Introduction
Cerebral ischemic stroke is a generic term for
cerebral tissue necrosis caused by cerebral supplying artery
stenosis or occlusion or insufficient cerebral blood supply. As the
pace of life speeds up, and as the way of life has changed
dramatically, the incidence rate of cerebral ischemic stroke is
increasing year by year, seriously affecting the quality and
threatening life (1,2). Therefore, it is urgent to find
effective methods and drugs for the treatment of cerebral ischemic
stroke. With the development of science of Chinese traditional
medicine, people increasingly focus on the study of natural
products. Because traditional Chinese medicine has the advantages
of high efficiency, low toxicity and multiple targets, work is
being carried out to finding an effective anti-cerebral ischemic
stroke drug using traditional Chinese medicine (3). Magnolol is a natural product with very
high activity extracted from Chinese traditional medicine. It has
good lipid solubility, so it can penetrate the blood-brain barrier,
enter into and work in the brain. Previous studies have confirmed
that magnolol has effects of anti-inflammation, anti-virus,
antitumor, and inhibition of apoptosis, but magnolol's
anti-ischemic stroke effect is still undetermined (4–6). In this
study, by constructing models of rat with cerebral ischemic stroke
and after administration of magnolol, the effect of magnolol on
rats with cerebral ischemic stroke was observed and the molecular
mechanism was studied, to deeply investigate the action mechanism
of magnolol.
Materials and methods
Experimental animals and grouping
A total of 60 male Sprague-Dawley (SD) rats
(4-week-old, weighing 200±20 g, purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd., Beijing, China) were
selected and randomly divided into sham operation, model and
magnolol administration groups, with 20 rats in each group. The
rats were kept in cage with controlled temperature and light cycles
(24°C and 12/12 light cycles) and free access to water and food.
The humidity was 60±10%. The rats in magnolol administration group
were intraperitoneally injected with magnolol (75 mg/kg) for 7
consecutive days, once per day. After 7 days of administration, the
rats were fasted for 12 h but were given drinking water. Middle
cerebral artery occlusion (MCAO) reperfusion models of rat were
established in model and magnolol groups by longa's animal model.
The study was approved by the Ethics Committee of Xingtai People's
Hospital (Xingtai, China).
Main reagents
A terminal-deoxynucleotidyl transferase mediated
nick end-labeling (TUNEL) kit (Roche, Basel, Switzerland); a
bicinchoninic acid (BCA) protein quantification kit (Beyotime
Biotechnology, Shanghai, China); a TRIzol total ribonucleic acid
(RNA) extraction kit and a reverse transcription-polymerase chain
reaction (RT-PCR) reverse transcription kit (both from Tiangen
Biotech, Beijing, China); and glyceraldehyde phosphate
dehydrogenase (GAPDH), anti-brain-derived neurotrophic factor
(BDNF) and Bcl-2-associated X protein (Bax) monoclonal antibodies
(ProteinTech Group, Inc.; Wuhan Sanying Biotechnology; Wuhan,
China).
Experiment methods
Determination of relative cerebral
index
The rats in each group were weighed and the body
weight was recorded. Then, the rats were sacrificed and the brain
was quickly removed to record the brain weight. The cerebral index
was calculated by: Cerebral index = brain weight/body weight.
Hematoxylin and eosin (H&E)
staining
Paraffin-embedded rat brain sections were routinely
dewaxed until the paraffin was replaced by water and then rinsed
with phosphate-buffered saline (PBS) (3 min/time ×3 times). Then,
sections were stained with hematoxylin for 5 min, de-stained with
1% hydrochloric acid (HCl), and rinsed with double distilled water
(5 min/times ×6 times). After that, sections were made blue with
lithium carbonate saturated solution for 1 to 2 min and washed with
double distilled water (5 min/times ×3 times). Color separation was
then done with 80% alcohol and rinsing was conducted with double
distilled water (5 min/times ×3 times). Later, sections were
stained with eosin for 5 min and rinsed with double distilled water
(5 min/times ×3 times). Last, sections were dewatered, hyalinized,
blocked with resin, and photographed using an upright microscope
(Olympus Corporation, Tokyo, Japan).
Detection of apoptosis
The detection was performed in accordance with the
instructions of the TUNEL kit. Paraffin-embedded sections were
routinely dewaxed until the paraffin was replaced by water, washed
with 10 mM PBS for 3 min/time ×3 times, incubated in 1%
H2O2 at room temperature for 20 min to
inhibit endogenous peroxidase, rinsed with PBS for 3 min/time ×3
times, digested with 20 µg/ml protease K for 20 min at 37°C, washed
with PBS for 3 min/time ×3 times, incubated with terminal
deoxynucleotidyl transferase (TdT) buffer for 10 min, immersed in
TUNEL mixture [100 µl mixture containing 1 µl TdT and 1 µl solution
of
biotin-epsilon-aminocaproyl-[5-{3-aminoallyl}-2′-deoxyuridine-5′-triphosphate]
(Biotin-11-dUTP)] at 37°C for 90 min, washed with PBS for 3
min/time ×3 times, and placed in (Tris-HCI buffer) TB for 15 min at
room temperature to terminate the reaction. Then, sections were
dropwise added with anti-Avidin-horseradish peroxidase (HRP)
solution and incubated at 37°C for 1 h, rinsed with PBS for 3
min/time ×3 times, subjected to color development with
diaminobenzidine (DAB) for 20 to 30 min, and rinsed to terminate
the reaction. Conventional dehydrating, hyalinizing and blocking
with resin were carried out. Three fields were observed randomly
under high power lens (×200 magnification), and the mean value was
calculated as the number of apoptotic cells in the animal.
RT-PCR analysis
Moderate amount of brain tissues in sham operation,
model and magnolol groups were transferred into 1 ml TRIzol reagent
and fully ground into homogenate. The homogenate was left to stand
for 5 min at room temperature and the sample was completely lysed
using Tissuelyser (Qiagen GmbH, Hilden, Germany). The homogenate
was centrifuged at 12,000 × g for 5 min at 4°C, and the supernatant
was carefully collected, added with chloroform, mixed well, and
placed at room temperature for 5 min. The supernatant was carefully
collected after centrifugation at 12,000 × g for 15 min at 4°C,
added with the same volume of isopropanol, left to stannd at room
temperature for 10 min, and centrifuged at 12,000 × g for 10 min at
4°C, and the precipitate was then collected. Ethanol (75%) was
added, with homogeneous mixing, to wash the RNA precipitation.
Later, RNase-free water was added to completely dissolve it. The
ratio of optical density (OD) 260/OD280 and the RNA concentration
were then measured. Finally, the reaction product was subjected to
RT-PCR analysis via stepwise amplification performed based on the
primer sequence templates shown in Table
I.
 | Table I.RT-PCR primer sequences for BDNF, Bax
and GAPDH mRNAs. |
Table I.
RT-PCR primer sequences for BDNF, Bax
and GAPDH mRNAs.
| Gene names | Primer sequences |
|---|
| BDNF | F: 5′-3′
GCCCATATGACCATCCTTTTCCTTA |
|
| R: 3′-5′
CTATCTTCCCCTTTTAATGGTCAGT |
| Bax | F: 5′-3′
CAGGATGCGTCCACCAAGAA |
|
| R: 3′-5′
CGTGTCCACGTCAGCAATCA |
| GAPDH | F: 5′-3′
GAGCCGGGAAATCGTGCGT |
|
| R: 3′-5′
GGAAGGAAGGCTGGAAGATG |
Western blot analysis
Protein extraction
Brain tissues of rats in each group were collected
and washed twice with frozen saline, respectively. Protein
extraction was run according to the instructions of the total
protein extraction kit. The tissue was added with lysate,
homogenized for 1 min by a homogenizer and centrifuged at 12,000 ×
g for 10 min at 4°C. Then, the supernatant, i.e., total protein in
liver tissue, was collected. The protein concentration was
determined by the BCA protein concentration kit and the supernatant
was sub-packaged and stored at −80°C until use.
Protein denaturation
The total protein extract was mixed evenly with 2X
loading buffer based on the volume ratio of 1:1, heated in boiling
water for 5 min, cooled naturally, and stored at 4°C until use.
Sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE)
SDS-PAGE separation gel in an appropriate proportion
was prepared according to the molecular weight of target protein,
with a coagulation of ~1 h. Then, 5% SDS-PAGE spacer gel was
prepared and coagulated for ~0.5 h. Electrophoresis buffer was
added, and the denatured protein samples were loaded onto sample
loading wells according to the protein concentration, so that the
total protein content per well was the same. Electrophoresis was
performed at a constant voltage of 220 V and stopped after
bromophenol blue reached the bottom of the gel.
Membrane transfer
The gel was cut based on the molecular weight of
target protein, and put into transfer buffer. And 1 layer of
polyvinylidene fluoride (PVDF) membrane and 6 layers of filter
paper were cut according to the size of the gel. The PVDF membrane
was firstly immersed in methanol for 10 sec, and then put into the
transfer buffer together with the filter paper. They were put into
a transfer unit in the following sequence: Positive electrode,
three layers of filter paper, PVDF membrane, gel, three layers of
filter paper and negative electrode, with attention paid to edge
alignment so as to prevent blistering. The membrane transfer was
for 2 h at a constant voltage of 110 V.
Blocking
The PVDF membrane containing protein was placed and
blocked in 5% skimmed milk on a shaker at room temperature for 2
h.
Immune response
The blocked membrane was washed with
Tween/Tris-buffered saline (TTBS) for 5 min, placed into the mouse
anti-rat BDNF, Bax and GAPDH primary monoclonal antibodies
(1:1,000; cat. nos. 66292-1-Ig, 60267-1-Ig and 60004-1-Ig,
respectively; ProteinTech Group, Inc.; Wuhan Sanying Biotechnology)
in corresponding proportion, and incubated overnight at 4°C. The
membrane was washed with TTBS 3 times (10 min/time), put into the
corresponding goat anti-mouse secondary polyclonal antibody
(1:1,000; cat. no. SA00001-1; ProteinTech Group, Inc.; Wuhan
Sanying Biotechnology), incubated on a shaker at room temperature
for 3 h, and rinsed with TTBS 3 times (10 min/time).
Enhanced chemiluminescence (ECL)
A gel imager was preheated for 30 min. Reagents A
and B in the ECL kit were mixed evenly at same volume, added
dropwise onto the PVDF membrane, with full contact, kept in the
dark and subjected to color development for 1 min. The filter paper
was used to blot up excess liquid around the membrane, and then the
membrane was placed into the gel imager. Later, dynamic integration
mode was applied to take pictures, and the results were observed.
Image analysis software was employed for image analysis.
Statistical analysis
Experimental data are expressed as mean ± standard
error of the mean (mean ± SEM). For experimental results,
Statistical Product and Service Solutions (SPSS) 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA) was utilized for
statistical analysis. The t-test was used for mean comparison
between the two groups. One-way analysis of variance (ANOVA) was
applied for comparison of sample average among multiple groups and
the post hoc test was SNK test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of magnolol on cerebral
index
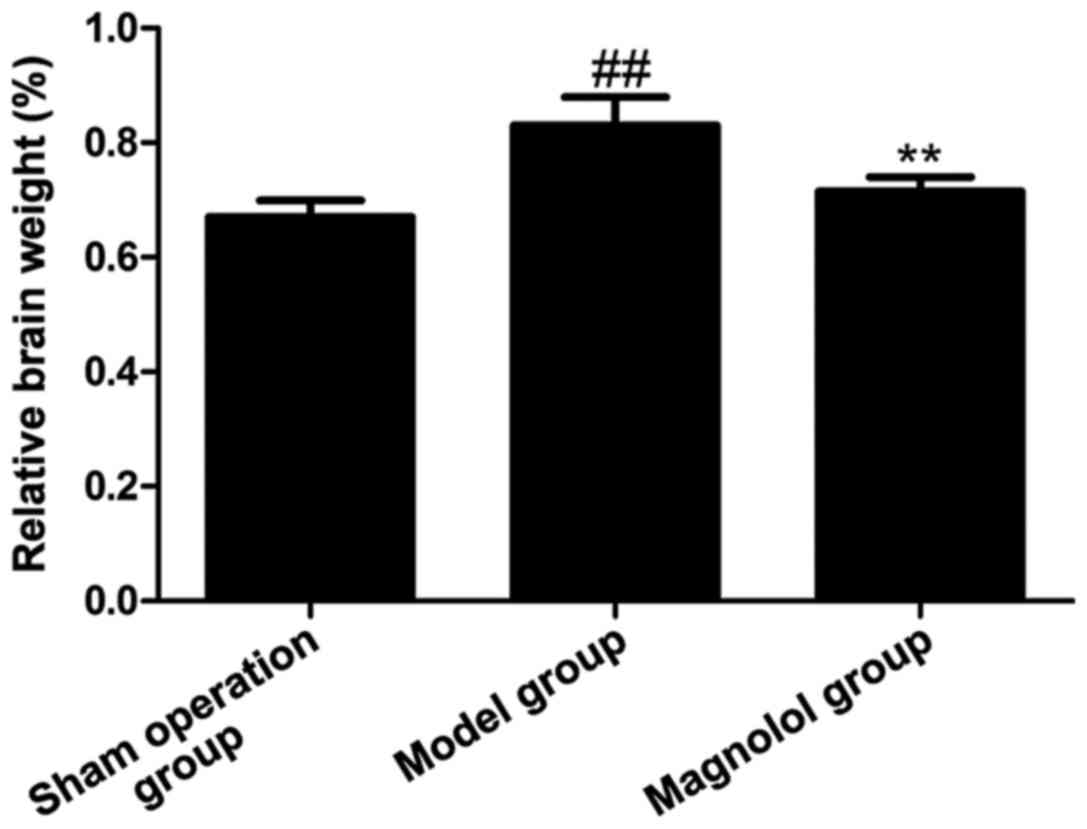
As shown in Fig. 1,
the cerebral index in model group was increased significantly
compared with that in sham operation group, while the cerebral
index was obviously lower in magnolol administration group than
that in model group. This result showed that magnolol can
effectively affect cerebral index and improve brain injuries.
Results of H&E staining
H&E stained brain tissue sections in sham
operation, model and magnolol groups were used to find differences
in pathological features among samples. According to Fig. 2, compared with the brain tissue
section of rats in sham group, a large number of inflammatory cells
were found in the brain tissue section of rats in model group, with
pyknotic, broken or even disappeared cell nuclei, and severe brain
injuries; after rats were given magnolol, the inflammatory cells
were overtly reduced, and the nuclei were more complete, suggesting
that magnolol can improve brain injuries.
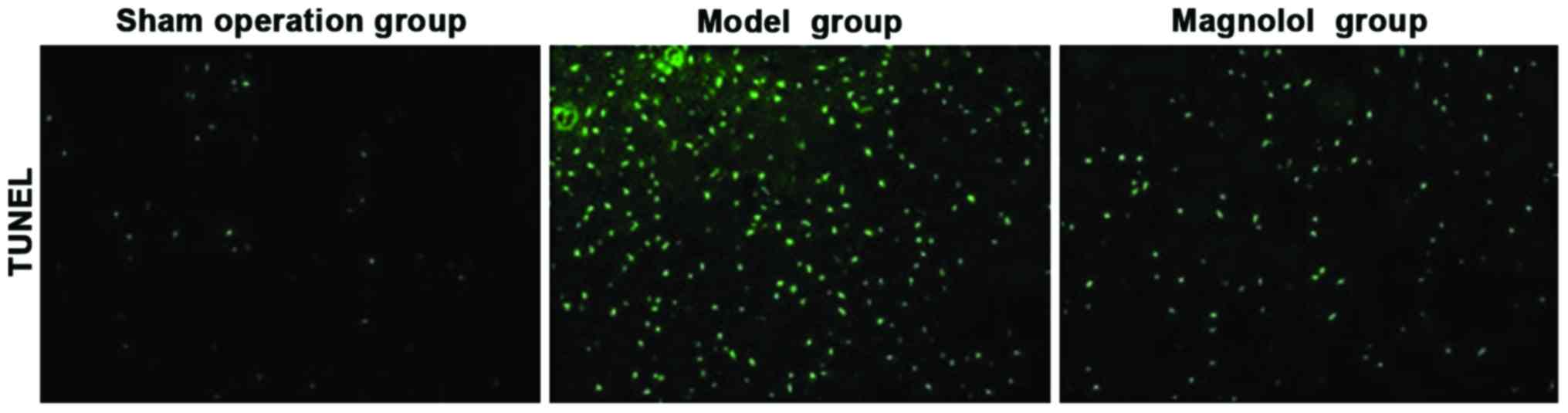
Results of TUNEL staining
The apoptosis of brain tissue of rats in sham
operation, model and magnolol groups was observed through TUNEL
staining. As shown in Fig. 3,
compared with the brain tissue of rats in sham operation group, the
brain tissue of rats in model group had abundant apoptotic cells,
but magnolol group had distinctly reduced apoptotic cells after the
administration of magnolol.
Results of RT-PCR
Total RNAs were extracted from brain tissue samples
of rats in sham operation, model and magnolol groups. Through
RT-PCR, it was found that BDNF was significantly decreased and Bax
was clearly increased in the model group, which were effectively
reversed after rats were given magnolol, indicating that magnolol
has a neuroprotective effect and can inhibit the production of
apoptosis (Fig. 4).
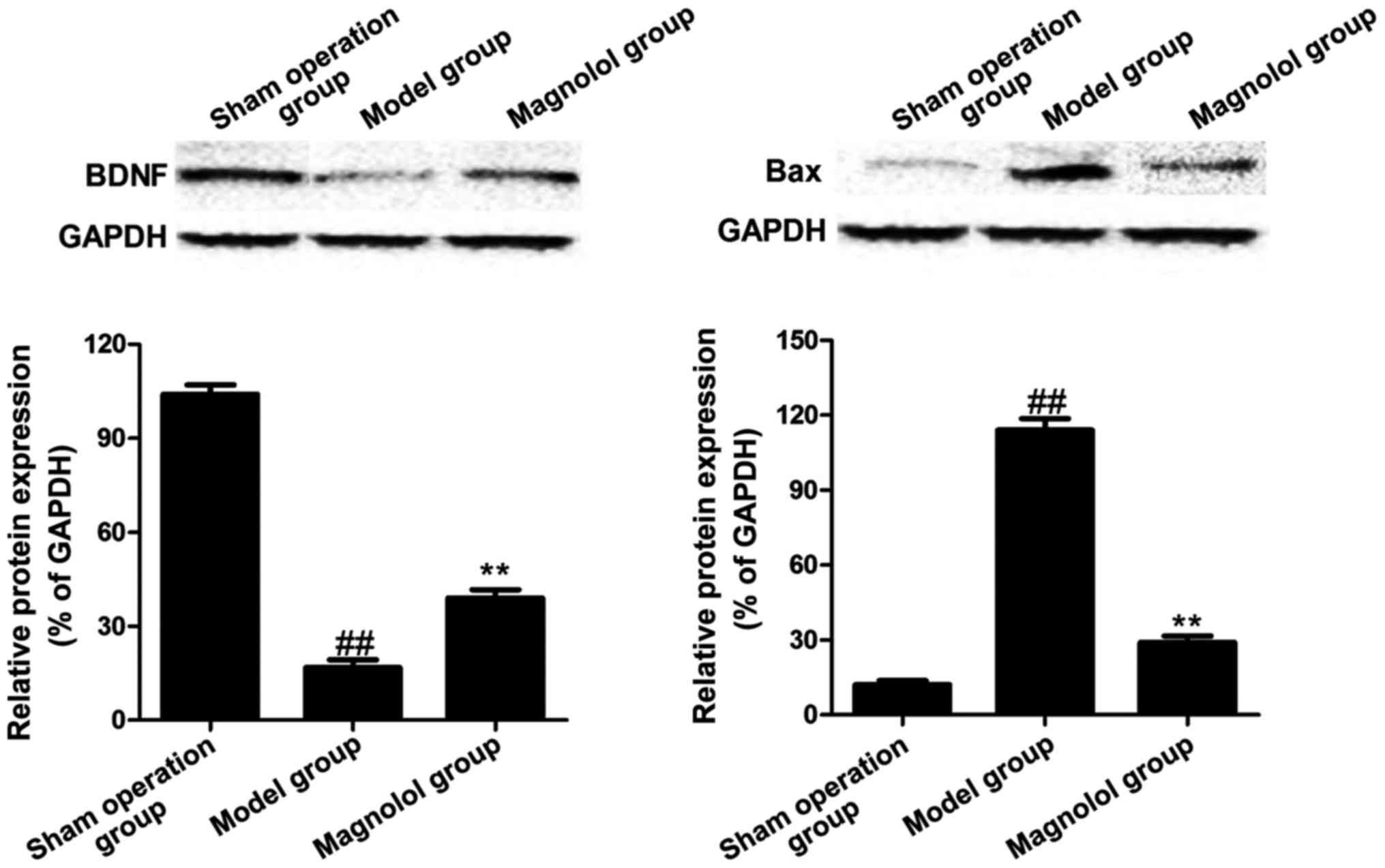
Protein expression levels of BDNF and Bax of rats in
sham operation, model and magnolol groups. Western blotting results
showed protein expression levels of BDNF and Bax of rats in sham
operation, model and magnolol groups. As shown in Fig. 5, sham operation group had a high
protein expression of BDNF but a low protein expression of Bax;
while model group showed a low protein expression of BDNF but a
high protein expression of Bax; in magnolol group, the protein
expression of BDNF was significantly higher than that in model
group, and the protein expression of Bax was clearly decreased
compared with that in the model group.
Discussion
Brain is a major part of the higher neural system of
the vertebrate and a higher nervous center to control movements,
produce sensations and achieve high brain functions (7). Cerebral ischemic stroke seriously
affects human health. Cerebral ischemic stroke refers to the sudden
onset of blood flow perfusion reduction or complete obstruction of
blood flow in supplying arteries in local brain tissues, leading to
stopping of blood supply, oxygen supply, sugar supply, thereby
resulting in disintegration and damage of local brain tissues
(8–10). At present, methods used for the
treatment of cerebral ischemic stroke are based on the improvement
of blood circulation of the brain, increase in blood and oxygen
supply in the penumbra area of ischemic area, control of brain
edema, and prevention and treatment of complications. Although
therapeutic methods for cerebral ischemic stroke are numerous, with
up to dozens of drugs, the ideal therapeutic regimen is still
controversial and uncertain (11).
Therefore, the development of new drugs with special effects is
necessary. However, new drugs in research and development mainly
are chemical synthetic drugs, with high development costs, great
risks, long cycles, and low success rates, so the annual number of
new drugs in research and development is extremely limited.
Active constituents extracted from traditional
Chinese medicine, especially Chinese herbal medicine, are not only
important sources for new drug development (12–14), but
also important means in preventing and treating modern diseases.
Moreover, active constituents also have a great significance for
the modernization and internationalization of Chinese traditional
medicine (15). In recent years,
natural products with significant anti-cerebral ischemic stroke
effect have been continuously found (16). A large number of studies have shown
that BDNF and Bax (an apoptosis-related factor) play important
roles in cerebral ischemic stroke. This study took BDNF and Bax as
targets to find effective Chinese traditional medicine. Magnolol is
a natural compound; modern studies have indicated that magnolol has
various pharmacological effects, such as antitumor,
anti-hyperlipidemia, and anti-fungal effects; many previous studies
have also revealed that magnolol has effects of anti-inflammation,
anti-apoptosis and immunoregulation; however, the effect, mechanism
of action, and target of action of magnolol on cerebral ischemic
stroke are still unknown (17–20).
Therefore, these unknown fields were studied in depth by the
authors.
Male SD rats were studied and they were randomly
divided into sham operation, model and magnolol administration
groups. The cerebral indexes of each group were detected,
respectively. The histopathological differences among each group
were detected by H&E staining. Apoptosis of each group was
measured via TUNEL staining. The expression of mRNA and protein of
BDNF and Bax in sham operation, model and magnolol groups was
detected through RT-PCR and western blot analysis. The results
showed that the cerebral index was dramatically decreased after the
administration of magnolol. The results of H&E staining
revealed that a large number of inflammatory cells were found in
the brain tissue of rats in model group, and the structure of cell
nucleus was destroyed; magnolol was effective in improving brain
injuries. The results of TUNEL staining indicated that plenty of
apoptotic cells were produced in model group, and it was obviously
improved after rats were given magnolol. RT-PCR and western
blotting, respectively confirmed that in model group, the
expression of mRNA and protein of BDNF was clearly decreased but
distinctly raised after the administration of magnolol; the
expression of mRNA and protein of Bax was overtly increased, but
clearly declined after the administration of magnolol. This study
shows that magnolol can improve cerebral ischemic stroke in rats;
this is because magnolol can increase the expression of BDNF and
decrease the expression of Bax. Magnolol, an anti-apoptotic
neuroprotective drug, provides a new regimen for the prevention and
treatment of cerebral ischemic stroke.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and LQ performed PCR and western blot analysis.
JX assisted in the detection of apoptosis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Xingtai People's Hospital (Xingtai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagai N, Kawao N, Okada K, Ishida C,
Okumoto K, Ueshima S, Suzuki Y, Umemura K and Matsuo O: Initial
brain lesion size affects the extent of subsequent
pathophysiological responses. Brain Res. 1322:109–117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arai K, Jin G, Navaratna D and Lo EH:
Brain angiogenesis in developmental and pathological processes:
Neurovascular injury and angiogenic recovery after stroke. FEBS J.
276:4644–4652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DiStefano PS, Friedman B, Radziejewski C,
Alexander C, Boland P, Schick CM, Lindsay RM and Wiegand SJ: The
neurotrophins BDNF, NT-3, and NGF display distinct patterns of
retrograde axonal transport in peripheral and central neurons.
Neuron. 8:983–993. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amin AR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH
and Lee WS: Magnolol suppresses proliferation of cultured human
colon and liver cancer cells by inhibiting DNA synthesis and
activating apoptosis. J Cell Biochem. 84:532–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang ES and Park KK: Magnolol suppresses
metastasis via inhibition of invasion, migration, and matrix
metalloproteinase-2/-9 activities in PC-3 human prostate carcinoma
cells. Biosci Biotechnol Biochem. 74:961–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Zhang C, Jiang H, Li Y, Zhang L,
Robin A, Katakowski M, Lu M and Chopp M: Atorvastatin induction of
VEGF and BDNF promotes brain plasticity after stroke in mice. J
Cereb Blood Flow Metab. 25:281–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Madinier A, Bertrand N, Mossiat C,
Prigent-Tessier A, Beley A, Marie C and Garnier P: Microglial
involvement in neuroplastic changes following focal brain ischemia
in rats. PLoS One. 4:e81012009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mattson MP: Glutamate and neurotrophic
factors in neuronal plasticity and disease. Ann N Y Acad Sci.
1144:97–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawamoto Y, Nakamura S, Nakano S, Oka N,
Akiguchi I and Kimura J: Immunohistochemical localization of
brain-derived neurotrophic factor in adult rat brain. Neuroscience.
74:1209–1226. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jean YY, Lercher LD and Dreyfus CF:
Glutamate elicits release of BDNF from basal forebrain astrocytes
in a process dependent on metabotropic receptors and the PLC
pathway. Neuron Glia Biol. 4:35–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kong CW, Tsai K, Chin JH, Chan WL and Hong
CY: Magnolol attenuates peroxidative damage and improves survival
of rats with sepsis. Shock. 13:24–28. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YM, Hsiao G, Chen HR, Chen YC, Sheu JR
and Yen MH: Magnolol reduces myocardial ischemia/reperfusion injury
via neutrophil inhibition in rats. Eur J Pharmacol. 422:159–167.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teng CM, Ko FN, Wang JP, Lin CN, Wu TS,
Chen CC and Huang TF: Antihaemostatic and antithrombotic effect of
some antiplatelet agents isolated from Chinese herbs. J Pharm
Pharmacol. 43:667–669. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ho KY, Tsai CC, Chen CP, Huang JS and Lin
CC: Antimicrobial activity of honokiol and magnolol isolated from
Magnolia officinalis. Phytother Res. 15:139–141. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamasaki Y, Kobayashi I, Zaitu M, Tsuji K,
Kita M, Hayasaki R, Muro E, Yamamoto S, Matsuo M, Ichimaru T, et
al: Magnolol inhibits leukotriene synthesis in rat basophilic
leukemia-2H3 cells. Planta Med. 65:222–226. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bang KH, Kim YK, Min BS, Na MK, Rhee YH,
Lee JP and Bae KH: Antifungal activity of magnolol and honokiol.
Arch Pharm Res. 23:46–49. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang JP, Hsu MF, Raung SL, Chen CC, Kuo JS
and Teng CM: Anti-inflammatory and analgesic effects of magnolol.
Naunyn Schmiedebergs Arch Pharmacol. 346:707–712. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuo DH, Lai YS, Lo CY, Cheng AC, Wu H and
Pan MH: Inhibitory effect of magnolol on TPA-induced skin
inflammation and tumor promotion in mice. J Agric Food Chem.
58:5777–5783. 2010. View Article : Google Scholar : PubMed/NCBI
|