Introduction
Budd-Chiari syndrome (BCS) is a clinical disorder
caused by the obstruction of the hepatic vein (HV) outflow system,
anywhere from the hepatic venules to the cavoatrial junction
(1–3). BCS may be caused by obstruction of the
HV and/or inferior vena cava (IVC) (1,3,4). Patients with BCS suffer from liver
injury secondary to the obstruction of venous outflow, leading to
progressive symptoms and even liver cirrhosis if not treated in a
timely manner. Patients with BCS in the acute phase may succumb to
hepatic failure. In chronic BCS, patients develop hepatocirrhosis
leading to various complications, including gastrointestinal
bleeding, refractory ascites and hepatocellular cancer (1,3,5).
The treatment of BCS has evolved considerably over
the past few decades, and the overall 5-year survival rate has
risen to 80–90% (4,6–8). Various
treatment options for BCS include the following: i) Anti-coagulant
therapy; ii) endovascular decompression therapy, including
thrombolysis, stent-graft placement, angioplasty and transjugular
intrahepatic portosystemic shunt (TIPS); and iii) orthotopic liver
transplantation. Due to differences in the etiopathogenesis of BCS
between China and western countries, treatment options vary widely.
In western countries, BCS mostly occurs due to thrombosis and most
patients receive thrombolysis, TIPS or liver transplantation
(9,10). However, in China, BCS is mostly
caused by membranous occlusion of the HV or IVC. Therefore, most
patients with BCS in China undergo angioplasty (11–13).
Formerly, BCS in China was thought to be due to IVC
obstruction only. However, recent Chinese studies have indicated
that most patients with BCS either present with an obstruction of
the HV alone, or of the IVC and HV combined (11,14).
Therefore, in China, the endovascular treatment of HV obstruction
(HVO) associated with BCS has become a new challenge in clinical
practice.
While endovascular interventional therapy for
patients with BCS due to IVC obstruction has been standardized
(15,16), it is still evolving for patients with
BCS due to HVO. Western countries advocate the use of TIPS as a
primary treatment for HVO in BCS (3,9,10), while Chinese physicians prefer
recanalization (4,13,17). The
present retrospective study assessed the efficacy of recanalization
in 69 consecutive patients with BCS due to HVO.
Materials and methods
Patient data
All procedures were performed in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional and national) and with the Helsinki
Declaration of 1975, as revised in 2008 (5). All patients provided written informed
consent prior to undergoing the procedure.
The prospectively maintained data of patients
diagnosed with BCS and treated at Anhui Provincial Hospital (Hefei,
China) or Affiliated Hospital of Xuzhou Medical University (Xuzhou,
China) and December 2010 and December 2012 were reviewed. For
inclusion in the present study, patients were required to conform
to the following: Primary BCS due to HV stenosis or HV occlusion as
confirmed by magnetic resonance imaging (Fig. 1) or digital subtraction angiography,
and symptoms associated with portal hypertension. Patients with any
of the following were excluded: Hepatic sinusoidal obstruction
syndrome; IVC thrombosis; recurrent BCS; or BCS due to any other
cause, including cancer, cysts or parasites. Patients who were lost
to follow-up within 5 years were also excluded (Fig. 2).
Recanalization of the HV via
transjugular and/or femoral vein approach
The venography of the IVC was performed first, via
the internal jugular and/or femoral vein, in order to identify the
ostium of the HV and correlate it with the pre-operative images. In
patients with HV stenosis, a 5F Cobra catheter and an ultra-slip
wire were used to explore the ostium of the target HV. For patients
with complete HVO (left, middle or right), a single-bend catheter
and a self-made single-bend needle (Fig.
3A and B) were used to puncture the major HV (left, middle or
right that was most affected by the obstruction) trunk, branch
and/or traffic branch (i.e., the connecting vessel between two
major HVs). After successful cannulation, HV angiography was
performed, and the HV pressure was measured in order to assess
luminal obstruction of the affected major HV and its branches.
After complete assessment, balloon angioplasty (Figs. 4–6)
and endovascular stenting were performed. The patients with BCS who
underwent balloon angioplasty only, were included in balloon
angioplasty group. The patients who received balloon angioplasty in
addition to other methods of angioplasty, including thrombolysis
and/orendovascular stenting, were included in the combination
therapy group.
Recanalization of HV via percutaneous
transhepatic and IVC approach
After successful percutaneous puncture of one of the
branches of the HV, the guide wire was passed through the
obstructed segment of the HV and then drawn out from the internal
jugular or femoral vein. Subsequently, recanalization of the HV was
performed in the opposite direction through the IVC using an
internal jugular or femoral vein approach.
Recanalization of HV with
thrombus
A 5F thrombolytic catheter was placed in the HV via
a transjugular approach and thrombolysis was performed with
urokinase (100,000 U; 4–6 times daily). Radiography was performed
every 3 days for review, and the catheter was adjusted so that the
catheter's lateral orifice was within the thrombus. Recanalization
of the HV was performed after the thrombosis was completely
dissolved, or if two consecutive reviews did not indicate any
progression of the thrombosis.
TIPS
Through the transjugular route, angiography was
performed to identify the major HVs. If the major HV could not be
identified by angiography of the HV, IVC and portal vein,
angiography was performed using ultrasound-guided percutaneous
liver puncture through an accessory HV. The puncture site of the HV
or IVC and portal vein was selected based on the angiography
results and TIPS was performed (9,10).
Second-stage treatment
For patients with BCS who did not undergo HV
angioplasty for the first time, a different treatment plan was
selected based on the patient's liver function (Child's score)
(18). If the patient's Child's
score was less than 12 points, medical treatment (including
diuresis and liver protection) was given. If medical treatment was
effective, HV angioplasty was performed again following 6 months of
medical treatment. If the patient's Child's score exceeded 12
points or medical treatment was not effective, TIPS or liver
transplantation was considered.
Post-angioplasty treatment
All patients received subcutaneous
low-molecular-weight heparin (5,000 IU; twice daily) for 3 days,
and then oral warfarin (5 mg; daily) for 12 months after treatment.
The dose of warfarin was adjusted such that the prothrombin time
was maintained at 20–25 sec.
Success criteria for angioplasty
After interventional therapy, if the HVs featured a
smooth blood flow and a transmembrane pressure difference of <4
cmH2O, the procedure was considered successful.
Follow-up
The two centers routinely followed the patients up
by telephone and through outpatient services every week for the
first month, monthly for the next 3 months, every 3 months
thereafter. Significant clinical events were recorded, including
clinical deterioration, new radiographic signs on the liver and new
BCS-associated interventions. Clinical deterioration was defined as
re-admission after discharge, occurrence of new symptoms,
recurrence of massive ascites, venous dilation over the trunk, leg
edema, variceal bleeding or hepatic encephalopathy. The deadline
for the follow-up was July 2017, the time-point of death or the
time after which the patient was lost to follow-up.
One assigned clinician was responsible for
collecting the data for each of the participating patients. The
recorded data included socio-demographic features, clinical
manifestations, radiology results, interventional treatments and
outcomes. Another clinician checked and assessed the data
monthly.
Statistical analysis
SPSS statistical software version 22.0 (IBM Corp.,
Armonk, NY, USA) was used for the analyses. Categorical dataare
presented as n (%) and quantitative data as the mean ± standard
deviation. Quantitative data that conformed to a normal
distribution (e.g., venous pressure) were analyzed using
Student'st-test. Quantitative data that did not conform to a normal
distribution were analyzed using the Wilcoxon rank-sum test, and
qualitative data (e.g., ascites grade) were analyzed using the
χ2 test. Kaplan-Meier curves were drawn to analyze
patient survival and the log-rank test was used to analyze
differences in survival rates. P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient enrolment
From December 2010 to December 2012, 350 patients
with BCS were treated at the two centers (Fig. 2). In accordance with the exclusion
criteria, patients with secondary BCS, patients with BCS were
diagnosed prior to admission and those with involvement of IVC were
excluded. Of the remaining 80 patients with BCS with HVO only, 11
were excluded from the study due to insufficient follow-up. Thus,
69 patients (43 male, 26 female; mean age, 43 years; mean duration
of symptoms, 98 months) were included in the analysis. The baseline
clinical characteristics and laboratory tests of patients with BCS
are presented in Tables I and
II).
 | Table I.Baseline clinical features of
patients with Budd-Chiari syndrome (n=69). |
Table I.
Baseline clinical features of
patients with Budd-Chiari syndrome (n=69).
| Characteristic | Value |
|---|
| Male/female | 43/26 (62/38) |
| Age (years) | 43 (15–72) |
|
Smokersa | 15 (22) |
|
Alcoholicsa | 12 (17) |
| Duration of
symptoms (months) | 98 (0.05–348) |
|
Acute/chronicb | 10/59 (14/86) |
| Abdominal pain | 15 (22) |
| Abdominal
distension | 23 (33) |
| Ascites | 35 (51) |
| Gastrointestinal
bleeding | 16 (23) |
| Varices of
abdominal wall | 37 (54) |
| Anorexia | 12 (18) |
| Hepatomegaly | 51 (74) |
| Splenomegaly | 59 (86) |
 | Table II.Laboratory results of patients with
Budd-Chiari syndrome at diagnosis (n=69). |
Table II.
Laboratory results of patients with
Budd-Chiari syndrome at diagnosis (n=69).
| Parameters | Median (range) | Normal range | Patients with
abnormal values, n (%) |
|---|
| Aspartate
aminotransferase (U/l) | 36 (17–677) | 15–40 | 24
(35)a |
| Alanine
aminotransferase (U/l) | 34 (8–648) | 9–51 | 17
(25)a |
| Glutamyl peptide
transferase (U/l) | 83 (19–536) | 3–50 | 52
(75)a |
| Alkaline
phosphatase (U/l) | 118 (54–351) | 45–125 | 18
(26)a |
| Total bilirubin
(µmol/l) | 35.6
(8.2–114.7) | 1.7–21 | 49
(71)a |
| Direct bilirubin
(µmol/l) | 14 (4.2–72.3) | 0–7.3 | 49
(71)a |
| Albumin (g/l) | 35.6
(18.5–48.7) | 35–51 | 24
(35)b |
| Prothrombin time
(sec) | 16.7
(12.5–24.3) | 11–13 | 44
(64)a |
| White blood
cells(×109/l) | 4.86
(1.07–12.85) | 4–10 | 4 (6)a + 25 (36)b |
| Hemoglobin
(g/l) | 116 (51–169) | 120–165 | 4 (6)a + 21 (30)b |
| Platelets
(×109/l) | 108 (18.3–718) | 100–300 | 5 (7)a + 34 (49)b |
| Cancer antigen-125
(U/l) | 35.1
(7.1–1,032.8) | 0–35 | 31
(45)a |
| α-fetoprotein
(ng/ml) | 4.1
(0.85–85.3) | 0–25 | 6 (9)a |
| Hepatitis B surface
antigen (ng/ml) (n)c | 6 | 0–0.18 | 6 (9) |
Success rate of endovascular
therapy
Among the 69 patients, primary HV recanalization was
successful in 63 cases. Primary therapy failed in 6 patients due to
extensive HVO. Of these patients, 2 with severe symptoms received
TIPS (post-operatively, the symptoms of one patient resolved and
the other one succumbed to liver failure). The remaining 4 patients
with mild symptoms received conservative treatment (3 successfully
received second-stage recanalization 6 months later). The
cumulative technical success rate for HV recanalization was 95.7%
(66/69; Fig. 7). No serious
complications, including pericardial tamponade or ruptured blood
vessels, were encountered.
A total of 66 patients successfully underwent
angioplasty. Recanalization was performed in 43, 14 and 9 patients
via the transjugular, femoral vein and percutaneous transhepatic
approach, respectively. In 35 and 13 patients, the target veins for
recanalization were 1 or >2 major HVs. In 7 patients, the target
veins were an accessory HV and in 11 patients a major HV and an
accessory HV were recanalized.
All of the patients underwent balloon angioplasty.
In 41 patients, balloon angioplasty constituted the sole treatment
(balloon angioplasty group). In addition, 14, 6 and 5 patients in
the combination therapy group respectively received thrombolysis,
endovascular stenting and a combination of thrombolysis and
endovascular stenting. The baseline characteristics (including sex,
age, duration of symptoms, albumin levels, incidence of ascites and
cancer antigen-125) of the balloon angioplasty and combination
therapy groups are presented in Table
III. The differences of incidence of ascites, albumin levels,
cancer antigen-125 levels and duration of symptoms between the two
groups were statistically significant (Table III).
 | Table III.Baseline characteristics of the
balloon angioplasty and combination therapy groups. |
Table III.
Baseline characteristics of the
balloon angioplasty and combination therapy groups.
|
Characteristics | Balloon angioplasty
(n=41) | Combination therapy
(n=25) |
χ2/Z | P-value |
|---|
| Males/female
(n) | 26/15 | 16/9 |
χ2=0.002 | 0.962 |
| Age (years) | 42 (16–72) | 32 (15–63) | Z=−1.199 | 0.231 |
| Duration of
symptoms (months) | 126 (56–348) | 12 (0.05–76) | Z=−2.854 | 0.004 |
| Ascites (n) | 15 (36.6) | 20 (80.0) |
χ2=11.752 | 0.001 |
| Aspartate
aminotransferase (U/l) | 34 (21–589) | 46 (17–677) | Z=−0.564 | 0.573 |
| Alanine
aminotransferase (U/l) | 32 (12–610) | 61 (8–648) | Z=−0.313 | 0.754 |
| Glutamyl peptide
transferase (U/l) | 74 (19–487) | 128 (21–536) | Z=−0.555 | 0.579 |
| Total bilirubin
(µmol/l) | 35.6
(8.2–83.5) | 48.7
(10.3–114.7) | Z=−0.716 | 0.474 |
| Albumin (g/l) | 36.8
(24.5–48.7) | 28.2
(18.5–32.6) | Z=−2.263 | 0.024 |
| Prothrombin time
(sec) | 15.7
(12.5–23.6) | 17.8
(12.8–24.3) | Z=−1.485 | 0.138 |
| Cancer antigen-125
(U/l) | 29.1
(7.1–367.5) | 421.5
(17.5–1,032.8) | Z=−2.711 | 0.007 |
Clinical efficacy
On admission, 3, 20 and 43 patients presented with
mild, moderate and massive ascites, respectively. After
endovascular therapy, resolution of ascites was achieved in 53
patients, while 13 patients had a small amount of residual ascites.
The difference in the grade of ascites prior to and after therapy
was statistically significant (χ2=122.250, P=0.001). The
mean HV pressure after HV recanalization was significantly lower
compared with that prior to the procedure (23±7 vs. 47±9
cmH2O; t=17.979, P=0.001; Table IV). The symptoms of 61 patients were
completely relieved after HV recanalization, while those of 5
patients were partially relieved.
 | Table IV.Pre- and post-operative clinical
efficacy according to severity of ascites and HV pressure. |
Table IV.
Pre- and post-operative clinical
efficacy according to severity of ascites and HV pressure.
| Parameters | Pre-operative n
(%) | Post-operative n
(%) |
χ2/t-value | P-value |
|---|
| Ascites (n) |
|
|
χ2=122.250 | 0.001 |
|
None | 0 (0) | 53 (80.3) |
|
|
|
Mild | 3 (4.5) | 13 (19.7) |
|
|
|
Moderate | 20 (30.3) | 0 (0) |
|
|
|
Massive | 43 (65.2) | 0 (0) |
|
|
| HV pressure
(cmH2O) | 47±9 | 23±7 | t=17.979 | 0.001 |
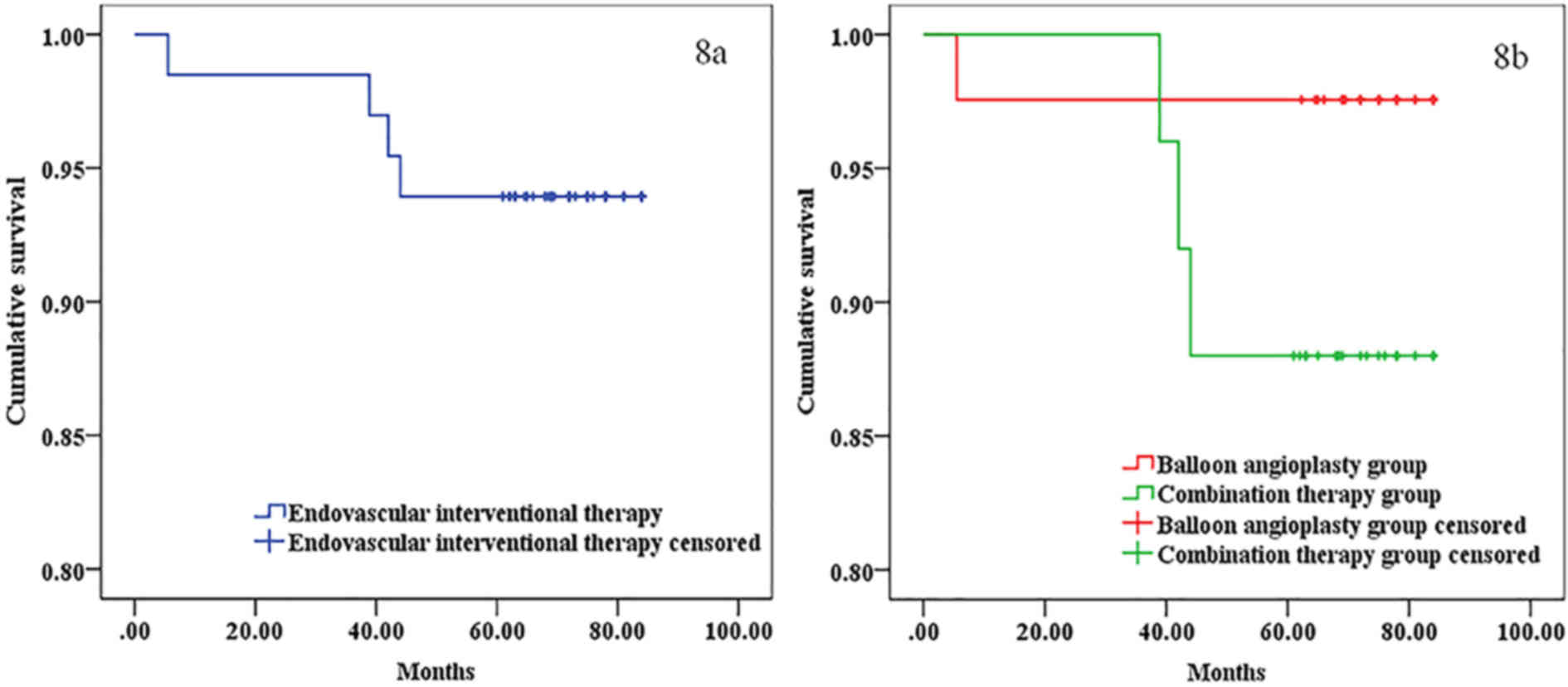
Survival rate
The median follow-up period was 75 months (range,
60–84 months). At 12, 36 and 60 months, the cumulative survival
rate of patients subjected to endovascular interventional therapy
was 98.5, 98.5 and 93.9%, respectively, while that in the balloon
angioplasty group was 97.6, 97.6 and 97.6%, respectively, and that
in the combination therapy group was 96.0, 96.0 and 88.0%,
respectively. No significant differences in all cumulative survival
rates between the balloon angioplasty group and combination therapy
group were identified (χ2=2.387; P=0.122; Fig. 8).
Discussion
In theory, angioplasty of an obstructed HV may
reduce congestion of the liver, decrease pressure of the HV and the
portal vein, and restore the patient's liver function, thereby
making it an ideal treatment for BCS caused by HVO (7,8).
However, endovascular therapy remains a challenging procedure in
these patients due to the anatomical characteristics of the HV
(13,19) and the difficulty in restoring HV
flow. However, in the present study, the technical success rate of
the operation in the Chinese BCS patients with HVO was high (95.7%)
and the post-operative asymptomatic survival rate (80.3% at 5
years) was also high after angioplasty of the obstructed HVs.
In each patient, the first task was to determine
which approach to use for recanalization of the obstructed HV. At
present, the 3 most commonly used therapeutic approaches are the
transjugular, transfemoral and percutaneous transhepatic approach
(4,7). In the majority of patients with BCS due
to HVO, the obstruction is in the proximal HV, resulting from
stenosis of the ostium or membranous occlusion (4,14,20).
These patients are ideally treated by recanalization via the
jugular or femoral vein.
The transjugular approach has a high success rate,
as the angle between the HV and the proximal IVC is usually
relatively large, and the guide wire may easily access the HV via
the jugular vein (4,17,21). If
recanalization of the HV via the transjugular fails, the femoral
vein may be used, but this is technically more difficult. In
certain patients with an accessory HV that intersects the IVC at an
obtuse angle, the transfemoral approach may be more appropriate
(4,11,19,21). The
percutaneous transhepatic approach may be used in patients with
mild ascites, if the jugular and femoral approaches have failed
(4,13). For patients with ascites whose extent
is more than mild, diuretics and other conservative treatments
should be offered initially, and the percutaneous transhepatic
approach could be employed once the ascites has significantly
reduced.
The second task is to select the appropriate target
HV for recanalization. Ideally, the obstructed vein should be the
first choice. The right HV drains a large area of the liver, has a
smaller angle with the IVC and is the preferred vein for
interventional therapy (7,13,19–21). If
all 3 major HVs are obstructed, it may be attempted to reopen the
right HV first. If the diameter and drainage range of the HVs
selected for recanalization are sufficiently large, reperfusion may
be effective for relieving the portal hypertension (4,17,21).
However, if the diameter of the HV is small, multiple HVs may be
considered for recanalization during the same procedure (4,7,11,13,18,21). If
all of the HV segments are occluded, leading to failure of the
interventional therapy, recanalization of the accessory HV may be
attempted to relieve portal hypertension (13,19).
The final task was to determine what type of
angioplasty was suitable for recanalization of the obstructed HV.
In the present study, it was observed that the symptoms completely
disappeared if the transmembrane pressure difference after
percutaneous transluminal angioplasty was 4 cmH2O.
Therefore, it is indicated that the transmembrane pressure
difference is a reliable predictor of successful treatment,
although specific indicators require to be verified (7). The choice of treatment depends on the
location and extent of the obstruction, as well as the
characteristics of the lesion (3,4,8,13,17,21,22).
For thrombus-free obstruction, balloon angioplasty has been
recommended, with the diameter of the selected balloon based on the
diameter of the vessel being treated, typically 12–20 mm (23). If the transmembrane pressure
difference is <4 cmH2O, the treatment is considered
successful. If the transmembrane pressure difference remains >4
cmH2O, an endovascular stent is placed. For the
thromboembolic HV, thrombolytic therapy has been recommended,
followed by balloon angioplasty and endovascular stent (8,17).
For patients with treatment failure and mild
clinical symptoms, conservative medical treatment prior to the
second stage of medullary treatment, performed after the formation
of collateral circulation in the compensated liver, has been
suggested (3,6,8). In the
present study, 3 patients were successfully subjected to
second-stage treatment. In patients with a severe condition, TIPS
was required to relieve portal hypertension and increase the chance
of survival (8–10,24). For
certain cases, liver transplantation has also been recommended
(5,8).
In the present study, no significant differences in
symptom-free survival rates were identified between the balloon
angioplasty and combination treatment groups. This may be due to
the small sample size. Furthermore, in the present cohort was not
randomized regarding the interventional treatment, but it was
selected based on the pathological features of each patient, which
may have introduced selection bias. Therefore, even if the
post-operative asymptomatic survival rate of patients receiving a
specific treatment was higher than that of patients receiving other
treatments, this does not confirm the superiority of the
treatment.
Re-obstruction of the vein is a post-operative
complication and the underlying cause of recurrence of BCS symptoms
(4). According to various studies,
HV stenting may reduce the incidence of HV re-obstruction (3,8,16,25).
However, stents are permanent devices that, increase the difficulty
of a subsequent interventional procedure in the case of a recurrent
stenosis, and should therefore be used with caution. A large
multicenter study indicated that the patency rates of covered and
bare stents in patients with BCS were comparable (6). In a patient with BCS due to HVO, a
covered stent affected the formation of a compensatory collateral
circulation and further aggravated hepatic ischemia (12). Therefore, the use of a covered stent
for recanalization of BCS caused by HVO may not be recommended.
BCS associated with HVO may be classified as
stenosis, membranous or segmental occlusion, thrombosis or wide
occlusion (1,3,4). For
stenosis or membranous/segmental occlusion, angioplasty has been
effective and has a high post-operative asymptomatic survival rate
(4,13,17). In
the present study, the majority of patients recovered after
endovascular interventional therapy, and the cumulative survival
rate at 60 months post-operatively was 93.9%. The cumulative
survival rate at 60 months in the balloon angioplasty group (97.6%)
was higher than that in the combination therapy group (88.0%). This
is probably due to the fact that most of the patients who received
combination therapy had more severe pre-operative symptoms such as
ascites and complicated baseline conditions (lower serum albumin),
which were associated with a relatively poorer prognosis. However,
since the sample size was relatively small, the difference between
the 2 groups was not statistically significant, and further study
with a larger population is required.
For patients with extensive obstruction, it is more
difficult to recanalize via angioplasty, with high rates of failure
and low rates of symptom-free survival. These patients are better
treated by TIPS, which directly reduces portal pressure, relieves
sinus pressure and has a higher efficacy (3,9,10).
In the present study, the post-operative
symptom-free survival rates at 12, 36 and 60 months were 98.5, 98.5
and 92.4%, respectively. These are slightly higher than the
survival rate (97.7, 92.2, and 90.0%) reported by Cui et al
(4). In the present study, as most
patients with BCS had HV stenosis or membranous or segmental
occlusion, the outcomes of recanalization were good. However, only
29–41% of Western patients with BCS have membranous or segmental
occlusion of the HV (4,26,27).
Therefore, percutaneous recanalization is not applicable to most
Western patients with BCS (4,26,27),
and TIPS is the recommended treatment (3,9,10). The putative 6- to- 120-month survival
rates of Western patients with HV-associated BCS after TIPS were
72–97% (10,26), which is slightly lower than the 12-,
36- and 60-month survival rate determined in the present study.
This may be due to the condition being more complicated in most
Western patients with BCS receiving TIPS, and therefore, their
survival rate is relatively low. These differences between Eastern
and Western patients with HV-associated BCS may explain for the
differences in the reported rates of recanalization. However,
regardless of these differences, in the present study, the mid-term
asymptomatic survival rate was high. This indicates that
recanalization was effective for the treatment of HV-associated BCS
in Chinese patients.
The present study is limited by its retrospective
nature and small sample size. In addition, the patients were not
randomized regarding the mode of endovascular treatment, but the
treatment was selected based upon the pathological features of the
patients, which may have introduced bias. Hence, infuture, large
sample prospective studies are required to validate the results of
the present study.
In conclusion, in the Chinese cohort of the present
study, the majority of patients with BCS had segmental or
membranous HVO. In the current study, the endovascular
interventional treatment of BCS patients with HVO that was applied
based on the characteristics of HV vascular lesions. These patients
experienced high symptom-free survival rates and low recurrence
rates after endovascular therapy. Larger prospective studies are
required to confirm the efficacy of endovascular therapy for BCS
caused by HVO.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Foundation of Anhui Province (grant no. 1708085QH218).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
DC, NZ and CL conceived and designed the study. HX,
WF and CL were responsible for the collection and analysis of the
patient data. DC, NZ and CL interpreted the data and drafted the
manuscript. WL contributed to the conception and design of this
study and revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethical approval and consent to
participate
All procedures were performed in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional and national) and with the Helsinki
Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all
patients for being included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martens P and Nevens F: Budd-Chiari
syndrome. United European Gastroenterol J. 3:489–500. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin N, Kim YH, Xu H, Shi HB, Zhang QQ,
Pons Colon JP, Kim D, Xu Y, Wu FY, Han S, et al: Redefining
Budd-Chiari syndrome: A systematic review. World J Hepatol.
8:691–702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goel RM, Johnston EL, Patel KV and Wong T:
Budd-Chiari syndrome: Investigation, treatment and outcomes.
Postgrad Med J. 91:692–697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui YF, Fu YF, Li DC and Xu H:
Percutaneous recanalization for HV-type Budd-Chiari syndrome:
Long-term patency and survival. Hepatol Int. 10:363–369. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akamatsu N, Sugawara Y and Kokudo N:
Budd-Chiari syndrome and liver transplantation. Intractable Rare
Dis Res. 4:24–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murad Darwish S, Plessier A,
Hernandez-Guerra M, Fabris F, Eapen CE, Bahr MJ, Trebicka J, Morard
I, Lasser L, Heller J, et al: Etiology, management, and outcome of
the Budd-Chiari syndrome. Ann Intern Med. 151:167–175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sang HF and Li XQ: Endovascular treatment
of Budd-Chiari syndrome with HV obstruction in China. J
Laparoendosc Adv Surg Tech A. 24:846–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seijo S, Plessier A, Hoekstra J, Dell'era
A, Mandair D, Rifai K, Trebicka J, Morard I, Lasser L, Abraldes JG,
et al: Good long-term outcome of Budd-Chiari syndrome with a
step-wise management. Hepatology. 57:1962–1968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fitsiori K, Tsitskari M, Kelekis A,
Filippiadis D, Triantafyllou K and Brountzos E: Transjugular
intrahepatic portosystemic shunt for the treatment of Budd-Chiari
syndrome patients: Results from a single center. Cardiovasc
Intervent Radiol. 37:691–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tripathi D, Macnicholas R, Kothari C,
Sunderraj L, Al-Hilou H, Rangarajan B, Chen F, Mangat K, Elias E
and Olliff S: Good clinical outcomes following transjugular
intrahepatic portosystemic stent-shunts in Budd-Chiari syndrome.
Aliment Pharmacol Ther. 39:864–872. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng D, Xu H, Lu ZJ, Hua R, Qiu H, Du H,
Xu X and Zhang J: Clinical features and etiology of Budd-Chiari
syndrome in Chinese patients: A single-center study. J
Gastroenterol Hepatol. 28:1061–1067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi XS, Ren WR, Fan DM and Han GH:
Selection of treatment modalities for Budd-Chiari syndrome in
China: A preliminary survey of published literature. World J
Gastroenterol. 20:10628–10636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui YF, Fu YF, Wei N, Zhu HC and Xu H:
Retrograde puncture assisted HV recanalization in treating
Budd-Chiari syndrome with segmental obstruction of hepatic venous.
Radiol Med. 120:1184–1189. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar Shalimar A, Kedia S, Sharma H,
Gamanagatti SR, Gulati GS, Nayak B, Thakur B and Acharya SK:
Hepatic venous outflow tract obstruction: Treatment outcomes and
development of a new prognostic score. Aliment Pharmacol Ther.
43:1154–1167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng X, Lv Y, Zhang B, He C, Guo W, Luo B,
Yin Z, Fan D and Han G: Endovascular management of Budd-Chiari
syndrome with IVC thrombosis: A 14-year single-center retrospective
report of 55 patients. J Vasc Interv Radiol. 27:1592–1603. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Q, Shen B, Zhang Q, Xu H, Zu M, Gu
Y, Wei N, Cui Y and Huang R: Comparison of long-term outcomes of
endovascular management for membranous and segmental IVC
obstruction in patients with primary Budd-Chiari syndrome. Circ
Cardiovasc Interv. 9:e0031042016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q, Xu H, Zu M, Gu Y, Wei N, Wang W,
Gao Z and Shen B: Catheter-directed thrombolytic therapy combined
with angioplasty for hepatic venous obstruction in Budd-Chiari
syndrome complicated by thrombosis. Exp Ther Med. 6:1015–1021.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siriwardana RC, Niriella MA, Dassanayake
AS, Liyanage C, Gunathilaka B, Jayathunge S and de Silva HJ:
Clinical characteristics and outcome of hepatocellular carcinoma in
alcohol related and cryptogenic cirrhosis: A prospective study.
Hepatobiliary Pancreat Dis Int. 14:401–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang W, Zhang XM, Yang L, Mitchell DG,
Zeng NL and Zhai ZH: Hepatic caudate vein in Budd-Chiari syndrome:
Depiction byusing magnetic resonance imaging. Eur J Radiol.
77:143–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mukund A and Gamanagatti S: Imaging and
interventions in Budd-Chiari syndrome. World J Radiol. 3:169–177.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao Y, Chen S and Yu C: Applicability of
different endovascular methods for treatment of refractory
Budd-Chiari syndrome. Cell Biochem Biophys. 61:453–460. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan X, Liu K, Che Y, Wang S, Wu X, Cao J
and Li J: Good clinical outcomes in Budd-Chiari syndrome with
hepatic venous occlusion. Dig Dis Sci. 61:3054–3060. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanaoka J, Shimada M, Uchiyama H, Ikegami
T, Imura S, Morine Y and Kanemura H: A simple formula to calculate
the liver drainage volume of the accessory right HV using its
diameter alone. Surgery. 146:264–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miraglia R, Maruzzelli L and Luca A:
Recanalization of occlusive transjugular intrahepatic portosystemic
shunts inaccessible to the standard transvenous approach. Diagn
Interv Radiol. 19:61–65. 2013.PubMed/NCBI
|
|
25
|
Gupta AC, Wang W, Shah C, Sands MJ, Bullen
J, Remer EM, Bayona PM, Carey W and Kapoor B: Added value of
covered stents in transjugular intrahepatic portosystemic shunt: A
large single-center experience. Cardiovasc Intervent Radiol.
40:1723–1731. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eapen CE, Velissaris D, Heydtmann M,
Gunson B, Olliff S and Elias E: Favourable medium term outcome
following hepatic vein recanalisation and/or transjugular
intrahepatic portosystemic shunt for Budd Chiari syndrome. Gut.
55:878–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valla D, Hadengue A, el Younsi M, Azar N,
Zeitoun G, Boudet MJ, Molas G, Belghiti J, Erlinger S, Hay JM and
Benhamou JP: Hepatic venous outflow block caused by short-length
hepatic vein stenoses. Hepatology. 25:814–819. 1997. View Article : Google Scholar : PubMed/NCBI
|