Introduction
Osteoarthritis (OA) is a degenerative disease, with
patients exhibiting joint pain, joint swelling, restricted movement
and joint deformities (1). OA is an
autoimmune disease and an increasing number of patients (~8%) with
OA have been diagnosed in the clinic since 2008 worldwide (2–4). OA is
divided into primary and secondary OA, according to the presence of
local and systemic risk factors, including inflammation and genetic
factors (5). The causes of OA are
complex, including genetic, physical and chemical factors, which
are difficult to classify with regards to a systematic model
(6). The pathogenesis associated
with this disease is not well understood and OA is frequently
misdiagnosed as rheumatoid arthritis or ankylosing spondylitis in
clinical settings (7–9).
Hesperidin is a flavanone glycoside, which is a
vitamin P flavonoid compound (10).
A previous study reported biological activities of hesperidin and
its molecular mechanisms, including involvement in the nuclear
factor erythroid 2-related factor/extracellular signal-regulated
kinase, No/cGFP and phosphoinositide 3-kinase/mitogen-activated
protein kinase signaling pathways in neuropharmacology (11). In addition, the therapeutic effects
of hesperidin on cardiac tissue and renal and hepatic damage have
been evaluated in several reports (12–14).
Another study revealed that hesperidin presented protective effects
against radiation-induced hepatic injury and damage to peripheral
blood lymphocytes (15).
Furthermore, hesperidin ameliorates oxidative stress and
mitochondrial dysfunction by activation of antioxidant enzymes,
including superoxide dismutase, catalase and
glutathione-S-transferase (16). In
addition, hesperidin alleviates acetaminophen-induced toxicity in
Wistar rats by abrogation of oxidative stress, apoptosis and
inflammation (17). However, the
anti-inflammatory effects of hesperidin on inflammation in human
osteoarthritis chondrocytes remain unclear.
A previous study indicated that cyclooxygenase
(COX)-2-selective inhibitors may be used for the treatment of
rheumatoid arthritis (18). Matrix
metalloproteinase (MMP) protein and activity levels are upregulated
in synovial fluid from patients with joint injury, inflammatory
arthritis and osteoarthritis compared to healthy controls (19). Notably, nuclear factor (NF)-κB
activation has been observed to increase in OA articular
chondrocytes (20). In the current
study, the efficacy of hesperidin in interleukin (IL)-1β-stimulated
human chondrocytes was investigated. The study further explored
potential mechanisms mediated by hesperidin in human chondrocytes
and reported an association between hesperidin and NF-κB signaling
pathway activation in IL-1β-stimulated human chondrocytes.
Materials and methods
Cell culturing and treatment
Human OA chondrocytes were obtained from the
Department of Anatomy & Neurobiology, Northeast Ohio Medical
University (Ohio, USA) and cultured in RPMI 1640 medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Thermo Fisher Scientific,
Inc.) at 37°C in 5% CO2. Cells were treated with IL-1β
(2.0 mg/ml, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and/or
hesperidin (2.0 mg/ml), NF-κB inhibitor (NF-κBIR; cat. no.
LY-294.002; 2.0 mg/ml, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) or PBS (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for
24 h at 37°C. The groups are as follows: PBS, IL-1β, NF-κBIR,
hesperidin and IL-1β+hesperidin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from human OA chondrocytes
(1×108 cells) was extracted using an RNAeasy Mini kit
(Qiagen Sciences, Inc., Gaithersburg, MD, USA) following the
manufacturer's protocol. RNA was reverse transcribed into cDNA at
42°C for 2 h using the High Capacity cDNA Reverse Transcription kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Expression levels of mRNAs in cells were measured by
qPCR. Forward and reverse primers were synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.; Table
I). PCR amplification started with a preliminary denaturation
step at 94°C for 2 min, followed by 45 cycles of 95°C for 30 sec,
56°C for 30 sec (annealing) and 72°C for 45 sec. The reaction
volume was 20 µl, containing 50 ng genomic cDNA, 200 µM dNTPs, 200
µM primers, 2.5 U Taq DNA polymerase and 2.5 U SYBR-Green (Thermo
Fisher Scientific, Inc.). Relative mRNA expression changes were
calculated by the 2−ΔΔCq method (21). Results are presented as the n-fold
change compared with β-actin.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| IL-6 |
TTCCATCCAGTTGCCTTCTTGG |
TTCTCATTTCCACGATTTCCCAG |
| IL-1β |
GGCTGCTTCCAAACCTTTGA |
GAAGACACGGATTCCATGGT |
| IL-17A |
ATGCACAGCCACCGCGACTT |
CTTCATGACTGCCTCCAAGTAG |
| IL-10 |
CAGTGCAGAAGAGTCGACTGCAAG |
CGCTTGAGATCCTGAAATATA |
| COX-2 | TCAAAACCGAGGTGTA |
GTGGGTAAGTATGTAGTGC |
| iNOS |
GTTGGTACATGGGCACTGAG |
TATGGTGGTCGGTAATGGTG |
| MMP-3 |
TCCCTCAGGAAGCTTGAACCTGAA |
AAACCTAGGGTGTGGATGCCTCTT |
| MMP-13 |
TGCTTCCTGATGACGATGTAC |
TCCTCGGAGACTGGTAATGG |
| β-actin |
CGGAGTCAACGGATTTGGTC |
AGCCTTCTCCATGGTCGTGA |
Cell viability
Human OA chondrocytes (1×103/well) were
seeded in 96-well plates following treatment with IL-1β (2.0 mg/ml)
and/or hesperidin (2.0 mg/ml; IL-1β, IL-1β+hesperidin and
hesperidin groups) for 24 h at 37°C, and treated with 10 µl MTT (5
mg/ml, Sigma-Aldrich; Merck KGaA) for 3 h at 37°C. Following
incubation, purple formazan crystals were dissolved using
isopropanol (15 µl). The absorbance was recorded using a microplate
reader (Multiskan FC, Thermo Fisher Scientific, Inc.) at 570 nm.
The effects of hesperidin on cell viability were determined by the
percent of cell viability, calculated as the ratio between mean
absorbance of three samples and mean absorbance of the IL-1β
group.
Cell transfection
Human OA chondrocytes (1×106/well,
untreated cells) were cultured in six-well plates for 12 h at 37°C.
The NF-κB overexpression plasmid was synthesized by cloning human
NF-κB cDNA into plasmid pcDNA3.1 (pNF-κB; Suzhou GenePharma Co.,
Ltd., Suzhou, China) and the empty plasmid pcDNA3.1 served as the
control (pControl). Cells were transfected pNF-κB (100 pmol) or
pControl (100 pmol) using Lipofectamine 3000 reagent (Invitrogen,
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Following 48 h transfection, NF-κB-overexpressed cells
were treated with hesperidin (2.0 mg/ml) for 24 h at 37°C further
analysis.
Production of nitric oxide (NO) and
prostaglandin E2 (PGE2)
After 48 h transfection, NF-kB-overexpressed cells,
treated with hesperidin or PBS, were used to detect NO and PGE2
production. Production of NO was assessed by measuring the nitrite
content in the culture supernatant was obtained via centrifugation
at 4,000 × g at room temperature for 10 min following mixing of the
culture with Griess reagent (1% sulphanilamide, 0.1%
N−1-naphthylenediamine dihydrochloride and 2.5% phosphoric
acid). The absorbance was measured at 540 nm following 10 min of
incubation at 37°C. The levels of PGE2 were analyzed using the
Prostaglandin E2 Parameter Assay kit (KGE004B, Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's instructions.
Western blot analysis
After 48 h transfection, NF-kB-overexpressed human
OA chondrocytes (1×107) treated with hesperidin or PBS
were lysed using radioimmunoprecipitation lysis buffer (Thermo
Fisher Scientific, Inc.). Protein concentrations were measured
using a BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Equal amounts of proteins (10 µg) were separated using 12% SDS-PAGE
and transferred to a polyvinylidene fluoride membrane (Merck KGaA).
Following blocking with 5% bovine serum albumin for 2 h at 37°C,
membranes were incubated with primary antibodies for 12 h at 4°C:
IL-1β (1:1,000; ab200478; Abcam, Cambridge, UK), IL-17A (1:1,000;
ab193955; Abcam), IL-6 (1:1,000; ab7737; Abcam), IL-10 (1:1,000;
ab19969; Abcam), COX-2 (1:1,000; ab15191; Abcam), PGE-2 (1:1,000;
ab181249; Abcam), inducible nitric oxide synthase (iNOS; 1:1,000;
ab15323; Abcam), MMP-3 (1:1,000; ab53015; Abcam), MMP-13 (1:1,000;
ab39012; Abcam) and β-actin (1:2,000; ab8226; Abcam). Horseradish
peroxidase-conjugated anti-rabbit IgG antibody (1:2,000; PV-6001;
OriGene Technologies, Inc., Beijing, China) was incubated with the
membrane for 24 h at 4°C. Membranes were washed with PBS and
visualized with an electrochemiluminescence western blotting
detection reagent (Amersham; GE Healthcare, Chicago, IL, USA). The
band intensities were analyzed by ImageJ software 1.0 (National
Institutes of Health, Bethesda, MD, USA).
NF-κB activity
NF-kB-overexpressed with or without hesperidin human
OA chondrocytes (1×106/well) were cultured in six-well
plates for 12 h at 37°C. The 3′-untranslated region (3′-UTR)
sequence of NF-κB was inserted into the pGL3 control vector
(Promega Corporation, Madison, WI, USA). The construct was referred
to as NF-κB-3′-UTR. Cells were washed with PBS and transfected with
NF-κB-3′-UTR or control (NF-κB-3′-mimic; Promega Corporation) using
Lipofectamine 3000 (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Cells were collected using
centrifugation in 4,000 × g at room temperature for 15 min after 48
h at 37°C and Renilla luciferase activity was measured using
the Dual-Luciferase Reporter Assay system (Promega Corporation)
according to the manufacturer's protocols. Results were obtained
from three independent experiments performed in duplicate and
normalized to Renilla.
Statistical analysis
Data are expressed as the mean ± standard deviation
and statistical analysis was performed with SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). Statistically significant differences between
groups were analyzed by one-way analysis of variance followed by
Tukey's honest significant difference test. P<0.01 was
considered to indicate a statistically significant difference.
Results
IL-1β stimulates inflammation in human
OA chondrocytes
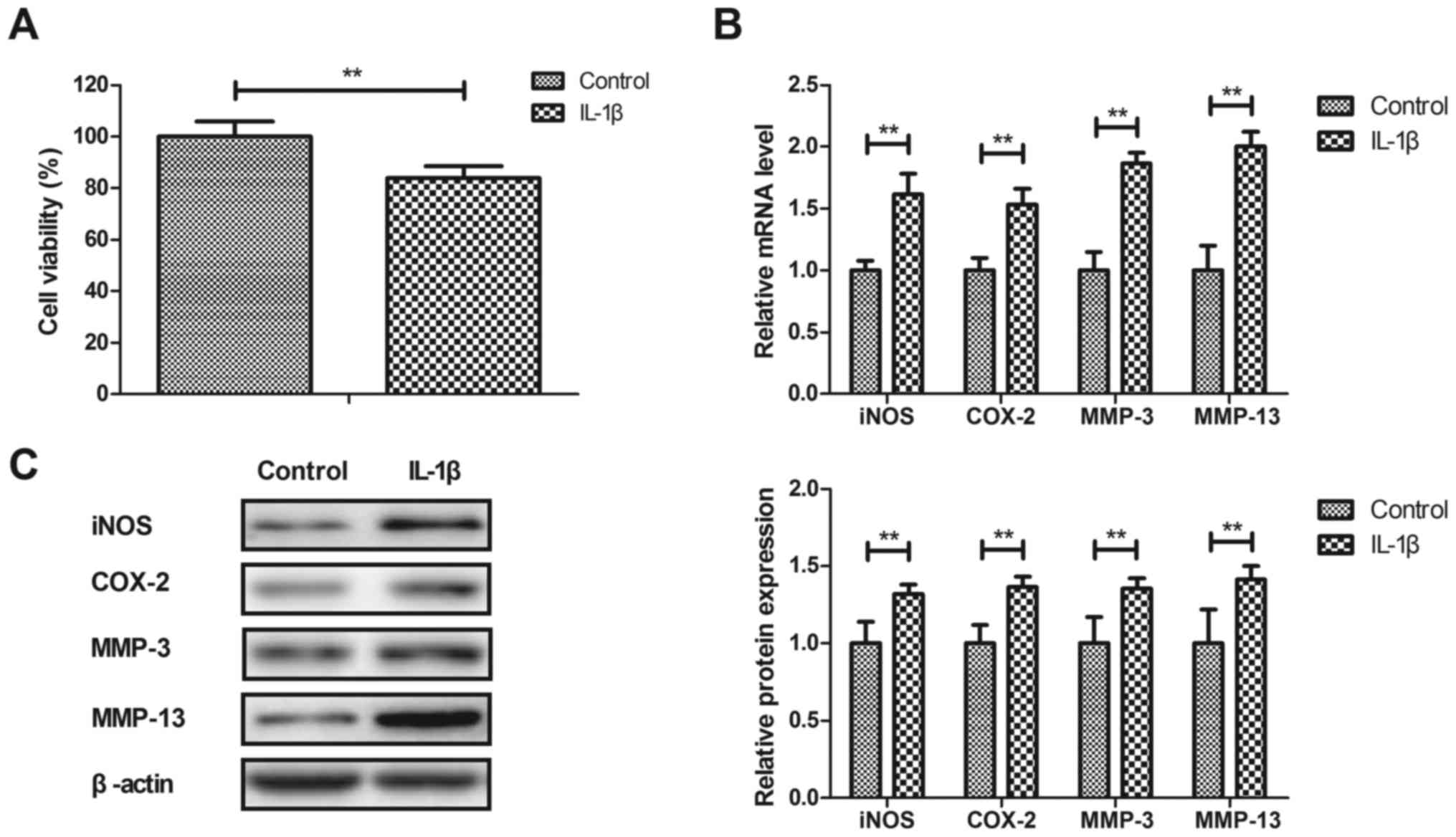
Anti-inflammatory effects of IL-1β on human OA
chondrocytes were investigated in vitro. As illustrated in
Fig. 1A, IL-1β significantly
decreased the viability of human OA chondrocytes after 24 h
compared with the control (P<0.01). IL-1β significantly
increased iNOS, COX-2, MMP-3 and MMP-13 gene and protein expression
in human OA chondrocytes compared with the PBS-treated cells
(P<0.01; Fig. 1B and C). These
results indicated that IL-1β stimulated inflammation in human OA
chondrocytes.
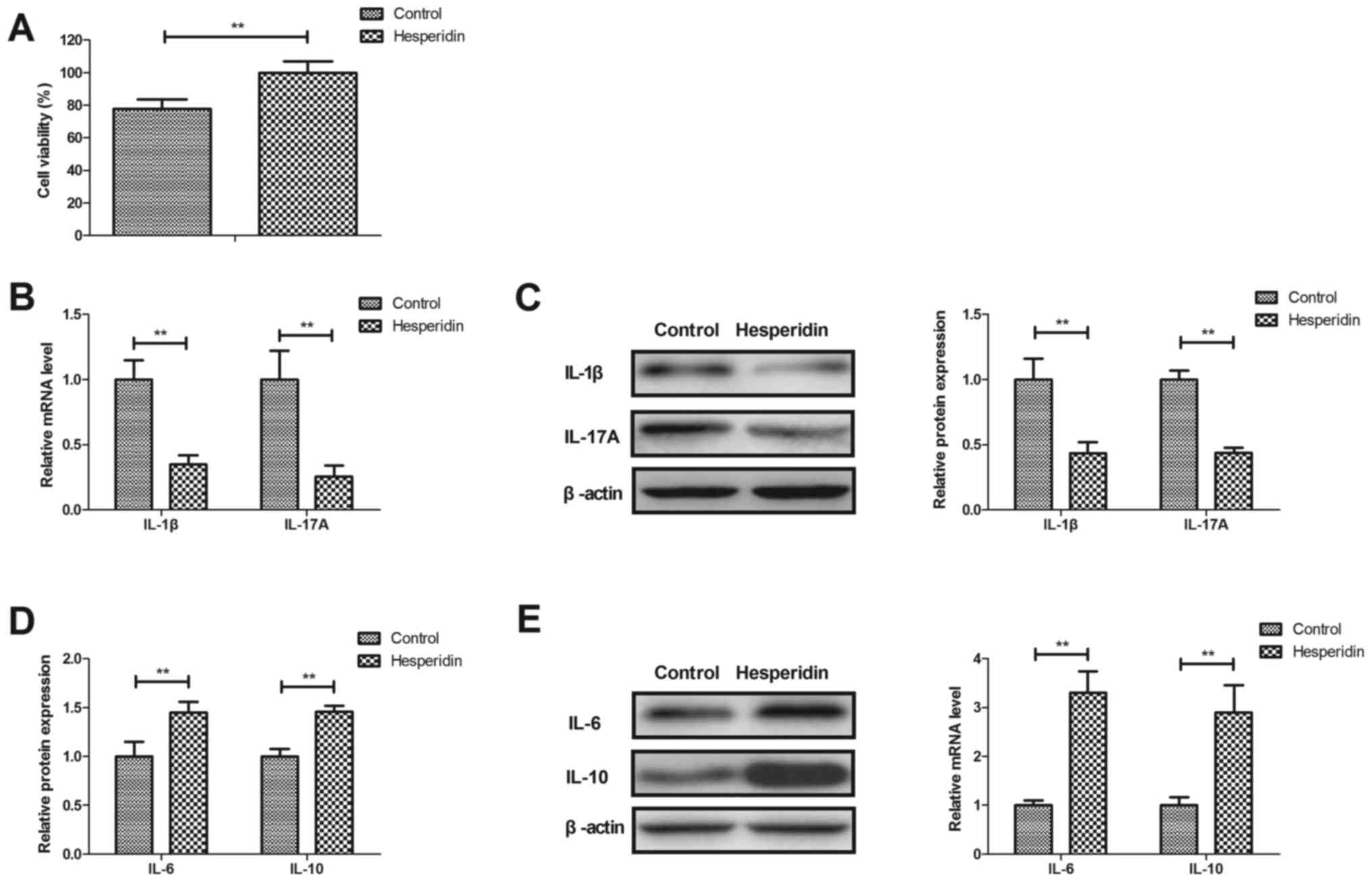
Hesperidin attenuates inflammation in
IL-1β-stimulated human OA chondrocytes
Effects of hesperidin on inflammation in
IL-1β-stimulated human OA chondrocytes were investigated in
vitro. Hesperidin significantly increased the viability of
human OA chondrocytes compared with the PBS-treated group
(P<0.01; Fig. 2A). Hesperidin
treatment significantly decreased IL-1β and IL-17A gene and protein
expression (P<0.01; Fig. 2B and
C) and significantly increased IL-6 and IL-10 gene and protein
expression in IL-1β-stimulated human OA chondrocytes compared with
the PBS-treated cells (P<0.01; Fig.
2D and E). These results indicated that hesperidin may
attenuate inflammation in IL-1β-stimulated human OA
chondrocytes.
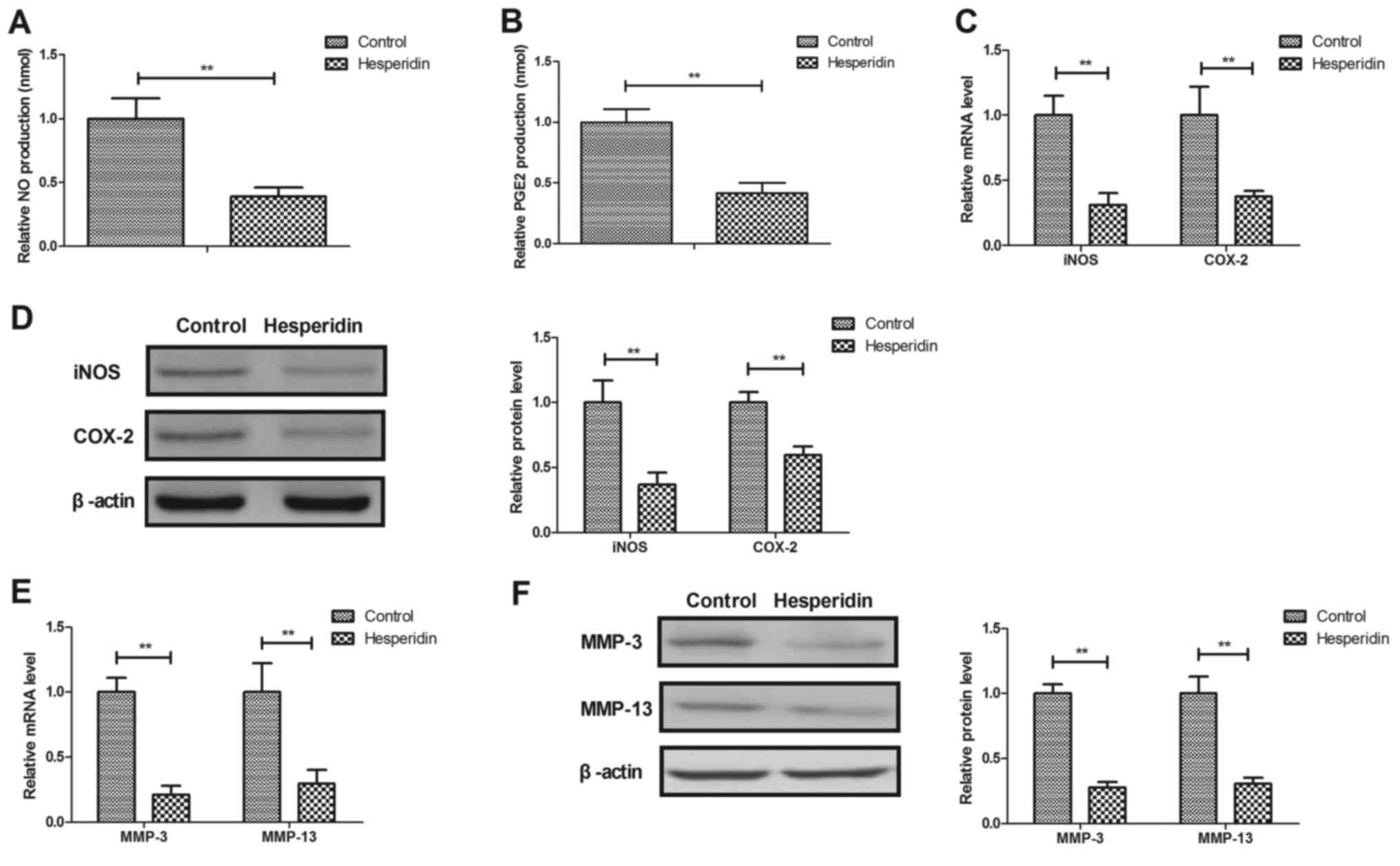
Hesperidin inhibits IL-1β-induced
MMP-3 and MMP-13 release in human OA chondrocytes
Hesperidin effects on NO, PGE2, iNOS and COX-2
expression in IL-1β-stimulated human OA chondrocytes were further
investigated. It was revealed that hesperidin significantly
decreased NO and PGE2 production (P<0.01; Fig. 3A and B) and significantly inhibited
iNOS and COX-2 gene and protein expression in IL-1β-stimulated OA
chondrocytes compared with the PBS-treated cells (P<0.01;
Fig. 3C and D). Hesperidin further
significantly inhibited IL-1β-induced MMP-3 and MMP-13 release in
human OA chondrocytes compared with the control (PBS-treated cells;
P<0.01; Fig. 3E and F). These
results suggested that hesperidin may inhibit the IL-1β-induced NO
and MMP signaling pathways in human OA chondrocytes.
Hesperidin affects IL-1β-stimulated
inflammation by inhibiting NF-κB activity in human OA
chondrocytes
The potential mechanism mediated by hesperidin was
analyzed in human OA chondrocytes. It was observed that hesperidin
significantly decreased the activity of NF-κB in IL-1β-stimulated
human OA chondrocytes compared with the PBS-treated cells
(P<0.01; Fig. 4A). Additionally,
NF-κBIR treatment significantly decreased iNOS, COX-2, MMP-3 and
MMP-13 gene and protein expression in IL-1β-stimulated human OA
chondrocytes compared with the PBS-treated cells (P<0.01;
Fig. 4B and C). NF-κB
overexpression, induced by transfection with pNF-κB, significantly
increased iNOS, COX-2, MMP-3 and MMP-13 gene and protein expression
in IL-1β-stimulated human OA chondrocytes compared with the
pControl (P<0.01; Fig. 4D and E).
The results also demonstrated that NF-κB overexpression reversed
hesperidin-inhibited iNOS, COX-2, MMP-3 and MMP-13 gene and protein
expression in IL-1β-stimulated human OA chondrocytes compared with
the PControl group (Fig. 4F and G).
These results indicated that hesperidin inhibited IL-1β-stimulated
inflammation by the inhibiting NF-κB activity in human OA
chondrocytes.
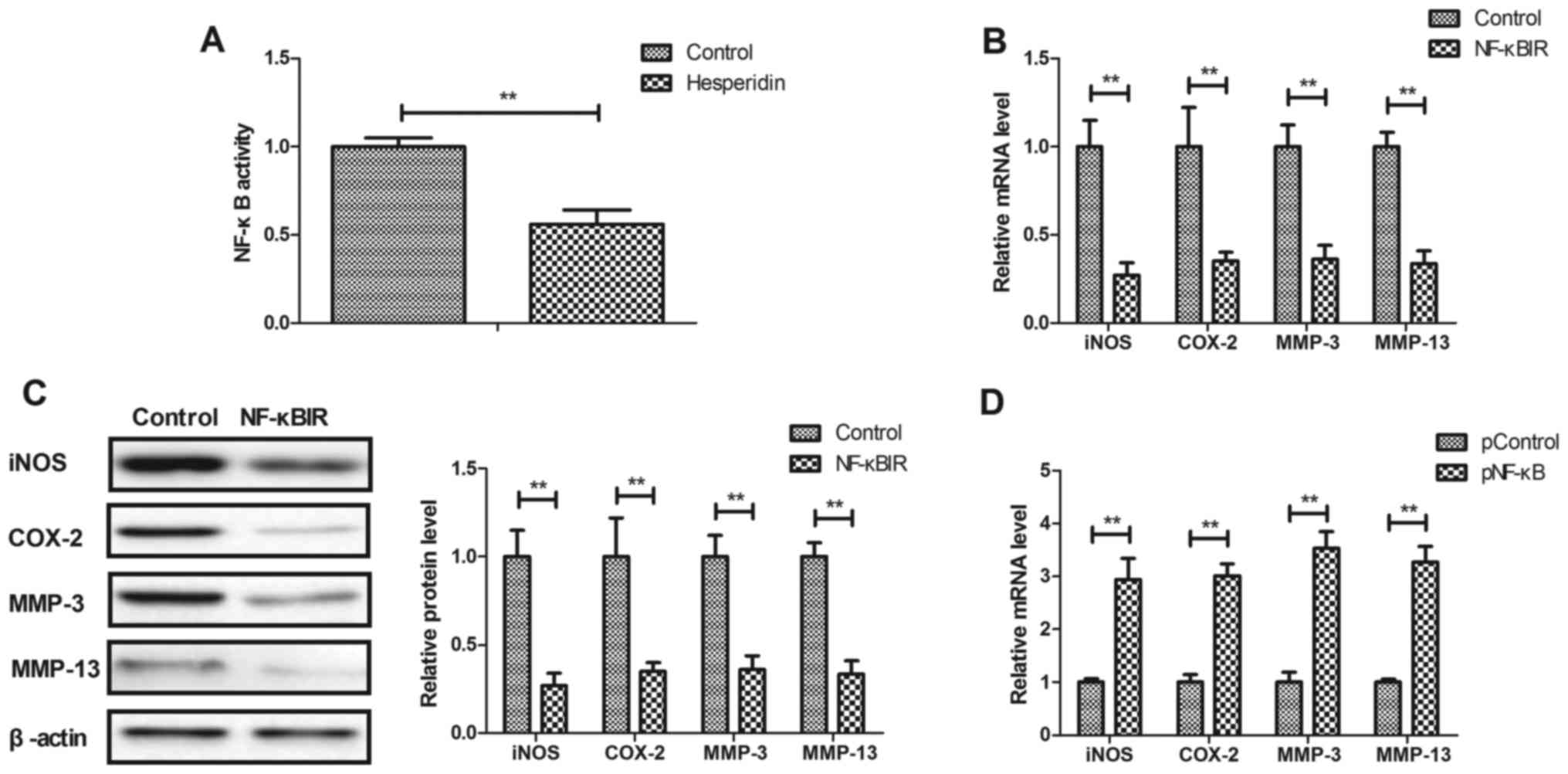 | Figure 4.Hesperidin inhibits IL-1β-stimulated
inflammation by reducing NF-κB activity in human OA chondrocytes.
(A) Hesperidin decreases the activation of NF-κB in
IL-1β-stimulated human OA chondrocytes. Effects of NF-κBIR on iNOS,
COX-2, MMP-3 and MMP-13 (B) gene and (C) protein expression in
IL-1β-stimulated human OA chondrocytes. Effects of pNF-κB on iNOS,
COX-2, MMP-3 and MMP-13 (D) gene and (E) protein expression in
IL-1β-stimulated human OA chondrocytes. Effects of pNF-κB on
hesperidin-inhibited IL-1β-stimulated iNOS, COX-2, MMP-3 and MMP-13
(F) gene and (G) protein expression in IL-1β-stimulated human OA
chondrocytes. **P<0.01. OA, osteoarthritis; IL, interleukin;
NF-κB, nuclear factor-κB; NO, nitric oxide; PGE2, prostaglandin E2;
iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2;
MMP, matrix metalloproteinase; NF-κBIR, NF-κB inhibitor; pNF-κB,
NF-κB overexpression; pControl, empty pcDNA3.1. |
Discussion
A previous study revealed that hesperidin attenuates
inflammation and oxidative damage in the pleural exudates and the
liver of a rat model of pleurisy (22). However, the role of hesperidin in
human OA chondrocytes is yet to be elucidated. In the current
study, the role of hesperidin in IL-1β-stimulated human OA
chondrocytes was analyzed. The findings revealed that hesperidin
significantly decreased IL-1β and IL-17A gene and protein
expression, while it increased IL-6 and IL-10 gene and protein
expression in IL-1β-stimulated human OA chondrocytes compared with
the PBS group. The current study observed that hesperidin inhibited
IL-1β-stimulated inflammation in human OA chondrocytes.
Currently, anti-IL-1β therapy is an efficient
treatment method for patients with OA (23). Liu et al (24) indicated that IL-17 serves a crucial
role in the pathogenesis of OA and is closely associated with pain,
which suggests that blocking the IL-17 signaling pathway may
contribute to the treatment of OA. It was reported in the current
study that hesperidin decreased IL-1β and IL-17A and increased IL-6
and IL-10 gene and protein expression in human OA chondrocytes,
which has previously been indicated to be beneficial in OA therapy
(24).
Suppressing NF-κB activity attenuates pain and
inflammation in rats with osteoarthritis (25). Hesperidin has been reported to
suppress oxidative stress and inflammation via activation of the
protein kinase B/nuclear factor erythroid 2-related factor 2
signaling pathway, inhibition of the receptor for advanced
glycation end products/NF-κB signaling pathway and further confers
neuroprotection (26). In the
current study, it was revealed that hesperidin significantly
inhibited NF-κB activity in IL-1β-stimulated human OA
chondrocytes.
Suppression of PGE2 and MMP expression was indicated
to inhibit cartilage degradation by pomegranate fruit extracts in a
model of posttraumatic osteoarthritis (27). Another study reported that patients
with OA exhibited significantly higher COX-2 levels compared with
the healthy control group (28). In
the current study, findings demonstrated that hesperidin
significantly decreased PGE2 and COX-2 expression in
IL-1β-stimulated human OA chondrocytes compared with PBS-treated
cells. A previous study has demonstrated that demethylation of an
NF-κB enhancer element induced iNOS production in OA and is
associated with an altered chondrocyte cell cycle (29). In the current study, results revealed
that hesperidin inhibited iNOS and COX-2 gene and protein
expression in IL-1β-stimulated human OA chondrocytes compared with
PBS-treated cells. Findings suggested that hesperidin regulated
iNOS and COX-2 expression via the inhibition of NF-κB activity in
IL-1β-stimulated human OA chondrocytes compared with PBS-treated
cells.
In conclusion, the findings of the current study
suggest that hesperidin downregulated inflammation in
IL-1β-stimulated human OA chondrocytes and highlight the potential
application of hesperidin for OA therapy based on decreasing NF-κB
activity. The current study suggests that hesperidin may be a
potential agent for the inhibition of inflammation in the treatment
of OA.
Acknowledgements
Not applicable.
Funding
The current study was supported by the Natural
Science Foundation of Guangdong Province (grant no.
2015A030310248).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZF designed the experiments. ZC and QX performed the
experiments. HL and SX acquired and interpreted all data in the
present study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethical
Committee of Jiangmen Central Hospital, Jiangmen (Jiangmen,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Onuora S: Osteoarthritis: Molecular
imaging detects activated macrophages. Nat Rev Rheumatol.
12:3132016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meheux CJ, McCulloch PC, Lintner DM,
Varner KE and Harris JD: Efficacy of intra-articular platelet-rich
plasma injections in knee osteoarthritis: A systematic review.
Arthroscopy. 32:495–505. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Monticone M, Frizziero A, Rovere G,
Vittadini F, Uliano D, LA Bruna S, Gatto R, Nava C, Leggero V and
Masiero S: Hyaluronic acid intra-articular injection and exercise
therapy: Effects on pain and disability in subjects affected by
lower limb joints osteoarthritis. A systematic review by the
italian society of physical and rehabilitation medicine (SIMFER).
Eur J Phys Rehabil Med 52: 389–399, 2016. Eur J Phys Rehabil Med
52: 389–399, 2016. 52: 389–399, 2016:389-399, 2016–399, 2016.
2016.
|
|
4
|
Hammond A, Jones V and Prior Y: The
effects of compression gloves on hand symptoms and hand function in
rheumatoid arthritis and hand osteoarthritis: A systematic review.
Clin Rehabil. 30:213–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang H, He S, Zhang X, Luo S, Zhang B,
Duan X, Zhang Z, Wang W, Wang Y and Sun Y: A network pharmacology
approach to uncover the pharmacological mechanism of XuanHuSuo
powder on osteoarthritis. Evid Based Complement Alternat Med 2016.
32469462016.
|
|
6
|
Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ,
Eckstein F, Grago J, Boudreau RM, Englund M and Guermazi A: Partial
meniscectomy is associated with increased risk of incident
radiographic osteoarthritis and worsening cartilage damage in the
following year. Eur Radiol. 27:404–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malfait AM: Osteoarthritis year in review
2015: Biology. Osteoarthritis Cartilage. 24:21–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maleki M, Arazpour M, Joghtaei M, Hutchins
SW, Aboutorabi A and Pouyan A: The effect of knee orthoses on gait
parameters in medial knee compartment osteoarthritis: A literature
review. Prosthet Orthot Int. 40:193–201. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Su Y, Chen S, Zhang Y, Zhang Z, Liu
C, Lu M, Liu F, Li S, He Z, et al: The effects of resistance
exercise in patients with knee osteoarthritis: A systematic review
and meta-analysis. Clin Rehabil. 30:947–959. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
La Torre F, Nicolai AP and Otti M:
Hemorrhoids and conservative treatment. Review of the literature on
the use of diosmin and micronized hesperidin. Minerva Chir.
54:909–916. 1999.(In Italian).
|
|
11
|
Roohbakhsh A, Parhiz H, Soltani F, Rezaee
R and Iranshahi M: Neuropharmacological properties and
pharmacokinetics of the citrus flavonoids hesperidin and
hesperetin-a mini-review. Life Sci. 113:1–6. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Antunes MS, Goes AT, Boeira SP, Prigol M
and Jesse CR: Protective effect of hesperidin in a model of
Parkinson's disease induced by 6-hydroxydopamine in aged mice.
Nutrition. 30:1415–1422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang D, Liu L, Zhu X, Wu W and Wang Y:
Hesperidin alleviates cognitive impairment, mitochondrial
dysfunction and oxidative stress in a mouse model of Alzheimer's
disease. Cell Mol Neurobiol. 34:1209–1221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar A, Chaudhary T and Mishra J:
Minocycline modulates neuroprotective effect of hesperidin against
quinolinic acid induced Huntington's disease like symptoms in rats:
Behavioral, biochemical, cellular and histological evidences. Eur J
Pharmacol. 720:16–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li G, Chen MJ, Wang C, Nie H, Huang WJ,
Yuan TD, Sun T, Shu KG, Wang CF, Gong Q and Tang SQ: Protective
effects of hesperidin on concanavalin A-induced hepatic injury in
mice. Int Immunopharmacol. 21:406–411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Poetini MR, Araujo SM, Trindade de Paula
M, Bortolotto VC, Meichtry LB, Polet de Almeida F, Jesse CR, Kunz
SN and Prigol M: Hesperidin attenuates iron-induced oxidative
damage and dopamine depletion in Drosophila melanogaster model of
Parkinson's disease. Chem Biol Interact. 279:177–186. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad ST, Arjumand W, Nafees S, Seth A,
Ali N, Rashid S and Sultana S: Hesperidin alleviates acetaminophen
induced toxicity in Wistar rats by abrogation of oxidative stress,
apoptosis and inflammation. Toxicol Lett. 208:149–161. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hochberg MC: Treatment of rheumatoid
arthritis and osteoarthritis with COX-2-selective inhibitors: A
managed care perspective. Am J Manag Care. 8 17 Suppl:S502–S517.
2002.PubMed/NCBI
|
|
19
|
Tchetverikov I, Lohmander LS, Verzijl N,
Huizinga TW, TeKoppele JM, Hanemaaijer R and DeGroot J: MMP protein
and activity levels in synovial fluid from patients with joint
injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis.
64:694–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu L, Sun C, Zhang S, Xu X, Zhai L, Wang
Y, Wang S, Liu Z, Cheng H, Xiao M, et al: Sam68 promotes NF-κB
activation and apoptosis signaling in articular chondrocytes during
osteoarthritis. Inflamm Res. 64:895–902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adefegha SA, Rosa Leal DB, Olabiyi AA,
Oboh G and Castilhos LG: Hesperidin attenuates inflammation and
oxidative damage in pleural exudates and liver of rat model of
pleurisy. Redox Rep. 22:563–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chevalier X, Conrozier T and Richette P:
Desperately looking for the right target in osteoarthritis: The
anti-IL-1 strategy. Arthritis Res Ther. 13:1242011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Peng H, Meng Z and Wei M:
Correlation of IL-17 level in synovia and severity of knee
osteoarthritis. Med Sci Monit. 21:1732–1736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai T, Shi K, Chen G, Shen Y and Pan T:
Malvidin attenuates pain and inflammation in rats with
osteoarthritis by suppressing NF-κB signaling pathway. Inflamm Res.
66:1075–1084. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong Y and An Z: Hesperidin attenuates
learning and memory deficits in APP/PS1 mice through activation of
Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch
Pharm Res. 41:655–663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akhtar N, Khan NM, Ashruf OS and Haqqi TM:
Inhibition of cartilage degradation and suppression of PGE2 and
MMPs expression by pomegranate fruit extract in a model of
posttraumatic osteoarthritis. Nutrition. 33:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fan HW, Liu GY, Zhao CF, Li XF and Yang
XY: Differential expression of COX-2 in osteoarthritis and
rheumatoid arthritis. Genet Mol Res. 14:12872–12879. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Andrés MC, Takahashi A and Oreffo RO:
Demethylation of an NF-κB enhancer element orchestrates iNOS
induction in osteoarthritis and is associated with altered
chondrocyte cell cycle. Osteoarthritis Cartilage. 24:1951–1960.
2016. View Article : Google Scholar : PubMed/NCBI
|