood RH: A novel mechanism of chemoprotection by sulforaphane:
Inhibition of histone deacetylase. Cancer Res. 64:5767–5774. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han CW, Kwun MJ, Kim KH, Choi JY, Oh SR,
Ahn KS, Lee JH and Joo M: Ethanol extract of Alismatis Rhizoma
reduces acute lung inflammation by suppressing NF-κB and activating
Nrf2. J Ethnopharmacol. 146:402–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu J, Wang Y, Li Z, Dong S, Wang D, Gong
L, Shi J, Zhang Y, Liu D and Mu R: Effect of heme oxygenase-1 on
mitofusin-1 protein in LPS-induced ALI/ARDS in rats. Sci Rep.
6:365302016.doi: 10.1038/srep36530. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shan Y, Akram A, Amatullah H, Zhou DY,
Gali PL, Maron-Gutierrez T, González-López A, Zhou L, Rocco PR and
Hwang D: ATF3 protects pulmonary resident cells from acute and
ventilator-induced lung injury by preventing Nrf2 degradation.
Antioxid Redox Signal. 22:651–668. 2015. View Article : Google Scholar : PubMed/NCBI
|