Introduction
Cerebrospinal fluid (CSF) hypovolemia, also referred
to as intracranial hypotension (IH), is a common neurosurgical
scenario, which may occur spontaneously or be iatrogenic. The
clinical manifestation ranges from headache, cranial nerve palsy to
mental state decline, and may result in death (1–4). Based
on the medical history and characteristic symptoms of affected
patients the diagnosis is relatively straight-forward. Regardless
of the causes, patients with CSF hypovolemia frequently have
pre-existent continuous CSF leakage (1–5).
Post-operative CSF hypovolemia is a specific type of CSF
hypovolemia and a substantial number of the reported cases of early
post-operative CSF hypovolemia were identified to had unintentional
or unrecognized post-operative continuous excessive CSF leakage
according to further investigation (1–3,6–9). The
most severe types of post-operative CSF hypovolemia are
pseudohypoxic brain swelling (PHBS) and post-operative
IH-associated venous congestion (PIHV), which is characterized by
effacement of basal cisterns, ventricular collapse, venous
congestion and changes in bilateral deep gray structures on imaging
(3,6–9). As a
result of the excessive loss of CSF through lumbar drainage or
sub-galeal suction drainage over a short period, patients with PHBS
or PIHV often experience irreversible progressive exacerbation and
death. The present study provided another series of patients who
were diagnosed with post-operative CSF hypovolemia after uneventful
intracranial surgery. Of note, in contrast to previous studies, no
post-operative lumbar or sub-galeal suction drainage was placed.
Due to early recognition and timely management, the affected
patients experienced relatively favorable outcomes.
Case report
Between January 2011 and December 2015, 2,748
emergencies or scheduled intracranial surgeries were performed at
the Department of Neurosurgery of the First Hospital of Jilin
University (Eastern Division; Changchun, China). A retrospective
review of the medical records of the patients was performed to
identify those who developed early post-operative CSF hypovolemia
without the existence of continuous CSF leakage. The present study
was approved by the Ethics Committee of The First Hospital of Jilin
University (Changchun, China). Written informed consent for the
publication of their data and any accompanying images was obtained
from the patients or their guardians. The definition of early
post-operative CSF hypovolemia without continuous CSF leakage was
as follows: i) Progressive decline in mental state or other
neurological symptoms that were not explainable by factors that may
cause an acute increase in intracranial pressure (e.g.,
intracranial bleeding, massive ischemic stroke and hydrocephalus);
ii) drainage modalities, as lumbar drainage and sub-galeal suction
drainage were absent; iii) evidence of CSF hypovolemia by computed
tomography (CT) (e.g., collapse of the ventricular system,
effacement of the cisterns, sub-dural collection, brain sag and
intracranial venous engorgement); iv) Low or normal intracranial
pressure (ICP) identified by ICP monitoring or lumbar puncture; v)
Exclusion of intra- and post-operative cerebral hypoxia; vi)
evident improvement of the clinical symptoms when management for
CSF hypovolemia was initiated.
Patient characteristics
A total of 7 patients, 5 of which were male, were
identified in the present retrospective review (Table I). The patient age ranged from 44 to
61 years (mean, 47.86 years). They experienced CSF hypovolemia
between days 1 and 7 after emergency or scheduled intracranial
surgery. Ventricular collapse, cisternal effacement and midline
shift are the most common radiological observations. Sub-dural
effusion (SDE) was identified in two patients, one of whom
experienced spontaneous absorption after mannitol ceasing and
intravenous hydration, and the other one underwent decompressive
craniectomy to halt irreversible progressive decline in mental
state. A total of 4 patients achieved a Glasgow Outcome Scale (GOS)
score of 5, 1 achieved a GOS of 4 and the other 2 a GOS of 3. No
mortality was noted in this series.
 | Table I.Patient characteristics and
outcome. |
Table I.
Patient characteristics and
outcome.
| Case no. | Sex/age (years) | Accompanying
diseases | Indication for
surgery | Type of surgery | Time interval to CSF
hypovolemia | Findings on
imaging | Management | Outcome (GOS) |
|---|
| 1 | M/47 | No | Acute SDH | Decompressive
craniectomy | 5 days PO | Ventricular collapse,
cisternal effacement, midline shift | Trendelenburg
position and intravenous hydration | 5 |
| 2 | M/44 | No |
Craniopharyngioma | Craniotomy and tumor
resection | 7 days PO | Ventricular collapse,
cisternal effacement, midline shift, subdural effusion | Decompressive
craniectomy and intravenous hydration | 5 |
| 3 | F/46 | No | Intracranial
aneurysm | Craniotomy and
aneurysm clipping | 3 days PO | Ventricular collapse,
cisternal effacement, midline shift, subdural effusion | Mannitol ceasing and
intravenous hydration | 5 |
| 4 | M/61 | No | Meningioma | Craniotomy and tumor
resection | 3 days PO | Ventricular collapse,
cisternal effacement | Bed rest and
intravenous hydration | 5 |
| 5 | M/52 | Diabetes | Acute SDH | Decompressive
craniectomy | 2 days PO | Ventricular collapse,
cisternal effacement, midline shift | Trendelenburg
position and intravenous hydration | 3 |
| 6 | F/37 | No | ICH | Decompressive
craniectomy | 3 days PO | Ventricular collapse,
cisternal effacement, midline shift | Mannitol ceasing and
intravenous hydration | 4 |
| 7 | M/48 | Hypertension | Intracranial
aneurysm | Decompressive
craniectomy | 1 day PO | Ventricular collapse,
cisternal effacement, midline shift | Mannitol ceasing,
Trendelenburg position and intravenous hydration | 3 |
Illustrative cases
Case 1
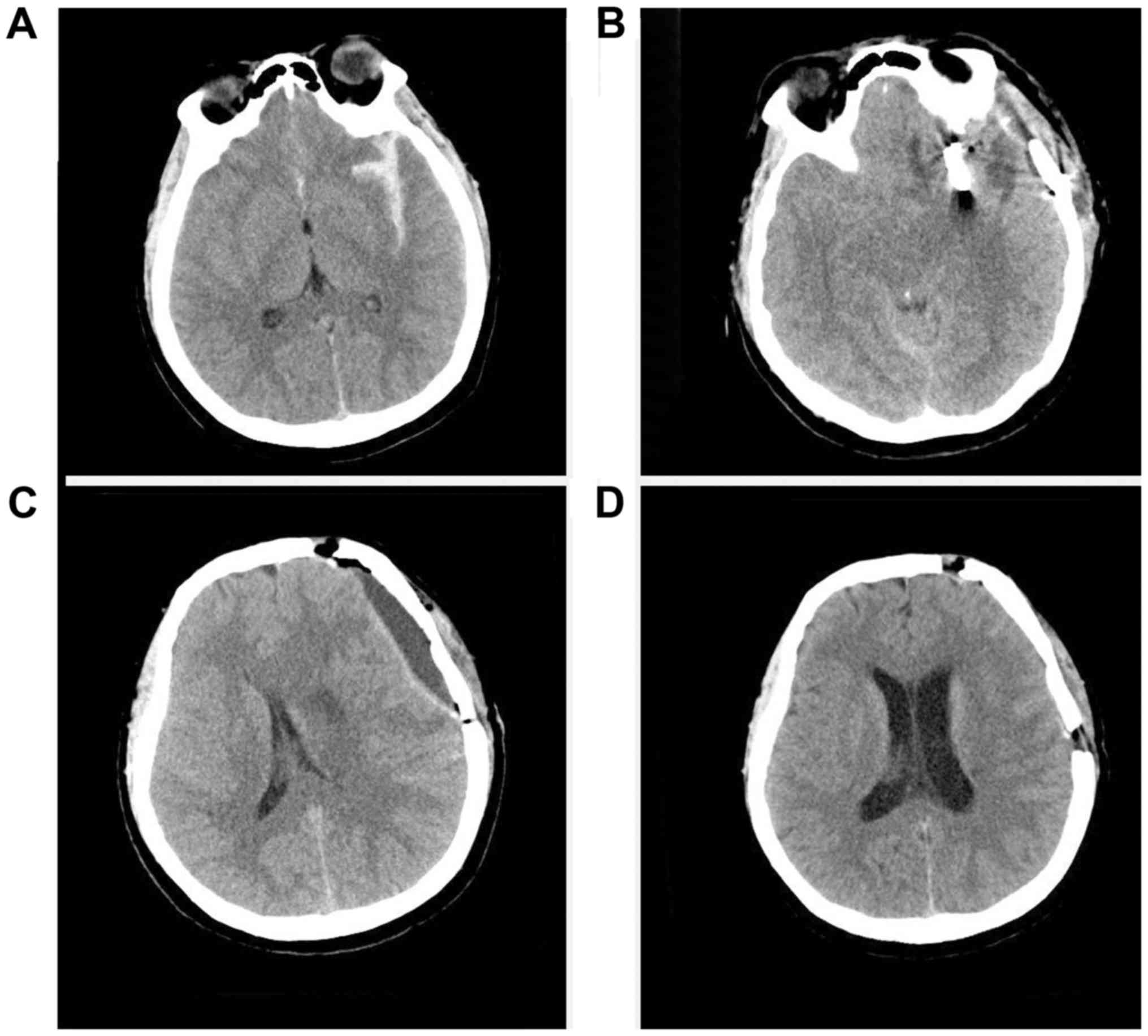
A 47-year-old healthy man was admitted after a car
accident. Head CT revealed an acute subdural hematoma at the right
frontoparietotemporal region and an evident midline shift to the
left side (Fig. 1A). An emergent
decompressive craniectomy was performed. A head CT performed at 1
day post-operatively revealed complete evacuation of the subdural
hematoma and restoration of the midline shift (Fig. 1B). The patient experienced an
uneventful recovery process until his consciousness began to
decline on post-operative day 5. Although the decompressive site
was soft on palpation and the ICP monitoring result was 80 mm
H2O, CT revealed brain swelling, collapse of the
ventricular system, effacement of the cisterns and midline shift to
the left side (Fig. 1C and D).
Intravenous (iv) administration of 50 g mannitol three times a day
(tid) was prescribed. The patient's mental state continued to
decline. CT performed at 10 days post-operatively revealed
exacerbation of the brain swelling and effacement of the cisterns,
and a more evident midline shift. The presence of CSF hypovolemia
was then considered. Intravenous mannitol was ceased and
intravenous hydration and Trendelenburg position were subsequently
initiated, following which the patient's consciousness was
obviously regained. CT performed at 14 days post-operatively
indicated re-expansion of the ventricular system and cisterns, as
well as restoration of the midline (Fig.
1E and F). The patient experienced a favorable recovery and a
custom-made titanium cranioplasty was performed after 4 months. His
GOS score was 5 at 1 year's follow-up.
Case 3
A 46-year-old woman was admitted to our department
for subarachnoid hemorrhage (Fig.
2A). The subarachnoid hemorrhage was demonstrated to be rupture
of a left middle cerebral aneurysm by further CT angiography. No
past medical history was reported. She underwent an uneventful
microsurgical clipping of the aneurysm. Administration of mannitol
(50 g, tid, iv) was prescribed to prevent post-operative brain
edema. Three days post-operatively, the patient's mental state
declined. CT revealed basal cistern effacement and left SDE
(Fig. 2B and C). The opening
pressure was 110 mm H2O on subsequent lumbar puncture in
the lateral decubitus position. As CSF hypovolemia was suspected,
mannitol was ceased and intravenous hydration was initiated,
following which the patient's consciousness began to recover
gradually. At 7 days post-operatively, the SDE disappeared
spontaneously with midline restoration (Fig. 2D). The patient was discharged with a
Glasgow Coma Scale (GCS) score of 15 (10).
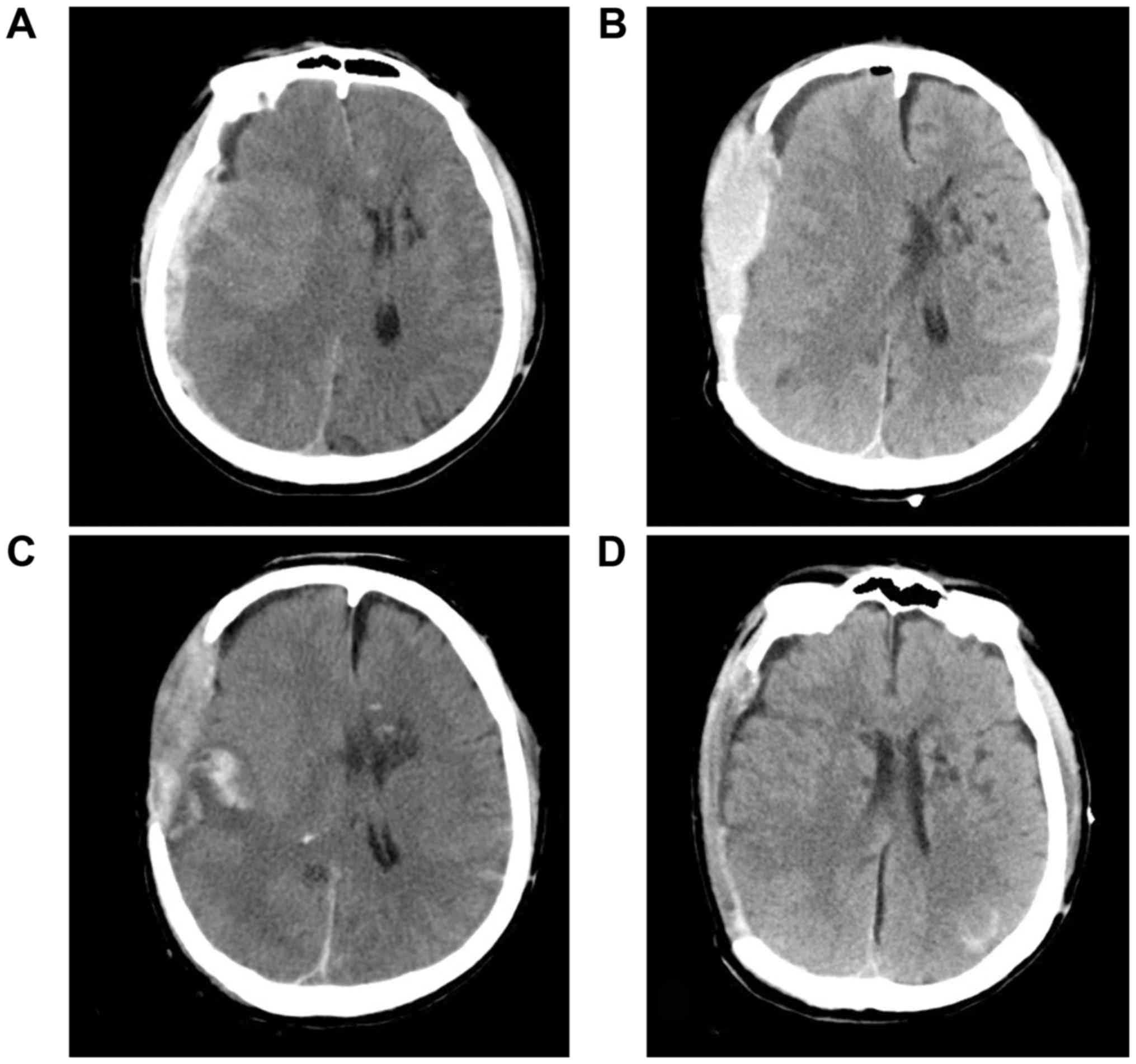
Case 5
A 52-year-old man was admitted to our department
following a car accident. Head CT performed at the emergency room
revealed an extensive subdural hematoma at the right side (Fig. 3A). Physical examination indicated
decerebrate rigidity, anisocoria and a GCS score of 4. He had a
history of diabetes for 2 years, as well as smoking and alcohol
abuse for >30 years. An emergent decompressive craniectomy was
performed. On day 1 post-operatively, he was able to withdraw from
painful stimuli and his pupils were symmetrical and reactive to
light. The ICP monitoring results ranged from 5 to 11 mm Hg. On day
2 post-operatively, he developed a decorticate response and
anisocoria. No elevation of the ICP was noted. Immediate CT
revealed epidural hematoma, collapse of the ventricular system and
a midline shift to the left side (Fig.
3B). An emergency epidural hematoma evacuation was performed.
However, his neurological state did not recover after the second
operation. Another CT indicated no improvement of the collapse of
the ventricular system and midline shift (Fig. 3C). The patient was placed in a
Trendelenburg position and given sufficient intravenous hydration.
His pupils began to become symmetrical and reactive. CT performed
on day 5 post-operatively indicated re-expansion of the ventricular
system and cisterns, as well as restoration of the midline
(Fig. 3D). A custom-made titanium
cranioplasty was performed 3 months later. His GOS score was 3 at 2
year's follow-up.
Discussion
According to a literature review performed as part
of the present study, there are two types of post-operative CSF
hypovolemia. The first one comprises an early decline in mental
state or even fatal incidents due to unintentional or unrecognized
continuous excessive CSF depletion (1–3,6–9). The
most typical cases were reported by Van Roost et al
(3), for which 17 cases of
post-operative diffuse brain swelling and sulcal effacement were
reported. Further investigation revealed that excessive sub-galeal
suction drainage of CSF was responsible for this phenomenon. As the
alterations on imaging were similar to those of cerebral hypoxia
(effacement of basal cisterns, ventricular collapse, venous
congestion, and changes in bilateral deep gray structures on
imaging investigations), PHBS or PIHV, which are terms adopted by
subsequent studies, was selected to describe this scenario to
distinguish the pathology from real cerebral hypoxia (6–9). The
second type features immediate deterioration in mental state after
CSF depletion or sampling (usually lumbar puncture) several weeks
after decompressive craniectomy (11–13). The
most typical study was published recently by Creutzfeldt et
al (13). Through performing a
study on patients at their institution and a literature review,
they concluded that lumbar puncture at ≥1 month after decompressive
craniectomy posed a risk of provoking paradoxical herniation.
However, none of the patients developed symptomatic CSF hypovolemia
in the early post-operative period of their study. Komotar et
al (14) reported 11 cases of
post-operative CSF hypovolemia after uneventful clipping of
intracranial aneurysms in 137 patients. Although intra-operative
lumbar drainage was deemed as the cause, issues regarding
post-operative sub-galeal suction drainage and hyperosmotic therapy
were not mentioned.
The present study provided a case series of a type
of post-operative CSF hypovolemia that requires early diagnosis and
management. Even though post-operative lumbar or sub-galeal
drainage were not present, CSF hypovolemia occurred in these cases.
Perhaps as a result of the absence of persistent excessive CSF
depletion and early management, favorable recovery was achieved.
According to our experience, the causes of CSF hypovolemia in this
case series may be as follows: i) Substantial loss of CSF during
surgery that was not readily compensated by post-operative CSF
production, ii) inappropriate early post-operative hyperosmotic
therapy exacerbated CSF depletion, iii) focal cerebral edema
exacerbated the condition of CSF hypovolemia, and iv) atmospheric
pressure imposed on the brain tissue due to decompressive
craniectomy.
The mechanism of post-operative CSF hypovolemia was
similar to that of spontaneous CSF hypovolemia. Two major
pathophysiological processes prevail in this scenario. First,
substantial depletion of CSF impairs its buoyancy effect on the
intracranial structures. Downward migration of the intracranial
contents results in traction and compression of the neurovascular
structures. Subsequently, according to the doctrine of
Monro-Kellie, a decrease in CSF must be compensated by other
intracranial contents (15).
Therefore, subdural fluid collection, engorgement of venous
structures, and cytotoxic as well as vasogenic brain edema occurs,
which leads to further deterioration of the mental state or even
irreversible brain edema and fatal outcome (3,4,6–9,16). Of note, patients with pre-existing
cerebellar tonsillar herniation are more likely to have a fatal
outcome (4,17,18). The
condition is more complex when a cranial defect is present
(18). Atmospheric pressure further
exacerbates compression of the neurovascular structure and downward
displacement of the intracranial contents.
The terminology for describing symptoms of CSF
depletion varies between different studies. Certain studies prefer
the term IH, while others prefer ‘CSF hypovolemia’. According to
Miyazawa et al (5), certain
patients with so-called IH demonstrated normal CSF pressure despite
the presence of typical symptoms. The buoyancy effect of CSF on the
intracranial contents depends on the CSF volume, not the
intracranial pressure. The typical alterations (subdural fluid
collection, engorgement of venous structures, as well as cytotoxic
and vasogenic brain edema) are also due to CSF volume depletion,
not intracranial pressure. According to our experience and the
descriptions in other studies, we prefer the term ‘CSF hypovolemia’
over ‘IH’ to describe this specific entity (1,2,5).
The management of early post-operative CSF
hypovolemia without continuous CSF leakage is similar to that of
CSF hypovolemia caused by spontaneous or iatrogenic continuous CSF
leakage. The key step is its correct and early diagnosis. When the
diagnosis of CSF hypovolemia is reached, factors including
continued CSF drainage, hyperosmotic agents and positions with head
elevation that may exacerbate the condition should be avoided.
Trendelenburg position and sufficient intravenous hydration are
practical and effective methods which are able to reverse the
pathology in a substantial number of patients (1,13,18). In
rare circumstances, an emergent decompressive sub-occipital
craniectomy is lifesaving (4).
In conclusion, although rare in incidence, early
post-operative CSF hypovolemia may occur without the existence of
intentional or unintentional post-operative continuous CSF
drainage. When the diagnosis of CSF hypovolemia is reached, factors
that may impair CSF compensation should be promptly terminated.
Trendelenburg position and sufficient intravenous hydration are
practical and effective treatments, and the pathology was thereby
reversed in a substantial number of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KH and XZ analyzed and interpreted the imaging data.
YZ, XG, and SS performed a thorough review of the medical records
and made substantial contributions to conception and acquisition of
data. JZ and GL interpreted the clinical data and drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee at
the First Hospital of Jilin University. Informed consent for
participation in the study or use of their medical data was
obtained from all participants or their legal guardian.
Consent for publication
Written informed consent was obtained from the
patients or their guardians for publication of this manuscript and
any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSF
|
cerebrospinal fluid
|
|
ICP
|
intracranial pressure
|
|
SDE
|
subdural effusion
|
|
GOS
|
Glasgow Outcome Scale
|
|
CT
|
computed tomography
|
|
GCS
|
Glasgow Coma Scale
|
|
IH
|
intracranial hypotension
|
References
|
1
|
Kawahara I, Tsutsumi K, Matsunaga Y,
Takahata H, Ono T, Toda K and Baba H: Early awareness of
cerebrospinal fluid hypovolemia after craniotomy for microsurgical
aneurysmal clipping. Acta Neurochir (Wien). 155:1543–1548. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li G, Zhu X, Zhang Y, Zhao J, Han Z and
Hou K: Cranial nerve palsy secondary to cerebrospinal fluid
diversion. Clin Neurol Neurosurg. 143:19–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Roost D, Thees C, Brenke C, Oppel F,
Winkler PA and Schramm J: Pseudohypoxic brain swelling: A newly
defined complication after uneventful brain surgery, probably
related to suction drainage. Neurosurgery. 53:1315–1326. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugrue PA, Hsieh PC, Getch CC and Batjer
HH: Acute symptomatic cerebellartonsillar herniation following
intraoperative lumbar drainage. J Neurosurg. 110:800–803. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyazawa K, Shiga Y, Hasegawa T, Endoh M,
Okita N, Higano S, Takahashi S and Itoyama Y: CSF hypovolemia vs
intracranial hypotension in ‘spontaneous intracranial hypotension
syndrome’. Neurology. 60:941–947. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parpaley Y, Urbach H, Kovacs A, Klehr M
and Kristof RA: Pseudohypoxic brain swelling (postoperative
intracranial hypotension-associated venous congestion) after spinal
surgery: Report of 2 cases. Neurosurgery. 68:E277–E283. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yokota H, Yokoyama K, Miyamoto K and
Nishioka T: Pseudohypoxic brain swelling after elective clipping of
an unruptured anterior communicating artery aneurysm. Clin Neurol
Neurosurg. 111:900–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Evins AI, Boeris D, Burrell JC and Ducati
A: Postoperative intracranial hypotension-associated venous
congestion: Case report and literature review. Clin Neurol
Neurosurg. 115:2243–2246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Snyder KA, Clarke MJ, Gilbertson JR and
Hocker SE: Prompt recognition and management of postoperative
intracranial hypotension-associated venous congestion: A case
report. Neurocrit Care. 24:448–453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teasdale G and Jennett B: Assessment of
coma and impaired consciousness. A practical scale. Lancet.
2:81–84. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oyelese AA, Steinberg GK, Huhn SL and
Wijman CA: Paradoxical cerebral herniation secondary to lumbar
puncture after decompressive craniectomy for a large
space-occupying hemispheric stroke: Case report. Neurosurgery.
57:E5942005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung HJ, Kim DM and Kim SW: Paradoxical
transtentorial herniation caused by lumbar puncture after
decompressive craniectomy. J Korean Neurosurg Soc. 51:102–104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Creutzfeldt CJ, Vilela MD and Longstreth
WT Jr: Paradoxical herniation after decompressive craniectomy
provoked by lumbar puncture or ventriculoperitoneal shunting. J
Neurosurg. 123:1170–1175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Komotar RJ, Mocco J, Ransom ER, Mack WJ,
Zacharia BE, Wilson DA, Naidech AM, McKhann GM 2nd, Mayer SA,
Fitzsimmons BF and Connolly ES Jr: Herniation secondary to critical
postcraniotomy cerebrospinal fluid hypovolemia. Neurosurgery.
57:286–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lundberg N: The saga of the Monro-Kellie
doctrine in intracranial pressure VIshii S, Nagai H and Brock M:
Proceedings of the fifth international symposium on intracranial
pressure. Springer; Tokyo: pp. 68–76. 1983, View Article : Google Scholar
|
|
16
|
Hadizadeh DR, Kovács A, Tschampa H,
Kristof R, Schramm J and Urbach H: Postsurgical intracranial
hypotension: Diagnostic and prognostic imaging findings. AJNR Am J
Neuroradiol. 31:100–105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dagnew E, van Loveren HR and Tew JM Jr:
Acute foramen magnum syndrome caused by an acquired Chiari
malformation after lumbar drainage of cerebrospinal fluid: Report
of three cases. Neurosurgery. 51:823–828. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Li G, Zhang Y, Zhu X and Hou K:
Sinking skin flap syndrome and paradoxical herniation secondary to
lumbar drainage. Clin Neurol Neurosurg. 133:6–10. 2015. View Article : Google Scholar : PubMed/NCBI
|