Introduction
The Chinese herbal injections are new preparations
of novel approaches to prepare traditional Chinese herbal medicines
designed within the past decade for convenience in practice in
terms of practitioners and patients. However, as injections of
Chinese herbs have been extensively used, there has been an
increase un adverse drug reactions (ADRs), including drug-induced
death, liver and kidney injury, shock, diarrhea, vomiting, asthma,
erythra, and itchy skin (1–5). Such preparations, guided by a qualified
practitioner of TCM, should have mild and low adverse effects, as
previously documented (2). The
majority of these ADRs however, correlate with irrational use
especially overdose (2,6,7). In
recent years, increasing attention has been paid to the mechanisms
for ADRs. Nevertheless, little information is available on the
constituent complexity of these injections.
Chlorogenic acid (CGA) is a ubiquitous component in
most Chinese herbal injections, also usually employed as a typical
marker to control the quality of TCM (8), such as Shuang-Huang-Lian (SHL)
injection (according to the Pharmacopeia of China, 2005). This
field remains problematic (9,10) though
the allergenicity has constantly counted as the primary factor
leading to adverse effects of CGA (11,12). On
the other hand, CGA in lower concentrations eliminated hydroxyl
radical and superoxide in vitro although CGA at higher
concentrations produced radicals and served as a pro-oxidant, as
previously reported (13). Oxidative
damage to normal cells is likely to be one of the mechanisms of
such drug-induced adverse reaction, which may be a good
interpretation for why herbal injection-induced ADRs occur in the
case of overdose more often than not (6). Nevertheless, which insults are bound by
the adverse effects triggered by CGA remain unclear.
The present study determined the functions taken on
by CGA in the oxidative stress injury triggered by injections of
Chinese herbs by controlling SHL injection in different doses,
i.e., the most frequently used and ADRs reported on Chinese herbal
injections (14), and equivalent
dose of CGA, respectively. The experimental flow diagram is shown
in Fig. 1.
Materials and methods
Animals and reagents
A total of 90 male Wistar rats with a weight ranging
200–220 g were obtained from the Animal Center, Health Science
Center, Peking University (Beijing, certificate no. SCXK
2006–0008). The rats were caged as per a 12-h light/dark
circulation 40±5% of humidity and at 22±2°C, and given standard
water and diet without advance preparation. Prior to the
experiment, the rats were fasted for 12 h. The animal model
preparation, under the guidance of Animal Research Committee of
Peking University, complied with the EU adopted Directive
2010/63/EU. Experimental Animal Ethics Branch subordinated by
Biomedical Ethics Committee governed by Peking University approved
the experiment protocols (LA2011-38). The study was also approved
by the Ethics Committee of the TCM Hospital of Shijiazhuang
Affiliated to Hebei University of Chinese Medicine (Shijiazhuang,
China).
CGA was dissolved in sterile 0.9% normal saline,
which was obtained from Sigma-Aldrich: Merck KGaA (St. Louis, MO,
USA) with a purity >98%. SHL lyophilized powder injection (24
ampoules, 1.2 g/ampoule) was obtained from Heilongjiang
Songhuajiang Pharmaceuticals Co., Ltd. (Harbin, China) also
dissolved in 0.9% normal saline.
Drug administration and experimental
groups
This study randomly split the rats into five groups
according to weight. Additionally, the high-dose SHL injection
[H-SHL; 420 mg/kg, 8 ml/kg/h, intravenous (i.v.) drip injection],
high-dose CGA (H-CGA; 7 mg/kg, 8 ml/kg/h, i.v. drip injection),
low-dose SHL injection (L-SHL; 20 mg/kg, 8 ml/kg/h, i.v. drip
injection), low-dose CGA (L-CGA; 0.336 mg/kg, 8 ml/kg/h, i.v. drip
injection) and normal saline (control, 8 ml/kg/h, i.v. drip
injection) were given within 1 h via the left catheter of jugular
vein, respectively. The body weight CGA with the concentration of
0.336 mg/kg was selected as a low dose as being a mean dosage of
CGA in injection of Chinese herb being most frequently adopted. In
addition, the adverse effect relative to this dose has been rarely
reported. The dose recommended in the instructions of Qingkaling
injection, an injection of traditional Chinese medicine, takes up
merely 1/6 of the dose 7 mg/kg body weight. Additionally, the
majority of the found adverse effects occur close to this dose
(15). The L-SHL and the H-SHL group
contained the same amount of CGA (0.336 and 7 mg/kg, according to
the Pharmacopeia of China, 2005) as L-CGA or H-CGA group,
respectively. This study administered animals in another isolated
experiment series by adopting saline or the drug whereby
intraperitoneal bolus injection QD was for 7 or 14 days.
Furthermore, the relevant examinations on the animals were carried
out. Table I lists the animal number
in line with the groups for the experiment.
 | Table I.No. of animals for different
experimental groups and various parameters. |
Table I.
No. of animals for different
experimental groups and various parameters.
| Groups | Oxidant stress | Western blotting,
ELISA and biochemical examination | Total |
|---|
| 2 h |
|
|
|
|
Control | 6 | – | 6 |
|
L-CGA | 6 | – | 6 |
|
L-SHL | 6 | – | 6 |
|
H-CGA | 6 | – | 6 |
|
H-SHL | 6 | – | 6 |
| Day 7 |
|
|
|
|
Control | – | 6 | 6 |
|
L-CGA | – | 6 | 6 |
|
L-SHL | – | 6 | 6 |
|
H-CGA | – | 6 | 6 |
|
H-SHL | – | 6 | 6 |
| Day 14 |
|
|
|
|
Control | – | 6 | 6 |
|
L-CGA | – | 6 | 6 |
|
L-SHL | – | 6 | 6 |
|
H-CGA | – | 6 | 6 |
|
H-SHL | – | 6 | 6 |
| Total | 30 | 60 | 90 |
Microcirculatory observation
Surgery was performed, complying with the previous
descriptions (16). This study used
intramuscular injection of 20% urethane (1 ml/100 g BW),
anesthetizing the rats. The abdomen of rats was opened via a 25 to
30 mm cut. The ileocecal junction of the mesentery was gently
exposed (10–15 cm caudal) outside. On that basis, the exposed
junction was mounted on a plastic transparent stage for rats.
Through continuous superfusion, the mesentery was preserved moist
and warm at 37°C with saline solution. An inverted microscope (DM
IRB; Leica Microsystems GmbH, Cologne, Germany) was used to observe
the microcirculation of mesentery via the objective lens (×20), and
a 12 V, 100 W, direct current-stabilized light source was used to
transilluminate the mesentery. A video camera with vibrant color
(JK-TU53H; Toshiba, Tokyo, Japan) was installed on the microscope
to capture the images from the microscopic angle. Subsequently, the
image was transmitted onto a monitor (J2118A; TCL, Huizhou, China).
A digital video disk video cassette recorder (DVR-R25; Malata,
Xiamen, China) recorded the images. Single and unbranched venules
(30–50 µm in diameter; 200 µm in length) were selected for the
study (16).
After the observation of baseline (10 min),
microcirculation was examined. For monitoring oxidant stress in the
venule walls, 5 min before observation, topical dihydrorhodamine
oxidant-sensitive, 123 fluorescent probe was applied to the surface
of mesentery (10 µmol/l) (DHR; Molecular Probes: Thermo Fisher
Scientific, Inc., Eugene, OR, USA). A fluorescence microscope
inverted for 455 nm excitation light (DM IRB; Leica Microsystems
GmbH), to record the fluorescence image 60 and 120 min after
infusion at baseline. Additionally, this study employed Image-Pro
Plus 5.0 software to, respectively, measure the extravenular
interstices (Ie) and fluorescence intensity of walls venule (Iv).
How Ie and Iv were different from each other was ascertained for
each point of time. Besides, the ratio of each value calculated to
the baseline (16).
ELISA analysis
After injections for 7 or 14 days, the blood was
extracted from a branch of the descending aorta of the rats. For
measuring the concentrations of tumor necrosis factor-α (TNF-α) and
interleukin-10 (IL-10), 1 ml arterial systemic serum was collected.
Using ELISA kits from RapidBio Systems, Inc. (Carlsbad, CA, USA),
the concentrations of TNF-α (pg/ml) and IL-10 (pg/ml serum) were
ascertained. The assays were conducted as instructed by the
manufacturer (17).
Determination of activity of
catalase
After 7 or 14 days of injection, the serum was
collected and the blood extracted from a branch of the descending
aorta of rats. The activity by catalase (CGAT) was evaluated by
exposing samples to excessive hydrogen peroxide with the purpose of
decomposition. Additionally, to produce a comoles compound, was
reacted with the ammonium molybdate with the residual hydrogen
peroxide, which was absorbed at 405 nm to the greatest extent
(18). The the antioxidase
activities as U/ml serum were noted. Through commercial kits, the
assessments of CGAT activity was performed (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). A bicinchoninic acid
(BCA) protein assay kit was used to analyze the overall content of
protein in samples (Sun Biomedical Technology Co., Ltd, Beijing,
China) (18).
Western blotting of Nox4,
p22phox, p47phox protein expression
The lung tissues, brain tissues and tissues of
terminal ileum of rats were removed 7 or 14 days after injection.
Tissues were homogenized and minced in lysis buffer on ice [pH 7.4,
0.1% sodium dodecyl sulfate (SDS), 1% Nonidet p40 (NP40) solution,
50 mM Tris/HCl, and 150 mM NaCl], and centrifuged for 10 min at
12,000 × g. The supernatant was isolated with cytosol proteins. BCA
protein assay kit was used to study and quantified the overall
protein in the homogenates (Sun Biomedical Technology Co., Ltd.).
The prepared samples were boiled for 5 min in gel loading buffer
[pH 6.8, 1% bromophenol blue, 1.56% dithiothreitol (DTT), 10%
glycerol, 2% SDS, and 12.5 mM Tris/HCl]. The proteins (50 µg) were
separated in equal amounts for each sample on a mini-gel of 10%
SDS-polyacrylamide for 2 h at a constant voltage of 100 V. The
proteins were transferred to membranes of polyvinylidene difluoride
(PVDF) through electrophoresis for 16 h at 30 V. The membranes were
blocked at room temperature in TBS-T in 5% (w/v) non-fat dry milk
for 1 h (pH 7.4, 0.1 mM Tween-20, 100 mM NaCl, and 10 mM Tris/HCl).
The membranes were cultured through using rabbit polyclonal IgG
against β-actin (1:500), p47phox (1:200),
p22phox (1:200) (all from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), Nox4 [2 µg/ml; Abcam (Hong-Kong), Ltd.,
Hong-Kong, China] overnight. The samples were washed, and the
membranes cultures for 2 h through the secondary antibodies
conjugated by enzyme used in immunohistochemistry to label antigens
and their antibodies (1:3,000; Santa Cruz Biotechnology, Inc.).
Radiographic film was subsequent exposured and a chemiluminescence
system was enhanced, and detected the antibody labeling. The
optical density was visualized for the bands. Besides, the density
was normalized to the density taken by β-actin (19).
Statistical analysis
Data were presented as the mean ± standard error
(SEM). Additionally, SPSS 17.0 software for statistics was employed
for analysis (SPSS, Inc., Chicago, IL, USA). Multiple comparisons
were made using ANOVA followed by Tamhane's T2 and LSD tests. This
study accepted statistical significance at p<0.05.
Results
Determination of intensity of
fluorescence by DHR in the walls of venules
The intensity of fluorescence by DHR in the walls of
venules at diverse times in the five groups were determined. In rat
mesenteric walls of venule, we failed to detect DHR fluorescence
prior to infusing the five groups. Additionally, the low
fluorescence intensity remained in the L-SHL, L-CGA and control
group. Conversely, a marked DHR fluorescence was triggered by H-CGA
in walls of the venules for 60–120 min as the groups were infused
taking on evident difference in contrast with control group.
Likewise, H-SHL induced an obvious DHR fluorescence at 120 min in
contrast with control group and a little under the H-CGA group
level, which indicated the inherent capacity by CGA and SHL to
trigger reactive oxygen species (ROS) production at high dose from
venules.
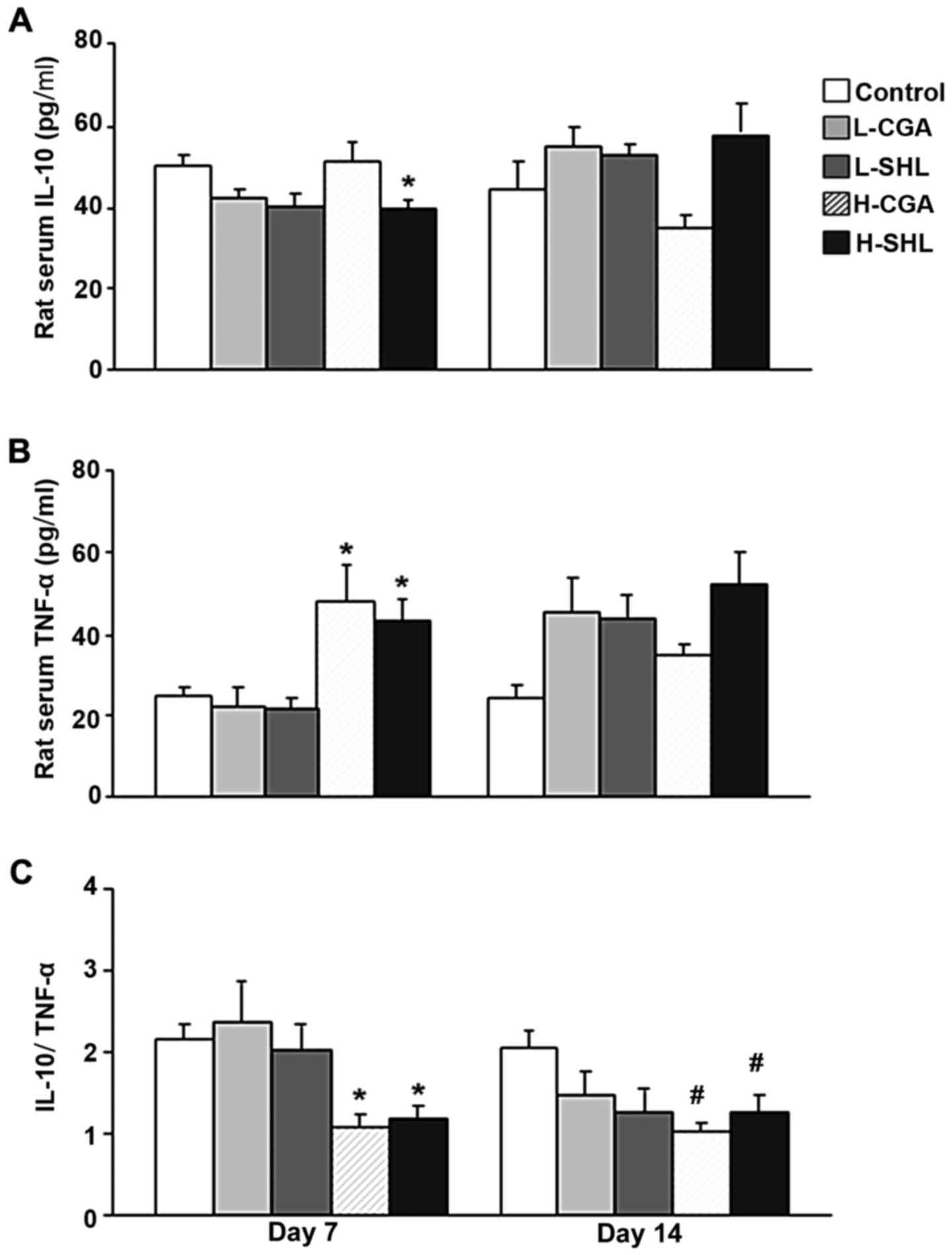
Analysis of IL-10, TNF-α and CGAT in rat serum.
Inflammatory factor parameters in rat serum were recorded according
to the experimental protocol. The changes of IL-10, TNF-α,
IL-10/TNF-α and CGAT in rat serum at day 7 and 14 are shown in
Figs. 2 and 3. H-SHL suppressed the concentration of
IL-10 after seven injections compared with control. Furthermore,
the expression of TNF-α was significantly higher in H-CGA and H-SHL
group than that in control group at day 7, and no significant
change was found in proinflammatory cytokines at day 14 point.
Moreover, the ratio of IL-10 and TNF-α decreased in H-CGA group at
day 7 and 14 as compared to control group, and, the ratio decreased
in response to injection with H-SHL, which was similar to the
change in H-CGA group at the two time points (Fig. 2).
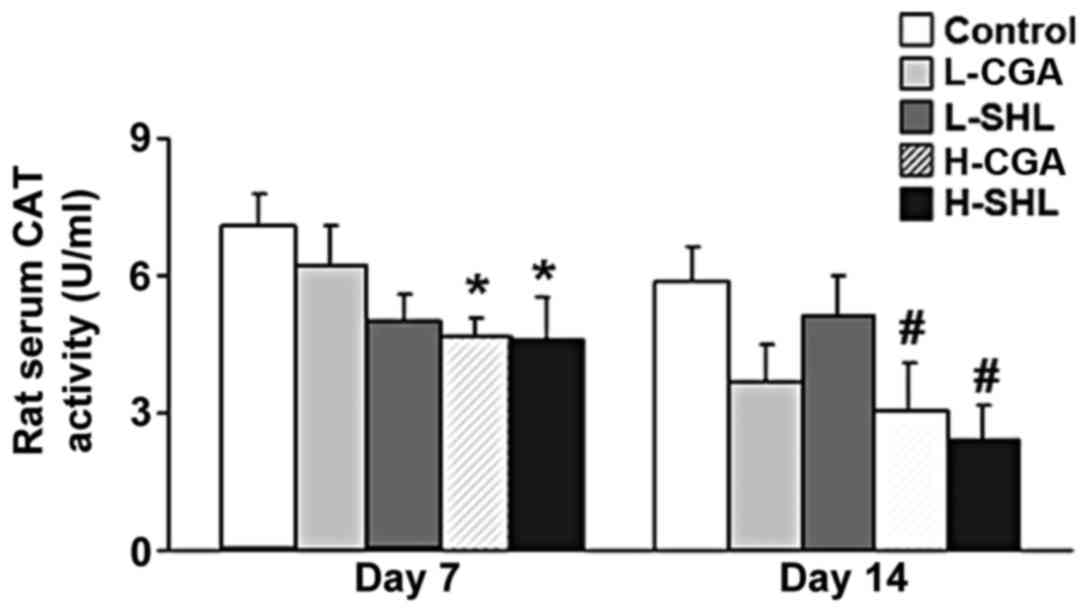
The CGAT in the antioxidant principle pertaining to
the organism was contained. As shown in Fig. 3, the activity of CGAT decreased in
H-CGA and H-SHL group after seven injections. At day 14 time point,
the activity of CGAT similarly decreased in the two groups. Other
groups did not affect the activity of the enzyme during the
observation.
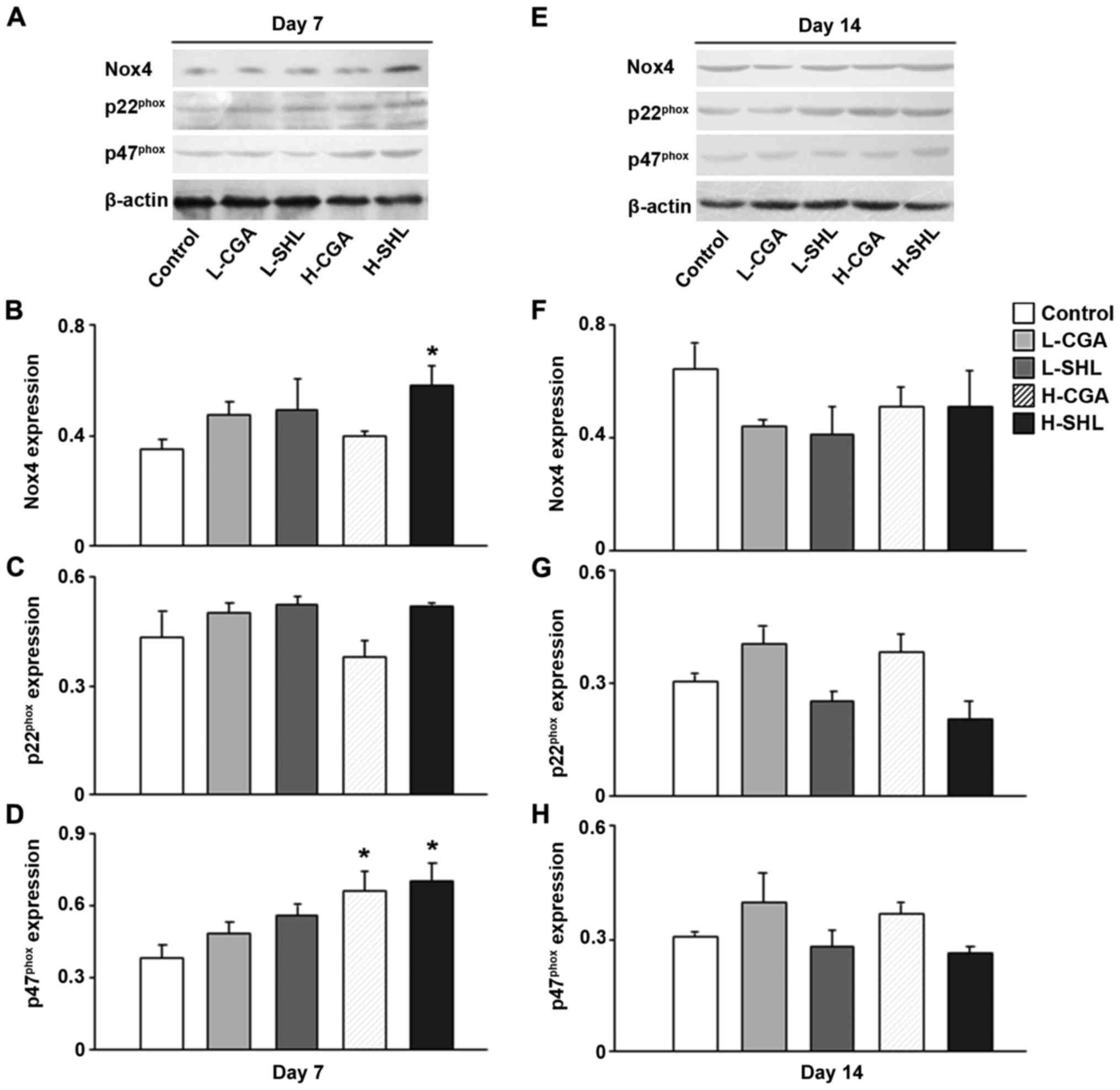
Determination of Nox4, p22phox, and
p47phox protein expression in the tissues. To consider
the function endowed with nicotinamide adenine dinucleotide
phosphate oxidase in CGA taking on high dose and SHL-triggered
production of ROS from circulatory system, the expression of the
three subunits of nicotinamide adenine dinucleotide phosphate
oxidase at protein level in the terminal ileum, p47phox
p22phox and Nox4, lung and brain tissues, and the
results are shown in Figs.
4–6. At the two time points
examined, no diversification was indicated as the foregoing
proteins were expressed between L-SHL, L-CGA and control group.
Comparatively, CGA in high dose facilitated the expression at day 7
and 14 studied for the protein Nox4 in the terminal ileum tissues,
and similarly H-SHL increased the expression of Nox4 at day 7 in
the same tissue. H-CGA also enhanced protein p22phox and
p47phox expression at day 7 time point (Fig. 4). In the brain tissues, injection
with H-CGA or H-SHL has the same effect increasing
p47phox protein expression at day 7. Furthermore, H-SHL
also enhanced protein Nox4 expression at the same time point
(Fig. 6).
Discussion
Injecting SHL or CGA in high dose probably result in
an inbalance between mechanism in antioxidant and oxidant, which
was proven in this study in rats. Such injection triggers oxidant
stress, inclusive of gained ROS production in wall of venules, and
decreased activity of CGAT. Promoted expression of
p47phox, p22phox and Nox4 was observed in
response to the injection of SHL or CGA in high dose, indicating
the containment of nicotinamide adenine dinucleotide phosphate
oxidase in the CGA-triggered oxidant stress. Moreover, exposure to
H-CGA or SHL undermined the balance of basal levels of pro-(TNF-α)
and anti-(IL-10) inflammatory cytokines, manifested as decreased
IL-10 to TNF-α ratio. As overdose drugs were added, adverse effects
observed overall showed up.
For monitoring the oxidative stress, the probe DHR
was employed on the basis of fluoresce and sensitive to
H2O2 to ascertain extent of ROS in other
types and intracellular H2O2 levels in other
cell types and in incubated endothelial cells (20–22). As
outcomes acquired in this report indicate, imbalance between the
systemic manifestation of ROS in microcirculatory system was
promoted in both groups of SHL and CGA taking on high dose starting
from 60 and 120 min administration, respectively. However,
injection with CGA or SHL in low dose did not enhance the
generation of ROS. This finding was consistent with previous
research (23). CGA-triggered
oxidative stress depends on the dose.
As the presented, CGA or SHL in high dose evidently
declined the activity of antioxidase CGAT in rat serum after 7 and
14 day injections. An equilibrium between clearance and ROS
production is of necessity for the standard cellular roles. As the
antioxidant capacity of ROS is overcome by its cellular production,
the equilibrium is broken, and an imbalance between the systemic
manifestation of ROS shows up. CGAT counts as the primary
antioxidant in cells produced by an enzyme and is of critical
significance for offering protection against stress generated in
oxidation (24). This antioxidase
can be evidently depleted, and its activity is remarkably reduced
as ROS is excessively accumulated. This finding conformed to the
outcomes detected in terms of intensity of fluorescence relative to
DHR (over ROS production). Accordingly, the injury by oxidant
stress exerted by high-dose drug is probably triggered by
dissipation of the antioxidant enzymes and ROS over production.
Scholars have always considered ROS over production
as a pathological process charged with organ dysfunction and
cellular damage (19). Given the
necessity to take by nicotinamide adenine dinucleotide phosphate
oxidase in producing stress related to oxidation of dysregulated
vascular oxidation-reduction circumstance (25), we investigated nicotinamide adenine
dinucleotide phosphate oxidase family members and explored the
potential of the enzyme as the source of high dose CGA or
SHL-induced ROS. It was found that H-CGA and SHL significantly
increased Nox4, p22phox or p47phox protein
expression in the terminal ileum, lung and brain tissues after 7 or
14 day injections. It was reported that Nox4 is present in all
vascular walls and is significantly more abundant than any other
Nox enzyme (26). The high
expression of Nox4, p22phox and p47phox
suggests that nicotinamide adenine dinucleotide phosphate oxidase
plays a role in high dose CGA and SHL-induced ROS production
especially derived from vascular walls. Even so, the possibility
remains for the involvement of other peroxidases present and other
mechanisms in vascular cells induced by CGA or SHL overdose.
This study observed a decrease of IL-10/TNF-α ratio
in the high dose CGA and SHL group after 7 and 14 day injections,
which suggested that the balance between IL-10 and TNF-α tipped
towards inflammation. The ratio between IL-10 and TNF-α has been
used as an accurate estimate of the inflammatory activity in the
systemic circulation (27). IL-10
demonstrates potent anti-inflammatory properties through inhibiting
the production of TNF-α and other pro-inflammatory cytokines
(28). It has revealed to possess
antioxidant-like properties in situations where oxidative stress is
increased (29). On the other hand,
TNF-α enhances oxidative stress both by increasing ROS generation
as well as by decreasing antioxidants. Moreover, it has been
postulated that the balance between pro- and anti-inflammatory
cytokines would be related with NF-κB mediation (30). In this experiment, we also measured
the protein expression of this factor and found that NF-κB p65
protein expression was increased in the lung and brain tissues when
exposed to high-dose CGA or SHL injection (further study is in
progress). NF-κB is one of the most important regulators of
pro-inflammatory cytokines inclusive of TNF-α, IL-1β, IL-6, IL-8,
and platelet activation factor gene transcription (31,32).
IL-10 acts as a natural antagonist to TNF-α, by inhibiting NF-κB
signaling through the preservation of inhibitory factor κB (IκB)
(30). Additionally, IL-10
alleviated inflammatory stimulus mediated increase in ROS (33) and ROS mediated IκB degradation and
thus activation of NF-κB (29). In
general, cytokines are interwoven and regulated through a feedback
mechanism (34), which suggests that
other inflammatory cytokines or mediators may be present in
high-dose CGA and SHL induced oxidant stress or inflammatory
injury.
In summary, the present study documented the
potential role of CGA in SHL injection overdose induced generating
excessive peroxide in vascular endothelial cells may well be
through nicotinamide adenine dinucleotide phosphate oxidase and
imbalance of basal levels of pro-(TNF-α) and anti-(IL-10)
inflammatory cytokines. These results illuminate mechanisms
underlying the unfavorable effects of CGA induced by irrational use
of Chinese herbal injection, and carefully reckon with the
clinician particularly as an injection of TCM involving high level
of CGA is adopted. We concluded that SHL injection induced
excessive generation of peroxides and imbalance between TNF-α and
IL-10 at high dose, which can be partly attributed to CGA.
Acknowledgements
We gratefully acknowledge the excellent technical
assistance of Professor Jing-yan Han of Peking University.
Funding
This study was supported by grant no. 90709052 from
the National Natural Science Foundation of China and the Production
of New Medicine Program of Ministry of Science and Technology of
the People's Republic of China (2008ZX09101-027) for Professor
Peng-Tao Li based on the Ministry of Education and Key laboratory
of Chinese Internal Medicine and Scientific and Technological
Innovation Team of Beijing University of Chinese Medicine.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WYD was responsible for the production of animal
models, revising and writing of the manuscript. YX contributed in
the calculation of the indexes. JJY, ZH and YBZ were responsible
for the collection of the data, the modification of the pictures
and a part of the statistical analysis. YZ was responsible for
revising and finalizing this report. All the authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the TCM Hospital of Shijiazhuang Affiliated to Hebei University of
Chinese Medicine (Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ai CL, Xie YM, Li MQ, Wang LX and Liao X:
Incidence rate of adverse reaction/event by Qingkailing injection:
A Meta-analysis of single rate. Zhongguo Zhong Yao Za Zhi.
40:4770–4778. 2015.(In Chinese). PubMed/NCBI
|
|
2
|
Wang WP, Yu M, Wang L, Jiang XR, Li XB,
Wang HW, Cao Y, Liu K and Huang LQ: Academic discussion of adverse
reaction of clinical trials of new traditional Chinese medicines
and relevant influencing factors. Zhongguo Zhong Yao Za Zhi.
40:346–350. 2015.(In Chinese). PubMed/NCBI
|
|
3
|
Tan LJ, Wang M and Zhu Y: Research
progress of adverse reactions of traditional Chinese medicine
injections. Zhongguo Zhong Yao Za Zhi. 39:3889–3898. 2014.(In
Chinese). PubMed/NCBI
|
|
4
|
Li BQ, Dong X, Yang GQ, Fang SH, Gao JY,
Zhang JX, Gu FM, Miao XM and Zhao H: Role of chlorogenic acid in
the toxicity induced by Chinese herbal injections. Drug Chem
Toxicol. 33:415–420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang ZF, Zhao W, Zhang Y and Xie YM:
Analysis of influencing factors on adverse reaction of Shengfu
injection based on prospective active safety monitoring. Zhongguo
Zhong Yao Za Zhi. 40:4746–4751. 2015.(In Chinese). PubMed/NCBI
|
|
6
|
Chang YP, Huo J, Xie YM, Zhang H and
Zhuang Y: Real world study of affect on liver function of overdose
of salvianolate extract injection. Zhongguo Zhong Yao Za Zhi.
38:3092–3098. 2013.(In Chinese). PubMed/NCBI
|
|
7
|
Wang ZF, Ai QH, Li YY, Jiang JJ, Wang LX,
Yang W and Xie YM: Analysis of the allergic reaction types of
Chinese medicine injection based on immunotoxicty. Zhongguo Zhong
Yao Za Zhi. 40:4762–4765. 2015.(In Chinese). PubMed/NCBI
|
|
8
|
Huang FH, Zhang XY, Zhang LY, Li Q, Ni B,
Zheng XL and Chen AJ: Mast cell degranulation induced by
chlorogenic acid. Acta Pharmacol Sin. 31:849–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Layton LL, Panzani R and Cortese TA:
Coffee-reaginic human sera tested in human volunteers and macaque
monkeys. Absence of reactions to chlorogenic acid. Int Arch Allergy
Appl Immunol. 33:417–427. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Layton LL, Panzani R, Greene FC, Green TW
and Smith JD: Castor bean allergy as cross-reactive
hypersensitivity to the spurges (euphorbiaceae): Absence of
reaction to chlorogenic acid in primary allergy to castor beans.
Int Arch Allergy Appl Immunol. 23:225–238. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Freedman SO, Shulman R, Krupey J and Sehon
AH: Antigenic properties of chlorogenic acid. J Allergy. 35:97–107.
1964. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freedman SO: Loss of allergenicity of
chlorogenic acid in the gastrointestinal tract. J Allergy.
35:108–116. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Y, Kusama K, Satoh K, Takayama E,
Watanabe S and Sakagami H: Induction of cytotoxicity by chlorogenic
acid in human oral tumor cell lines. Phytomedicine. 7:483–491.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Cheng L, Yuan Q, Cui X, Shang H,
Zhang B and Li Y: Adverse drug reactions of Shuanghuanglian
injection: A systematic review of public literatures. J Evid Based
Med. 3:18–26. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun SG, Li ZF, Xie YM, Liu J, Lu Y, Song
YF, Han YH, Liu LD and Peng TT: Analysis of rational clinical uses
of traditional Chinese medicine injections and factors influencing
adverse drug reactions. Zhongguo Zhong Yao Za Zhi. 38:2969–2973.
2013.(In Chinese). PubMed/NCBI
|
|
16
|
Wang MX, Liu YY, Hu BH, Wei XH, Chang X,
Sun K, Fan JY, Liao FL, Wang CS, Zheng J, et al: Total salvianolic
acid improves ischemia-reperfusion-induced microcirculatory
disturbance in rat mesentery. World J Gastroenterol. 16:5306–5316.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan Y, Guo H, Zhang Y, Zhou D, Gan P,
Liang DM and Chen JY: Protective effects of L-carnitine on
intestinal ischemia/reperfusion injury in a rat model. J Clin Med
Res. 3:78–84. 2011.PubMed/NCBI
|
|
18
|
Wang Y, Jiang YF, Huang QF, Ge GL and Cui
W: Neuroprotective effects of salvianolic acid B against
oxygen-glucose deprivation/reperfusion damage in primary rat
cortical neurons. Chin Med J (Engl). 123:3612–3619. 2010.PubMed/NCBI
|
|
19
|
Collins-Underwood JR, Zhao W, Sharpe JG
and Robbins ME: NADPH oxidase mediates radiation-induced oxidative
stress in rat brain microvascular endothelial cells. Free Radic
Biol Med. 45:929–938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan Q, Liu YY, Sun K, Chen CH, Zhou CM,
Wang CS, Li A, Zhang SW, Ye ZL, Fan JY, et al: Improving effect of
pretreatment with yiqifumai on LPS-induced microcirculatory
disturbance in rat mesentery. Shock. 32:310–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han JY, Horie Y, Fan JY, Sun K, Guo J,
Miura S and Hibi T: Potential of 3,4-dihydroxy-phenyl lactic acid
for ameliorating ischemia-reperfusion-induced microvascular
disturbance in rat mesentery. Am J Physiol Gastrointest Liver
Physiol. 296:G36–G44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han JY, Miura S, Akiba Y, Higuchi H, Kato
S, Suzuki H, Yokoyama H and Ishii H: Chronic ethanol consumption
exacerbates microcirculatory damage in rat mesentery after
reperfusion. Am J Physiol Gastrointest Liver Physiol.
280:G939–G948. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng LF, Dai F, Zhou B, Yang L and Liu
ZL: Prooxidant activity of hydroxycinnamic acids on DNA damage in
the presence of Cu(II) ions: Mechanism and structure-activity
relationship. Food Chem Toxicol. 46:149–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crack PJ and Taylor JM: Reactive oxygen
species and the modulation of stroke. Free Radic Biol Med.
38:1433–1444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai DZ and Dai Y: Role of endothelin
receptor A and NADPH oxidase in vascular abnormalities. Vasc Health
Risk Manag. 6:787–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haurani MJ, Cifuentes ME, Shepard AD and
Pagano PJ: Nox4 oxidase overexpression specifically decreases
endogenous Nox4 mRNA and inhibits angiotensin II-induced
adventitial myofibroblast migration. Hypertension. 52:143–149.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stumpf C, Lehner C, Yilmaz A, Daniel WG
and Garlichs CD: Decrease of serum levels of the anti-inflammatory
cytokine interleukin-10 in patients with advanced chronic heart
failure. Clin Sci (Lond). 105:45–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bolger AP, Sharma R, von Haehling S,
Doehner W, Oliver B, Rauchhaus M, Coats AJS, Adcock IM and Anker
SD: Effect of interleukin-10 on the production of tumor necrosis
factor-alpha by peripheral blood mononuclear cells from patients
with chronic heart failure. Am J Cardiol. 90:384–389. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dokka S, Shi X, Leonard S, Wang L,
Castranova V and Rojanasakul Y: Interleukin-10-mediated inhibition
of free radical generation in macrophages. Am J Physiol Lung Cell
Mol Physiol. 280:L1196–L1202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schottelius AJ, Mayo MW, Sartor RB and
Baldwin AS Jr: Interleukin-10 signaling blocks inhibitor of kappaB
kinase activity and nuclear factor kappaB DNA binding. J Biol Chem.
274:31868–31874. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cook-Mills JM and Deem TL: Active
participation of endothelial cells in inflammation. J Leukoc Biol.
77:487–495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Souza DG, Fagundes CT, Amaral FA,
Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ,
Fierro IM, et al: The required role of endogenously produced
lipoxin A4 and annexin-1 for the production of IL-10 and
inflammatory hyporesponsiveness in mice. J Immunol. 179:8533–8543.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gunnett CA, Heistad DD, Berg DJ and Faraci
FM: IL-10 deficiency increases superoxide and endothelial
dysfunction during inflammation. Am J Physiol Heart Circ Physiol.
279:H1555–H1562. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamaoka M, Yamaguchi S, Okuyama M and
Tomoike H: Anti-inflammatory cytokine profile in human heart
failure: Behavior of interleukin-10 in association with tumor
necrosis factor-alpha. Jpn Circ J. 63:951–956. 1999. View Article : Google Scholar : PubMed/NCBI
|