Introduction
The lysosome is an important organelle in cells; it
has previously been regarded as the ‘garbage disposal’ organelle in
cells (1), as it contains >50
soluble acid hydrolases. The lysosome is now regarded as a key
subcellular organelle (2), acting to
degrade cellular components through initiation by phagocytosis,
autophagy and other pathways (3).
The characteristic acidic environment (pH 4.5–5.0) of lysosomes
provides an optimal environment for lysosomal hydrolase activity,
and this contributes to macromolecular degradation (4). If the internal pH changes, the activity
of internal hydrolytic enzymes will change, thus affecting the
function of the lysosomes. The change of lysosomal function can
lead to reactions inside the cell. The lysosomal membrane proteins
that are responsible for sustaining membrane integrity and
regulating lysosomal function are not completely known. As
lysosomal membrane integrity is important for the fate of cells,
once it is destroyed by a procedure known as lysosomal membrane
permeabilization (LMP), lysosomal content leakage will occur
(5). The leakage of lysosomal
constituents may be sufficient to trigger cell death (5).
SID1 transmembrane family member 2 (sidt2), a
lysosomal membrane protein, has previously been studied (3). Sidt2 is a lysosomal membrane protein.
In a previous study, sidt2 was identified as a novel integral
lysosomal membrane protein with a molecular weight of 94 kDa
(6). Sidt2 functions as an integral
protein and is associated with signaling pathways, including the
PTEN-induced putative kinase and CUP-5 proteins that regulate
lysosomal autophagy and apoptosis (3). The present study utilized a sidt2
deficient mouse model to explore the function and mechanisms of
sidt2 action in liver lipid metabolism and changes of LMP.
Materials and methods
Animals
Cre mice mated with sidt2
LoxP-Flox-LoxP−/+ mice to obtain
sidt2−/+Cre+/− mice. A total of 100 male and
200 female mice (age, 8–10 weeks; weight, 25–30 g) were purchased
from the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). Normal male rats can be used for breeding offspring after 8
weeks and females after 10 weeks. The mice were maintained in a
controlled temperature (22–25°C) and humidity (50–60%) with a 12 h
light/dark cycle and fed a controlled diet and water. The animals
had free access to food and water under basic feeding conditions.
To prevent the phenotypic effects of Cre mice, the F2 generation of
Sidt−/+ mice was used to mate with wild-type strain 129
mice (a total of 100 Cre mice and 100 sidt2
LoxP-Flox-LoxP−/+ mice were used) and F3
sidt2−/+Cre−/− mice were established. Through
the next generation and wild-type mice of the same strain, the Cre
genotype was removed and the heterozygous sidt2−/+ mice
of the sidt2 knockout were obtained. The sidt2−/+ mice
were bred with each other to obtain full knockout homozygous
sidt2−/− mice. Adult F3 generation mice mated with each
other to produce the sidt2−/− mice. Anesthesia was
administered in every operation to minimize the pain. Animal
experiments were reviewed and approved by Animal Ethics Committee
of Wannan Medical College (Wuhu, China).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
The mice were sacrificed and total RNA was extracted
from tissue in the liver, stomach, spleen, heart, kidney,
intestine, brain, pancreas and lung, and prepared using an RNA
extraction kit (cat. no. SK8652; Sangon Biotech Co., Ltd.,
Shanghai, China) according to the manufacturer's instructions. A
Reverse Transcription kit was purchased from Takara Biotechnology
Co., Ltd. (Dalian, China; cat. no. RR037A). The reverse
transcription reaction system was made up of 1 µl primer and 1 µl
dNTP, denaturation occurred at 65°C for 5 min then the mixture was
placed on ice for 5 min. A total of 2 µl DTT, 4 µl reverse
transcription buffer and 1 µl RNAase inhibitor were added. The
mixture was centrifuged at 10,000 × g for 1 min at room temperature
and incubated at 37°C for 2 min. A total of 1 µl reverse
transcriptase was added and incubated at 70°C for 15 min to perform
PCR amplification. The primers used were designed with Primer 5.0
software (Premier Biosoft International, Palo Alto, CA, USA) and
synthesized by Sangon Biotech Co., Ltd. Primer sequences for sidt2
were as follows: Forward, 5′-ATGTGGTGGTGGTAGTGAAG-3′, and reverse,
5′-AGATACACCACCACCATCAC-3′. PCR was performed as follows: 5 min at
95°C, followed by 34 cycles of 45 sec at 94°C, 45 sec at 56°C, 1
min at 72°C and 10 min at 72°C.
Analysis of blood lipids, serum
bilirubin, and concentration of H2O2, NO and iron in lysosomes
Blood was obtained via retro-orbital bleeding. ELISA
kits were used to measure the serum levels of ALT (cat. no.
DL-ALT-Mu-48T), HDL-C (cat. no. CSB-E12874m) and LDL-C (cat. no.
CSB-E16561r) according to the manufacturer's protocol (all Beyotime
Institute of Biotechnology, Haimen, China). Plasma total
cholesterol (T-CHOL; cat. no. A111-1), triglyceride (TG; cat. no.
F001; Nanjing Jiancheng Institute of Biological Engineering,
Nanjing, China), serum bilirubin and serum aspartate
aminotransferase (AST; cat. no. P3636; Beyotime Institute of
Biotechnology) were determined by ELISA according to the
manufacturer's protocol. H2O2 (cat. no.
A007-2), NO (cat. no. A012-1) (both Nanjing Jiancheng Institute of
Biological Engineering) and iron (Fe2+, Fe3+
and total Fe; cat. no. ab83366; Abcam, Cambridge, UK) levels in
lysosomes were measured using colorimetric assay kits and an iron
assay kit according to the manufacturer's protocol. Lysosomes were
isolated from mouse livers as previously described (7).
Western blot analysis
To evaluate protein expression, tissues from the
liver, stomach, spleen, heart, kidney, intestine, brain, pancreas
and lung were homogenized in a lysis buffer (Beyotime Institute of
Biotechnology). Homogenates were centrifuged at 12,000 × g at 4°C
for 10–20 min. The protein concentration of the test sample was
calculated using a BCA assay kit (Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Western blot
analysis was performed as described previously (8). Briefly, 30 µg total soluble proteins
were separated on 12.5% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% skimmed milk at room temperature for 90 min. Membranes were
incubated with rabbit anti-sidt2 specific antibodies (1:1,000;
SAB1304608; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 4°C
overnight and then with horseradish peroxidase-conjugated goat
anti-rabbit secondary antibodies (1:3,000; A0208; Beyotime
Institute of Biotechnology) at room temperature for 1 h. Antibodies
specific to β-actin (1:5,000; A5441; Sigma-Aldrich; Merck KGaA)
served as an internal control. The target proteins were visualized
using an enhanced chemiluminescence Western Blotting Substrate
(32106; Thermo Fisher Scientific, Inc., Waltham, MA, USA). ImageJ
1.48u software (National Institutes of Health, Bethesda, MD, USA)
was used for densitometry analysis.
Histological studies
Liver tissue samples were stored in liquid nitrogen.
The sections were fixed with formaldehyde-calcium for 10 min at 4°C
and cryostat sectioned at a thickness of 10 µm onto poly-L-lysine
slides for lipid deposition analyses using Oil Red O staining. The
frozen sections were rewarmed and dried for 10 min and incubated
with 100% isopropanol for 5 min, then incubated with 0.5% oil red O
solution for 7–8 min at 60°C. Additional sections were stained with
hematoxylin and eosin (H&E) for 5 min at room temperature and
examined by light microscopy at magnification, ×200.
Transmission electron microscopy
(TEM)
Liver tissue samples were fixed in 2.5%
glutaraldehyde for 2 h at 4°C, treated with 1% osmium tetroxide,
dehydrated and embedded in Durcupan (Sigma-Aldrich; Merck KGaA) for
48 h at 60°C, then sectioned (60 nm). The sections were stained
with dioxygen staining for 20 min and lead citrate for 7 min at
room temperature, then mounted on Gu-grids and examined by electron
microscopy (EM-1200EX; JEOL, Ltd., Tokyo, Japan; magnification,
×12,000).
Statistical analyses
All data are expressed as the mean ± standard error
of the mean. One-way analysis of variance followed by Tukey's post
hoc test was used for comparisons among multiple groups. The
comparison between two groups of data was performed using the
Student's t-test for pairwise comparison. P<0.05 was considered
to indicate a statistically significant difference. SPSS 16.0
(SPSS, Inc., Chicago, IL, USA) was used to perform analysis.
Results
Tissue distribution of sidt2 protein
and production of sidt2 knockout mice
In the brain, intestinal, and lung tissues the
expression levels are lower, with the lowest expression observed in
the heart (Fig. 1A and B). Sidt2
gene whole body knockout mice (sidt2−/−) were generated
using the Cre/LoxP system. The mRNA and protein expression levels
of sidt2 were examined using RT-PCR and western blotting,
respectively. The results indicated that negligible sidt2 mRNA and
protein expressions were observed in sidt2−/− mice
(Fig. 1C and D). To identify
RNA-level knockout mice, cDNA was obtained via RT-PCR by extracting
RNA from sidt2+/− and sidt2−/− mouse liver
tissues and using this as a template. The identification of the
reaction system was performed via PCR. Primers were designed with
sidt2 gene exon 2 as a template. The PCR product was 250 bp in
length. Sidt2+/+ mice yielded products 250 bp in size.
The sidt2 knockout homozygous mouse were not able to amplify the
product, as indicated in Fig.
1C.
Changes of serum biochemical
parameters in sidt2−/− mice
Measurements of sidt2−/− mouse serum AST,
alanine transaminase (ALT), T-CHOL, TG, high density lipoprotein
cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C),
total bilirubin (T-Bil), indirect bilirubin (I-Bil) and direct
bilirubin (D-Bil) were performed. The levels of sidt2−/−
mouse serum AST, ALT, T-CHOL, TG, LDL-C, T-Bil, I-Bil and D-Bil
were significantly higher than those of sidt2+/+ mice
(Fig. 2). However, the serum HDL-C
in the sidt2−/− mouse group was significantly lower
compared with the sidt2+/+ control group (Fig. 2E). Serum TG, T-CHOL, HDL-C and LDL-C
levels reflect the status of lipid metabolism in the body. Compared
with sidt2+/+ mice, the serum TG, T-CHOL and HDL-C
levels were increased in the sidt2−/− mice, whereas
LDL-C was decreased, thus indicating lipid metabolic disorder. The
serum T-Bil, D-Bil, I-Bil, AST and ALT levels were higher in the
sidt2−/− mice, and were indicators of hepatic cell
function. By measuring the levels of these serum components in the
mice, it was demonstrated that the sidt2−/− mice had
abnormal liver functions.
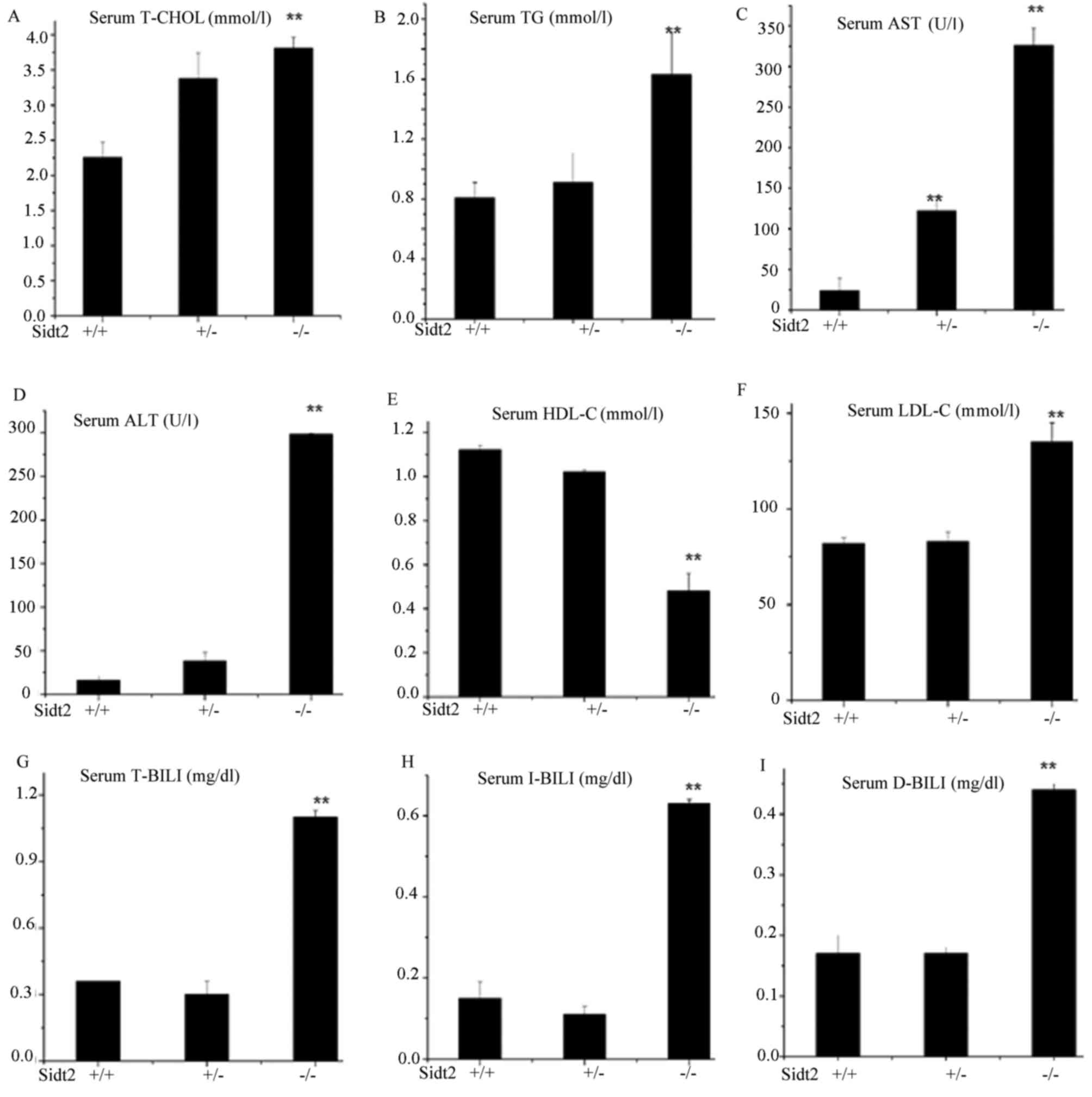 | Figure 2.Changes of serum biochemical
parameters in sidt2−/− mice. (A) T-CHOL, (B) TG, (C)
AST, (D) ALT, (E) HDL-C, (F) LDL-C, (G) T-BIL, (H) I-BIL and (I)
D-BIL concentrations were analyzed by enzymatic methods. Data are
expressed as the mean ± standard error of the mean (n=8).
**P<0.01 vs. sidt2+/+. Sidt2, SID1 transmembrane
family member 2; T-CHOL, total cholesterol; TG, total
triglycerides; AST, aspartate transaminase; ALT, alanine
transaminase; HDL-C, high density lipoprotein cholesterol; LDL-C,
low density lipoprotein cholesterol; T-BIL, total bilirubin; I-BIL,
indirect bilirubin; D-BIL, direct bilirubin. |
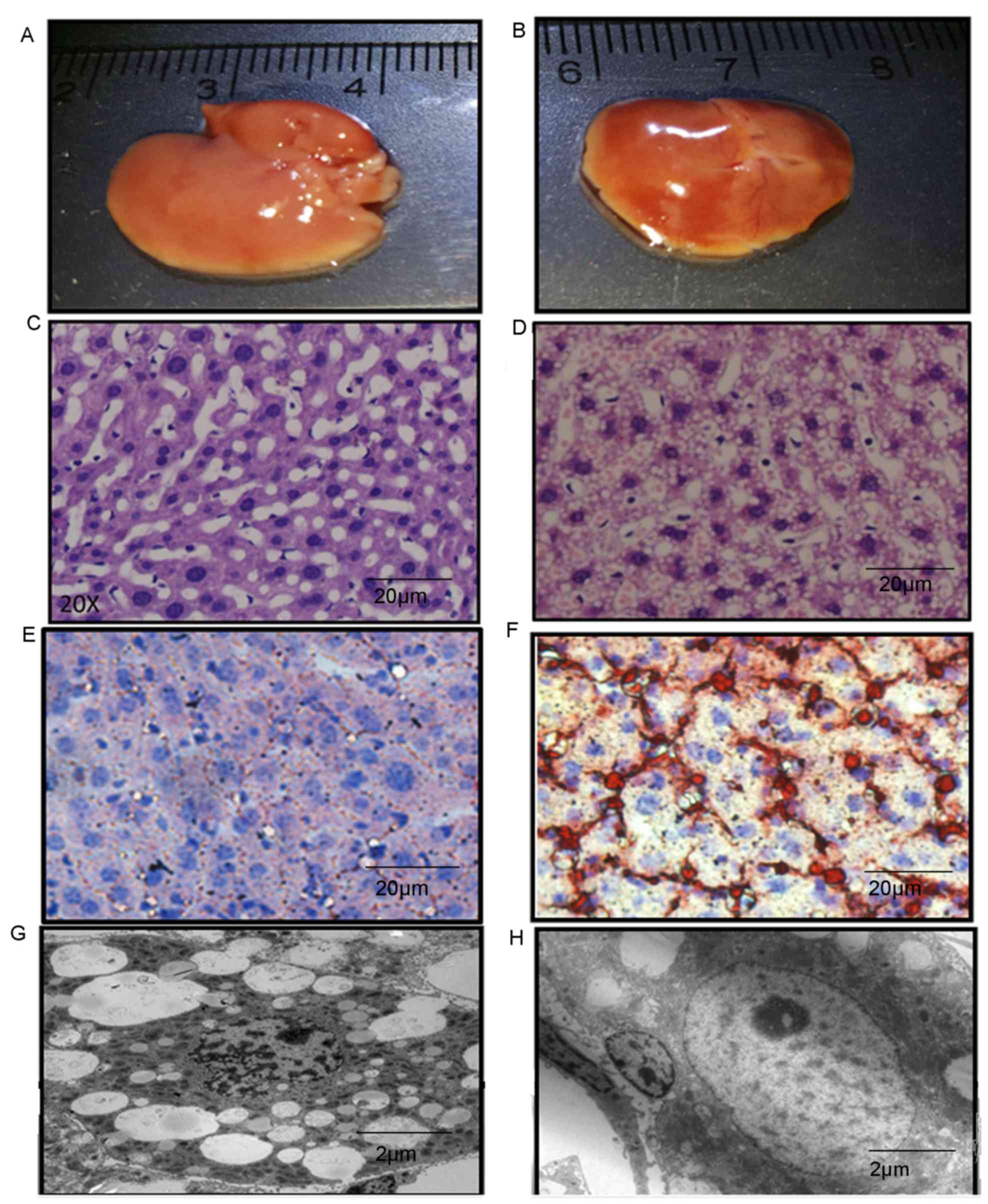
Morphological changes of the liver in
sidt2−/− mice
The colors of sidt2+/+ mouse livers were
pink, whereas in sidt2−/− mice the livers were more
yellow and appeared fatty. Sidt2+/+ mouse livers
appeared uniformly soft, but sidt2−/− mouse livers were
not. The envelopes were tight and smooth, and the edges of the
liver appeared dull (Fig. 3A and B).
There was no marked difference in liver volume of the
sidt2−/− mice compared with the sidt2+/+
mice. The morphologies of sidt2+/+ and
sidt2−/− mice liver frozen sections stained with H&E
and Oil Red O were observed via optical microscopy.
sidt2+/+ liver cells were polygonal in shape, nuclei
were large and round, and were located in the middle of the cell,
and the cytoplasm was eosinophilic, with basophilic briquettes
distributed in the cytoplasm. The sidt2−/− mouse liver
samples exhibited large fatty drops in the cytoplasm of the
hepatocytes, abutting the nucleus and the borders of the cytoplasm
towards the cell membrane. Liver cell swelling and cytoplasmic loss
was also observed. The cytoplasm appeared transparent and blebs
were noted. Occasionally, the cell volume appeared smaller and
dehydrated, with strong eosinophilic deep red staining around
necrotic bodies known as Mallory bodies (Fig. 3C and D). The morphology of livers in
the sidt2+/+ mice were also observed via optical
microscopy and Oil Red O staining, and no lipid droplet deposition
was observed. However in the sidt2−/− mice, many large
lipid droplets were observed (Fig. 3E
and F). TEM observations indicated liver steatosis (Fig. 3G), as revealed by membrane lipid
droplets in the cell liver cytoplasm, and some apoptotic body
formation (Fig. 3H).
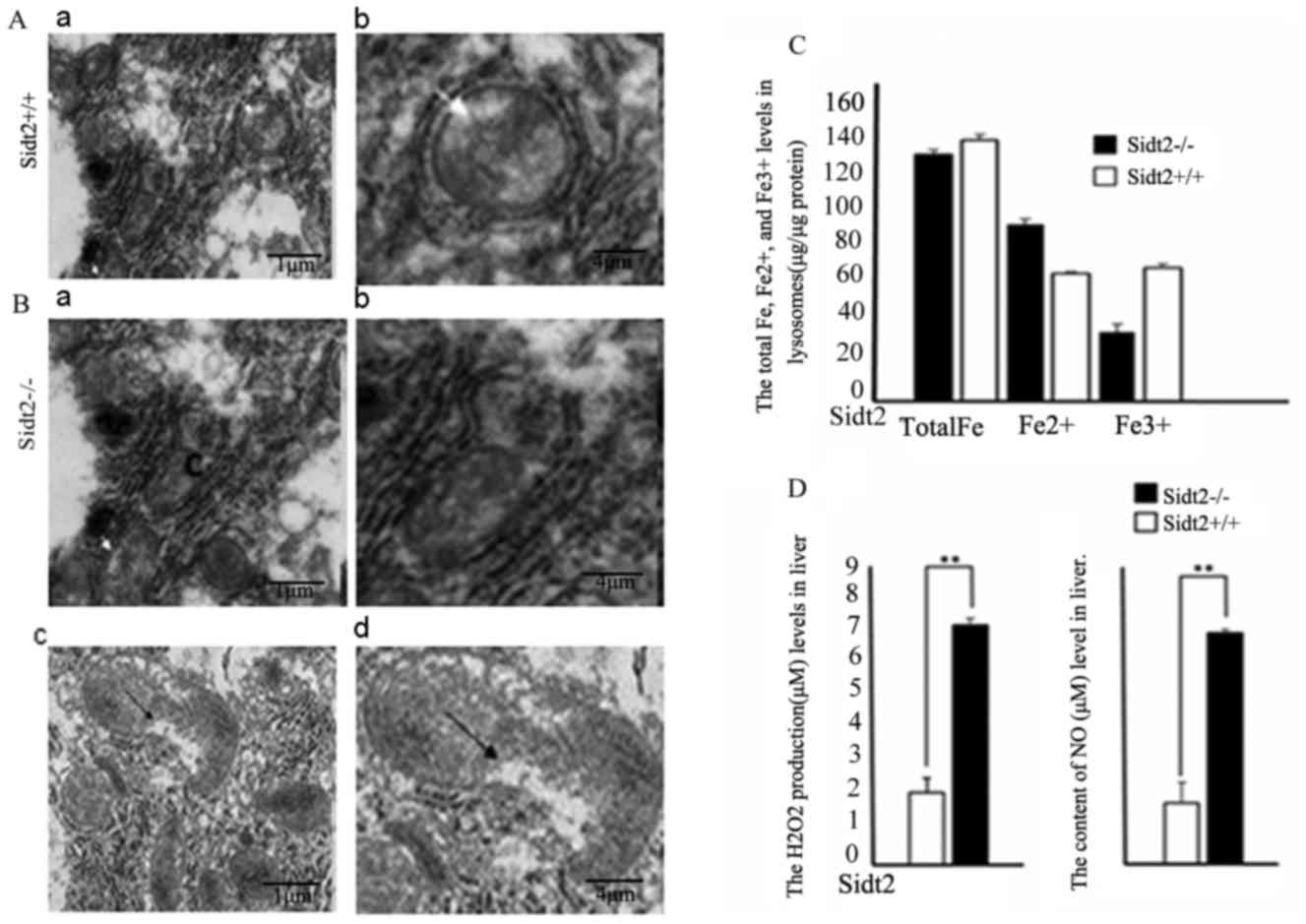
Mitochondrial damage and LMP-related
indices in sidt2−/− mice
Mitochondria are enclosed by two membranes; a smooth
outer membrane and an inner membrane that is folded into an array
of contiguous layers, which are known as cristae.
Sidt2−/− mouse liver samples were observed with TEM and
demonstrated to have mitochondrial edema, eventually destroying the
mitochondrial integrity (Fig. 4A and
B). The Fe2+ levels in the sidt2−/− mouse
livers were increased and the Fe3+ levels were decreased
when compared with the sidt2+/+ mouse livers (Fig. 4C). However, the total Fe levels were
not significantly different in the two groups. Hydrogen peroxide
(H2O2) and NO typically originate from the
mitochondria. Because of this mitochondrial damage,
H2O2 and NO levels were detected in liver
tissue homogenates, and observed that they increased significantly
compared with the levels in sidt2+/+ mice (Fig. 4D).
Discussion
In the present study, the changes of serum basal
levels of T-CHOL, TG, LDL-C and HDL-C were investigated in
6-month-old male sidt2−/− mice maintained on a normal
diet. It was demonstrated that sidt2−/− serum T-CHOL, TG
and LDL-C levels were increased significantly compared with
sidt2+/+ mice, but serum HDL-C was decreased. This was
demonstrated as a spontaneous disorder of lipid metabolism.
However, in sidt2−/− mice, serum AST, ALT, T-Bil, D-Bil
and I-Bil increased compared with those of sidt2+/+
mice, suggesting that the sidt2−/− mice not only had a
disorder of lipid metabolism, but that liver function was also
impaired.
Light microscopy of sidt2−/− mouse liver
sections observed following H&E staining demonstrated that
numerous lipid droplets accumulated in liver cells. The liver cells
were round and swollen and Mallory bodies, a sign of liver cell
necrosis, were observed. Oil Red O staining and light microscopy
revealed numerous large lipid droplets, suggesting that
pathological changes had occurred in the livers of the
sidt2−/− mice, and that liver function was impaired and
was accompanied by necrosis. Using TEM of sidt2−/− liver
sections, mitochondria edema was observed, and mitochondrial
cristae were separated from the mitochondrial matrix and exhibited
vacuole-like changes.
Mitochondrial cristae act as folding units to create
the mitochondrial matrix, lying inside of the inner membrane, and
an outer compartment known as the intermembrane space, which lies
between the mitochondrial membranes. Mitochondria are important
subcellular organelles involved in lipid metabolism (9). The free fatty acids for β-oxidation
that occur in the mitochondria of liver cells may be decreased from
swelling or vacuole-like changes to the liver cell mitochondria of
sidt2−/− mice. Free fatty acids will then accumulate to
form lipid droplets, eventually causing apoptosis, fatty liver
disease, and permanent damage to the liver (10).
Under normal physiological conditions, the lysosome
uses many proteins for endocytosis (11) and autophagy (12). These proteins bind to redox-reactive
iron (Fe2+) leading to a concentration decrease of
Fe2+ in the lysosome, thereby stabilizing the lysosomal
membrane (13). Normally, the
lysosome is not sensitive to oxidative stress. However, in the
present study, it was demonstrated that in sidt2−/−
mouse liver tissues and in liver cell lysosomes the redox-reactive
iron (Fe2+) increased. Increased Fe2+ can
change H2O2 to hydroxyl radicals via the
Fenton reaction (14). Hydroxyl
radicals attack the lysosomal membrane, making it more sensitive to
oxidative stress (15). If oxidative
stress occurs in the cell, the resulting lysosomal membrane
instability may lead to lysosomal membrane peroxidation (16). Due to the damaged mitochondria in
sidt2−/− mice liver cells, the resulting abnormal
release of H2O2 and NO even at low
concentrations would induce LMP (17) once they diffused into the cytoplasm
and directly damaged the lysosome membranes. Hydroxyl radicals
attack the lysosomal membrane, destroy its integrity, and lead to
lysosomal membrane disintegration through LMP (18). The lysosome contains >50 soluble
acid hydrolases, and when its membrane collapses the lysosomal
contents leak. The leakage of lysosomal constituents is suggested
to be sufficient to trigger other organelle damage, particularly
the mitochondria (19).
Mitochondrial damage may also cause the release of
H2O2 (18),
which, in turn, destroys the lysosome membrane (10), and leads to cell death in a
caspase-dependent or independent manner. These changes lead to
liver cell apoptosis and functional liver disorders.
It has previously been demonstrated that sidt2 is a
novel lysosomal membrane protein (3). It was demonstrated in the present study
that when this protein is deleted LMP occurs, thus confirming that
sidt2 is a key protein in the LMP response. However, the specific
mechanism whereby sidt2 causes LMP is unclear, and requires further
study. The changes of liver function and lipid metabolism can be
observed under a normal diet. To better understand the role of
sidt2, sidt2 deficiency mice can be challenged by HFD, or MCD. The
use of a normal diet is a limitation of the present study, and
future studies should make use of a high-fat diet to observe
changes in liver function. The results of the present study
revealed that sidt2 knockout mice exhibit pathological and
metabolic changes, which provides an important theoretical basis
for further study and a basis for the understanding of lysosomes
and disease.
Acknowledgements
The authors would like to thank Dr Jialin Gao
(Department of Endocrinology and Genetic Metabolism, Yijishan
Hospital of Wannan Medical College, Wuhu, China) for their
technical assistance.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81200632
and 81471002), the Natural Science Foundation of Anhui, China
(grant no. 1308085QH134), and the Introduction of Talents
Foundation of Yijishan Hospital (grant no. YR201104).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW conceived and designed the experiments and
provided relevant materials and analytical tools. YM performed the
experiments. LL analyzed the data.
Ethics approval and consent to
participate
Animal experiments were reviewed and approved by the
Animal Ethics Committee of Wannan Medical College.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu F, Chen Z, Wang B, Jin Z, Hou Y, Ma S
and Liu X: The role of lysosome in cell death regulation. Tumour
Biol. 37:1427–1436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Appelqvist H, Waster P, Kagedal K and
Ollinger K: The lysosome: From waste bag to potential therapeutic
target. J Mol Cell Biol. 5:214–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao J, Yu C, Xiong Q, Zhang Y and Wang L:
Lysosomal integral membrane protein Sidt2 plays a vital role in
insulin secretion. Int J Clin Exp Pathol. 8:15622–15631.
2015.PubMed/NCBI
|
|
4
|
Pryor PR and Luzio JP: Delivery of
endocytosed membrane proteins to the lysosome. Biochim Biophys
Acta. 1793:615–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oberle C, Huai J, Reinheckel T, Tacke M,
Rassner M, Ekert PG, Buellesbach J and Borner C: Lysosomal membrane
permeabilization and cathepsin release is a Bax/Bak-dependent,
amplifying event of apoptosis in fibroblasts and monocytes. Cell
Death Differ. 17:1167–1178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao J, Gu X, Mahuran DJ, Wang Z and Zhang
H: Impaired glucose tolerance in a mouse model of sidt2 deficiency.
PloS One. 8:e661392013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jialin G, Xuefan G and Huiwen Z: SID1
transmembrane family, member 2 (Sidt2): A novel lysosomal membrane
protein. Biochem Biophys Res Commun. 402:588–594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas DM, Ferguson GD, Herschman HR and
Elferink LA: Functional and biochemical analysis of the C2 domains
of synaptotagmin IV. Mol Biol Cell. 10:2285–2295. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fulda S, Galluzzi L and Kroemer G:
Targeting mitochondria for cancer therapy. Nat Rev Drug Discov.
9:447–464. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Berk M, McIntyre TM, Gores GJ and
Feldstein AE: The lysosomal-mitochondrial axis in free fatty
acid-induced hepatic lipotoxicity. Hepatology. 47:1495–1503. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antunes F, Cadenas E and Brunk UT:
Apoptosis induced by exposure to a low steady-state concentration
of H2O2 is a consequence of lysosomal rupture. Biochem J.
356:549–555. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurz T, Terman A and Brunk UT: Autophagy,
ageing and apoptosis: The role of oxidative stress and lysosomal
iron. Arch Biochem Biophys. 462:220–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Terman A and Kurz T: Lysosomal iron, iron
chelation, and cell death. Antioxid Redox Signal. 18:888–898. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schubert D and Chevion M: The role of iron
in beta amyloid toxicity. Biochem Biophys Res Commun. 216:702–707.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Risin SA and Penkratova NN: Effect of
unfractionated histones on isolated mouse liver lysosomes. Vopr Med
Khim. 26:752–755. 1980.(In Russian). PubMed/NCBI
|
|
16
|
Boya P and Kroemer G: Lysosomal membrane
permeabilization in cell death. Oncogene. 27:6434–6451. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin L, Stearns R and Gonzalez-Flecha B:
Lysosomal and mitochondrial pathways in H2O2-induced apoptosis of
alveolar type II cells. J Cell Biochem. 94:433–445. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mak IT, Misra HP and Weglicki WB: Temporal
relationship of free radical-induced lipid peroxidation and loss of
latent enzyme activity in highly enriched hepatic lysosomes. J Biol
Chem. 258:13733–13737. 1983.PubMed/NCBI
|
|
19
|
Groth-Pedersen L and Jäättelä M: Combating
apoptosis and multidrug resistant cancers by targeting lysosomes.
Cancer Lett. 332:265–274. 2013. View Article : Google Scholar : PubMed/NCBI
|