Introduction
Renal ischemia reperfusion (IR) injury is a common
cause of acute kidney injury (AKI) (1) and characterized by high morbidity and
mortality (2). In clinical settings,
patients subjected to kidney transplantation and renal tumor
resection inevitably suffer from renal IR injury (3). Renal tubular cell apoptosis and
inflammatory response are the most important pathophysiological
process of ischemic AKI (4).
Following IR, the tubular cells in the outer medulla suffer the
most severe injury, leading to renal dysfunctions (5). In addition, inflammatory response
promotes renal dysfunctions and progressive chronic kidney disease
(6). Therefore, inhibiting tubular
cell apoptosis and inflammatory response may be an effective
treatment of renal IR injury.
Chrysin is a naturally occurring flavonoid with
anti-inflammatory, anti-oxidant and anti-cancer properties
(7). It ameliorates
indomethacin-induced inflammatory response and oxidative injury
(8), and suppresses tumor growth of
murine melanoma (9). Additionally,
it attenuates focal cerebral IR injury in mice (10). However, its effect on renal IR injury
remains unknown.
In this study, a renal IR injury model was
established in mice and the effects of chrysin on renal IR injury
were investigated. Results demonstrated that chrysin remarkably
attenuated IR-induced renal dysfunctions and morphological
abnormalities. Furthermore, chrysin inhibited renal IR-induced
tubular cell apoptosis and inflammatory response. Therefore,
chrysin may protect against renal IR-induced ischemic AKI.
Materials and methods
Animals and treatment
All experiments were approved by the Institutional
Animal Care and Use Committee at Hubei University of Arts and
Science (Xiangyang, China). The surgical procedures were performed
in accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (NIH Publications no. 8023,
revised 1978). A total of 30 male C57BL/6 mice (8–10 weeks old)
were purchased from the Center of Experimental Animals of Wuhan
University (Wuhan, China) and housed in a humidity (50–60%) and
temperature-controlled environment with a 12-h light/dark cycle and
free access to food and water. Mice were randomly divided into
three groups (each, n=10): Sham, IR and IR+chrysin. To induce renal
IR injury in vivo, the mice were abdominally anesthetized
with phenobarbital sodium (60 mg/kg) and their body temperature was
maintained at 37°C. Flank incisions were also conducted to expose
the pedicels. The IR and IR+chrysin group mice were subjected to
bilateral renal pedicel clamping for 30 min and reperfusion for 48
h. The sham group mice only underwent exposed pedicles without
pedicle clamping and received injections of an equal volume of
saline. Blood and kidney samples were collected for analysis.
Chrysin was purchased from Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany (95082) and IR+chrysin group mice were injected with
chrysin for 3 days (100 mg/kg each time) prior to IR operation.
Renal function assay
The blood (200 µl) was collected and centrifugal
(3,500 × g) at 4°C for 15 min. Thereafter, the supernatant was
harvested and stored at −80°C. The serum concentrations of
creatinine (Cr) and blood urea nitrogen (BUN) were tested using
creatinine and urea assay kits (Nanjing Jiancheng Bioengineering
Research Institute, Nanjing, China) in accordance with the
manufacturer's protocol.
Hematoxylin and eosin (H&E)
assay
To evaluate kidney injury score, renal samples were
fixed in 4% formaldehyde at room temperature for 24 h, embedded in
paraffin and cut into 4 µm sections, stained with hematoxylin (8
min), and eosin (2 min) at room temperature. Histological features
were imaged using a light microscope (Olympus Corporation, Tokyo,
Japan) and a total of 10 random fields of view were obtained from
the cortico-medullary region. For assessment of renal injury score,
tubular apoptosis, cellular casts and tubular injury were included.
The scoring was as follows: 0 (<10%), 1 (10–25%), 2 (25–50%), 3
(50–75%) and 4 (>75%). The percentage of renal injury was
quantified and conducted in a blinded manner.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
TUNEL is the method of using the TdT enzyme to
covalently attach a tagged form of dUTP to 3′ends of double- and
single-stranded DNA breaks in cells. It is a reliable and useful
method to detect DNA damage and cell death in situ. In the
present study, renal samples were collected and fixed in 4%
paraformaldehyde at room temperature for 24 h, paraffin embedded,
and sectioned at 4 µm. Then the slides were deparaffinized,
rehydrated and the antigen was exposed. The death of tubular cells
was detected with Cell Death Detection kit (Fluorescein; Roche
Diagnostics, Indianapolis, IN, USA) in accordance with the
manufacturer's protocol. Incubated with the tunnel reagent at 37°C
for 2 h and mounted with antifade. Image Pro-Plus software version
6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used for
positive cell counting.
Immunohistochemistry (IHC) assay
Renal samples were collected and fixed in 4%
paraformaldehyde at room temperature for 24 h, paraffin embedded,
and sectioned at 4 µm. Then, the slides were deparaffinized,
rehydrated in a descending alcohol series and the antigen was
exposed by 20 min of incubation at 100°C in sodium citrate.
Thereafter, the slides were incubated with 3%
H2O2 at 37°C for 10 min to block the
endogenous peroxidase activity. Sequentially, the slides were
incubated with bovine serum albumin (cat. no. abs957; Absin
Bioscience Inc., Shanghai, China) and the primary antibodies
(cleaved Caspase 3, cat. no. 9664, 1:200, Cell Signaling
Technology, Inc., Danvers, MA, USA; MPO, cat. no. abs120616, 1:200,
Absin Bioscience Inc.) overnight at 4°C. After incubation with
biotin-labeled secondary antibody (abs957, Absin Bioscience Inc.)
at room temperature for 10 min, the color was developed with
3,3′-diaminobenzidine at room temperature for 30 sec. Then, the
tissues were imaged by fluorescence microscopy (Olympus
Corporation). The staining was evaluated in a blinded manner.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was isolated using TRIzol reagent
(#15596-026; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and then reverse-transcribed into cDNA by using a
Transcriptor First-Strand cDNA Synthesis kit (#04896866001; Roche)
in accordance with the manufacturer's protocol. SYBR Green
(#04887352001; Roche) was used to quantify the PCR-amplification
products. qPCR was performed under the following conditions:
Initial denaturation at 95°C for 30 sec, followed by 40 cycles of 5
sec at 95°C, 30 sec at 60°C, and 60 sec at 72°C. β-actin was used
as the reference gene. The threshold cycle values of the samples
were measured using the 2−∆∆Cq data analysis method
(11). The results were presented as
mean ± standard deviation and SPSS software version 19.0 (IBM
Corp., Armonk, NY, USA) was used in the analysis. The primer pairs
used in this study are listed in Table
I.
 | Table I.Primers for qPCR detection. |
Table I.
Primers for qPCR detection.
| Gene | Sequence 5′-3′ |
|---|
| β-actin | F:
GTGACGTTGACATCCGTAAAGA |
|
| R:
GCCGGACTCATCGTACTCC |
| IL-1β | F:
CCGTGGACCTTCCAGGATGA |
|
| R:
GGGAACGTCACACACCAGCA |
| IL-6 | F:
AGTTGCCTTCTTGGGACTGA |
|
| R:
TCCACGATTTCCCAGAGAAC |
| TNF-α | F:
CATCTTCTCAAAATTCGAGTGACAA |
|
| R:
TGGGAGTAGACAAGGTACAACCC |
ELISA
The blood was collected and centrifuged (3,500 × g)
at 4°C for 15 min. Thereafter, the supernatant was harvested and
stored at −20°C. The protein levels of TNF-α (cat. no. EK0527),
IL-1β (cat. no. EK0394) and IL-6 (cat. no. EK0411) were detected by
ELISA kits (Boster Biological Technology, Wuhan, China) at 450 nm
in accordance with the manufacturer's protocol.
Western blot analysis
Total protein (kidney) was isolated using
radioimmunoprcipitation lysis buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology, Haimen, China) and the concentration
was detected with bicinchoninic acid reagent (cat. no. #23225,
Thermo Fisher Scientific, inc.). Equal amounts of proteins (30 µg)
were loaded, proteins were separated with 10% SDS-PAGE gels and
then transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Thereafter, such proteins were
blocked in 5% skim milk for 1 h and incubated overnight at 4°C with
primary antibodies (Bax, Bcl-2, cleaved caspase-3, p-IκBα, IκBα,
p-P65, P65 and GAPDH). Following incubation in secondary antibodies
(cat. no. 926-32211; IRDye 800CW goat anti-rabbit IgG (H+L); LI-COR
Biosciences, Lincoln, NE, USA) for 1 h at room temperature, washed
three times and the membranes were scanned with the Odyssey
infrared imaging system (LI-COR Biosciences).
Antibodies
The antibodies against Bax (cat. no. ab32503), Bcl-2
(cat. no. ab32124) and GAPDH (cat. no. ab181602) were obtained from
Abcam and used in western blotting. The antibodies against cleaved
caspase-3 (cat. no. 9661; used in IHC and western blotting) and
phosphorylated (p)-IκBα (cat. no. #2859; used in western blotting)
were purchased from Cell Signaling Technology, Inc. The antibodies
against IκBα (cat. no. sc-371), P65 (cat. no. sc-372) and p-P65
(cat. no. sc-33020) were obtained from Santa Cruz Biotechnology,
Inc., (Dallas, TX, USA) and were used in western blotting. The
antibody against myeloperoxidase (MPO; cat. no. abs120616; used in
IHC) was purchased from Absin Bioscience Inc. The second antibody
(cat. no. 926-32211; used in western blotting) was purchased from
LI-COR Biosciences.
Statistical analysis
All experiments were repeated at least three times
independently. Continuous data were expressed as mean ± standard
deviation. Difference between groups were statistically analyzed
using one-way analysis of variance followed by
Student-Newman-Keuls' method with SPSS software version 19.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Chrysin pretreatment attenuates
IR-induced renal dysfunction
To investigate the effect of chrysin on renal
functions in renal IR injury, the chrysin pretreatment mice were
injected with chrysin (Fig. 1A) for
3 days (100 mg/kg each time) prior to the renal IR operation. The
serum levels of BUN and Cr were significantly increased in the IR
group compared with the sham group (P<0.05). However, chrysin
pretreatment significantly decreased the levels of BUN and Cr
(P<0.05; Fig. 1B and C). These
results indicate that Chrysin pretreatment attenuates IR-induced
renal dysfunction.
Chrysin pretreatment attenuates renal
IR-induced morphological abnormalities
Following renal IR, more severe tubular damage was
observed in the IR group compared with the sham group. However, the
abnormalities and tubular injury were significantly attenuated in
the chrysin pretreatment group compared with in the IR group
(P<0.05; Fig. 2A and B). These
results indicate that Chrysin pretreatment attenuates renal
IR-induced morphological abnormalities.
 | Figure 2.Chrysin pretreatment attenuates renal
IR-induced morphological abnormalities. (A) The H&E staining of
sham, IR and IR+chrysin group. Scale bar, 20 µm. (B) Tubular injury
score of the sham, IR and IR+chrysin group. For assessment of renal
injury, tubular apoptosis, cellular casts and tubular injury were
included. Score 0–4 represent the injury area <10%, 10–25%,
25–50%, 50–75% and >75%. *P<0.05 vs. the sham group;
#P<0.05 vs. the IR group. H&E, hematoxylin and
eosin; IR, ischemia reperfusion. |
Chrysin pretreatment attenuates renal
IR-induced apoptosis of tubular cells
The results presented above demonstrate that chrysin
administration attenuates renal tubular damage. To investigate the
effect of chrysin on tubular cell apoptosis, TUNEL staining was
performed. The number of TUNEL positive cells was significantly
increased in the IR group compared with in the sham group
(P<0.05). However, chrysin pretreatment significantly decreased
the number of apoptosis cells compared with the IR group
(P<0.05; Fig. 3A and B).
Considering that cleaved caspase-3 protein is a marker of cell
apoptosis and the protein level of cleaved caspase-3 was
investigated by immunohistochemistry (IHC). Results demonstrated
that the protein level of cleaved caspase-3 was significantly
increased following renal IR injury (P<0.05). However, the
expression of cleaved caspase-3 was significantly decreased in the
IR+chrysin group compared with the IR group (P<0.05; Fig. 3C and D). These results indicate that
Chrysin pretreatment attenuates renal IR-induced apoptosis of
tubular cells.
Chrysin pretreatment decreases the
expression of Bax and cleaved caspase-3 and increases the
expression of Bcl-2 in renal IR injury
To detect the effect of chrysin on pro-apoptosis and
anti-apoptosis associated proteins, a western blot assay was
conducted (Fig. 4A). The protein
levels of Bax and cleaved caspase-3 were increased and the
expression of Bcl-2 was decreased in the IR group compared with the
sham group. However, the protein levels of Bax and cleaved
caspase-3 were significantly decreased, and the protein level of
Bcl-2 was significantly increased in the chrysin pretreatment group
compared in the IR group (P<0.05; Fig. 4B-D). These results indicate that
Chrysin pretreatment decreases the expression of Bax and cleaved
caspase-3 and increases the expression of Bcl-2 in renal IR
injury.
Chrysin pretreatment attenuates renal
IR-induced inflammatory response
To investigate the effect of chrysin on renal IR
induced inflammatory response, the level of myeloperoxidase (MPO)
by IHC was detected. The expression of MPO was significantly
increased in the IR group compared with the sham group (P<0.05).
However, the expression of MPO was significantly decreased in the
chrysin pretreatment group compared with the IR group (P<0.05;
Fig. 5A and B). Consistently, the
mRNA levels of TNF-α, IL-1β and IL-6 were significantly decreased
in the chrysin administration group compared with the IR group
(P<0.05; Fig. 5C-E). Furthermore,
the protein levels of proinflammatory cytokines were measured by
ELISA. Results demonstrated that the protein levels of TNF-α, IL-1β
and IL-6 were significantly induced in renal ischemia reperfusion
injury. However, chrysin pretreatment significantly decreased the
expression of TNF-α, IL-1β and IL-6 in renal ischemia reperfusion
injury (P<0.05; Fig. 5F-H). These
results indicate that Chrysin pretreatment attenuates renal
IR-induced inflammatory response.
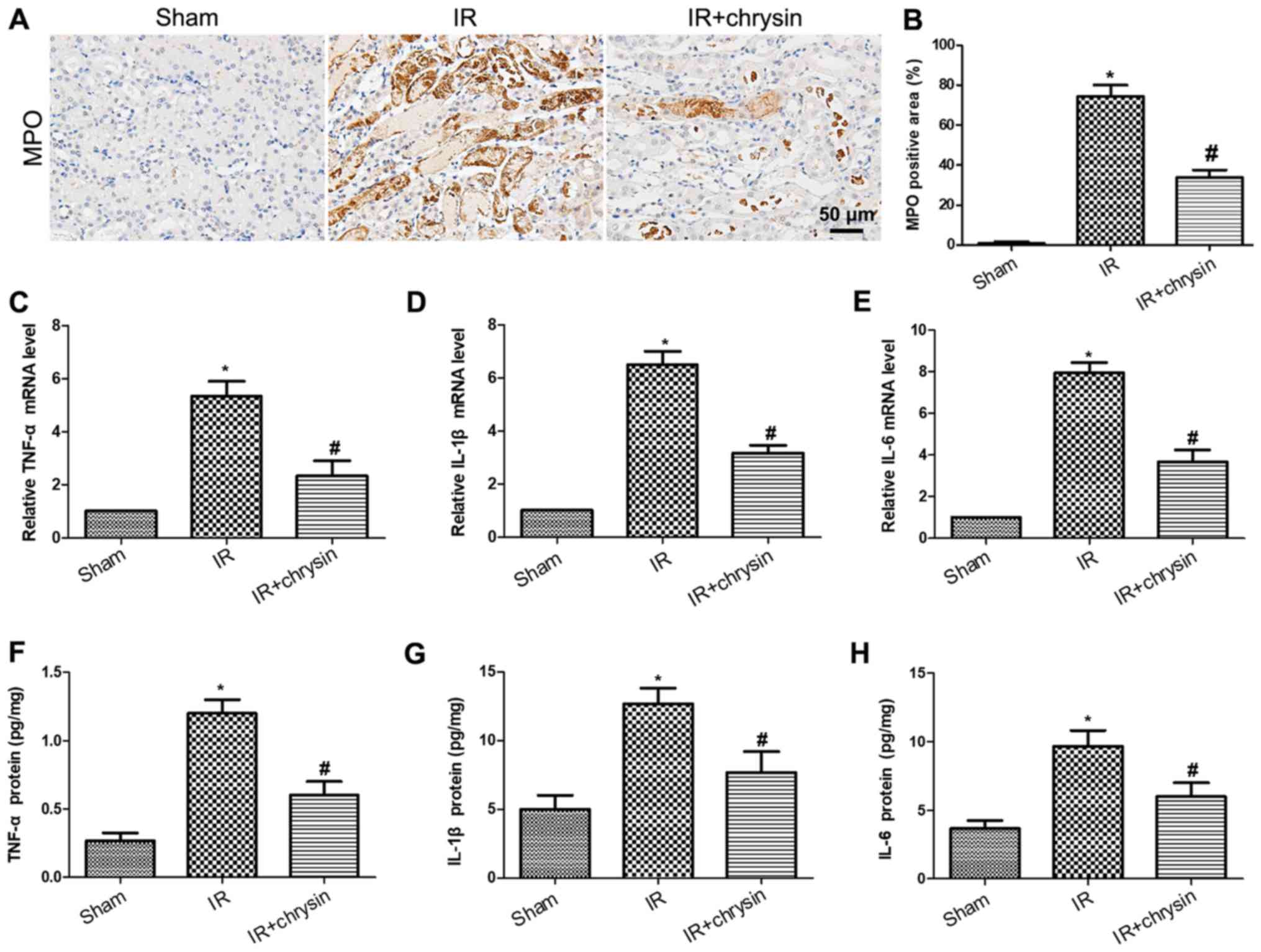 | Figure 5.Chrysin pretreatment attenuates the
renal IR-induced inflammatory response. (A) The
immunohistochemistry staining of MPO in the sham, IR, and
IR+chrysin group. Scale bar, 50 µm. (B) The assessment of MPO
positive areas in the sham, IR and IR+chrysin group. The mRNA
levels of (C) TNF-α, (D) IL-1β, and (E) IL-6 from the sham, IR, and
IR+chrysin group by reverse transcription-quantitative polymerase
chain reaction analysis. The serum protein levels of (F) TNF-α, (G)
IL-1β and (H) IL-6 from the sham, IR, and IR+chrysin group by ELISA
analysis. *P<0.05 vs. the sham group; #P<0.05 vs.
the IR group. IR, ischemia reperfusion; IL, interleukin; TNF, tumor
necrosis factor; MPO, myeloperoxidase. |
Chrysin pretreatment suppresses the
NF-κB signaling in renal IR injury
The NF-κB signaling is closely associated with renal
IR injury (12). To investigate
whether chrysin is involved in renal IR-induced NF-κB signaling
activation, the protein levels of IκBα/NF-κB signaling were
measured (Fig. 6A). The
phosphorylation levels of IκBα and P65 were significantly increased
in the IR group compared with the sham group (P<0.05). However,
the phosphorylation levels of IκBα and P65 were significantly
decreased in the chrysin pretreatment group compared with the IR
group (P<0.05; Fig. 6B and C).
These results indicate that Chrysin pretreatment suppresses the
NF-κB signaling in renal IR injury.
Discussion
Ischemic AKI is a complex syndrome with multiple
cellular abnormalities, resulting in accelerating cycles of tubular
apoptosis, inflammation and renal injury (13). Clinically, no agent can effectively
prevent renal injury following ischemia (14). In this study, the protective effects
of chrysin on IR-induced renal dysfunctions and morphological
abnormalities were reported. Furthermore, chrysin attenuated
tubular cell apoptosis and inflammatory response in renal IR
injury.
Chrysin is a bioflavonoid with anti-inflammatory
(15,16), anti-oxidant (17) and anti-carcinogenic (18) properties. In this study, a renal IR
model was established in mice and pretreated with chrysin. Chrysin
attenuated IR-induced renal dysfunction and morphological
abnormalities. Chrysin particularly suppressed tubular apoptosis,
inflammation and the IκBα/NF-κB signaling pathway in renal IR
injury.
Tubular cell apoptosis is a major pathogenic
mechanism in ischemic AKI (19). The
outer medulla suffers the most severe injury and apoptosis of
tubular cells leading to renal dysfunction in renal IR injury
(5). The levels of Bax and cleaved
caspase-3 increase and the level of Bcl-2 decrease in renal IR
injury, resulting in tubular cell apoptosis (20,21). In
the present study, the protein levels of Bax and cleaved caspase-3
were decreased and the protein level of Bcl-2 was increased in the
chrysin pretreatment group compared with the IR group. Consistent
with these results, the number of TUNEL positive cells was
decreased in the chrysin pretreatment group compared with the IR
group. The results of the present study suggest that chrysin
protects against renal tubular cell apoptosis induced by renal IR
in mice.
The inflammatory response is another important part
of the pathophysiology implicated in renal IR injury (22). Following renal IR, neutrophil
infiltration markedly increases and pro-inflammatory cytokines,
including TNF-α, IL-6 and IL-1β, are induced (23). In the present study, neutrophil
infiltration and TNF-α, IL-6, and IL-1β levels were decreased in
the chrysin pretreatment mice compared with the IR group mice. The
IκBα/NF-κB signaling pathway serves an important role in the renal
IR-induced inflammatory response (12). In the present study, the
phosphorylation levels of IκBα and P65 were increased in the IR
group compared with the sham group. However, the phosphorylation
levels of IκBα and P65 were significantly decreased in the chrysin
pretreatment group compared with the IR group. Results suggest that
chrysin functions as an anti-inflammatory agent in renal IR injury
by suppressing the IκBα/NF-κB signaling pathway.
In conclusion, to the best of our knowledge this
study provides the first evidence that chrysin attenuates renal
dysfunction and morphological abnormalities in ischemic AKI.
Furthermore, chrysin suppresses tubular cell apoptosis,
inflammatory response and the NF-κB signaling pathway in renal IR
injury. Therefore, chrysin may be a promising therapeutic agent for
renal IR injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data and materials are available from the
corresponding author on reasonable request.
Authors' contributions
DL designed the research. MX performed the
experiments and wrote the manuscript. HS analyzed the data. All
authors read and approved the manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Institutional
Animal Care and Use Committee at Hubei University of Arts and
Science. The surgical procedures were performed in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
References
|
1
|
Arai S, Kitada K, Yamazaki T, Takai R,
Zhang X, Tsugawa Y, Sugisawa R, Matsumoto A, Mori M, Yoshihara Y,
et al: Apoptosis inhibitor of macrophage protein enhances
intraluminal debris clearance and ameliorates acute kidney injury
in mice. Nat Med. 22:183–193. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raup-Konsavage WM, Wang Y, Wang WW,
Feliers D, Ruan H and Reeves WB: Neutrophil peptidyl arginine
deiminase-4 has a pivotal role in ischemia/reperfusion-induced
acute kidney injury. Kidney Int. 93:365–374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rabadi M, Kim M, D'Agati V and Lee HT:
Peptidyl arginine deiminase-4-deficient mice are protected against
kidney and liver injury after renal ischemia and reperfusion. Am J
Physiol Renal Physiol. 311:F437–F449. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen H, Wang L, Wang W, Cheng C, Zhang Y,
Zhou Y, Wang C, Miao X, Wang J, Wang C, et al: ELABELA and an
ELABELA fragment protect against AKI. J Am Soc Nephrol.
28:2694–2707. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qin C, Xiao C, Su Y, Zheng H, Xu T, Lu J,
Luo P and Zhang J: Tisp40 deficiency attenuates renal ischemia
reperfusion injury induced apoptosis of tubular epithelial cells.
Exp Cell Res. 359:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Brooks CR, Xiao S, Sabbisetti V,
Yeung MY, Hsiao LL, Ichimura T, Kuchroo V and Bonventre JV:
KIM-1-mediated phagocytosis reduces acute injury to the kidney. J
Clin Invest. 125:1620–1636. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deldar Y, Pilehvar-Soltanahmadi Y,
Dadashpour M, Montazer Saheb S, Rahmati-Yamchi M and Zarghami N: An
in vitro examination of the antioxidant, cytoprotective and
anti-inflammatory properties of chrysin-loaded nanofibrous mats for
potential wound healing applications. Artif Cells Nanomed
Biotechnol. 46:706–716. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
George MY, Esmat A, Tadros MG and
El-Demerdash E: In vivo cellular and molecular gastroprotective
mechanisms of chrysin; Emphasis on oxidative stress, inflammation
and angiogenesis. Eur J Pharmacol. 818:486–498. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sassi A, Maatouk M, El GD, Bzéouich IM,
Abdelkefi-Ben HS, Jemni-Yacoub S, Ghedira K and Chekir-Ghedira L:
Chrysin, a natural and biologically active flavonoid suppresses
tumor growth of mouse B16F10 melanoma cells: In vitro and in vivo
study. Chem Biol Interact. 283:10–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao Y, Chen L, Xiao J, Wang C, Jiang W,
Zhang R and Hao J: Chrysin protects against focal cerebral
ischemia/reperfusion injury in mice through attenuation of
oxidative stress and inflammation. Int J Mol Sci. 15:20913–20926.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia Y, Zhao J, Liu M, Li B, Song Y, Li Y,
Wen A and Shi L: Brazilin exerts protective effects against renal
ischemia-reperfusion injury by inhibiting the NF-κB signaling
pathway. Int J Mol Med. 38:210–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dominguez JH, Liu Y, Gao H, Dominguez JN,
Xie D and Kelly KJ: Renal tubular cell-derived extracellular
vesicles accelerate the recovery of established renal ischemia
reperfusion injury. J Am Soc Nephrol. 28:3533–3544. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan X, Wang X, Chen C, Zhou J and Han M:
Bone mesenchymal stem cells ameliorate ischemia/reperfusion-induced
damage in renal epithelial cells via microRNA-223. Stem Cell Res
Ther. 8:1462017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramirez-Espinosa JJ, Saldana-Rios J,
Garcia-Jimenez S, Villalobos-Molina R, Avila-Villarreal G,
Rodriguez-Ocampo AN, Bernal-Fernandez G and Estrada-Soto S: Chrysin
induces antidiabetic, antidyslipidemic and anti-inflammatory
effects in athymic nude diabetic mice. Molecules. 23:672017.
View Article : Google Scholar
|
|
16
|
Choi JK, Jang YH, Lee S, Lee SR, Choi YA,
Jin M, Choi JH, Park JH, Park PH, Choi H, et al: Chrysin attenuates
atopic dermatitis by suppressing inflammation of keratinocytes.
Food Chem Toxicol. 110:142–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Missassi G, Dos Santos Borges C, de Lima
J, Villela E, Silva P, da Cunha Martins A Jr, Barbosa F Jr and De
Grava Kempinas W: Chrysin administration protects against oxidative
damage in varicocele-induced adult rats. Oxid Med Cell Longev.
2017:21729812017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim W, Ryu S, Bazer FW, Kim SM and Song G:
Chrysin attenuates progression of ovarian cancer cells by
regulating signaling cascades and mitochondrial dysfunction. J Cell
Physiol. 233:3129–3140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Cai T, Xu J, Jiang L, Wu J, Sun Q,
Zen K and Yang J: UCP2 attenuates apoptosis of tubular epithelial
cells in renal ischemia-reperfusion injury. Am J Physiol Renal
Physiol. 313:F926–F937. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi DE, Jeong JY, Choi H, Chang YK, Ahn
MS, Ham YR, Na KR and Lee KW: ERK phosphorylation plays an
important role in the protection afforded by hypothermia against
renal ischemia-reperfusion injury. Surgery. 161:444–452. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Mu Y, Zhou X, Ji H, Gao X, Cai WW,
Guan Q and Xu T: SIRT2-mediated FOXO3a deacetylation drives its
nuclear translocation triggering FasL-induced cell apoptosis during
renal ischemia reperfusion. Apoptosis. 22:519–530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Hu F, Wen J, Wei X, Zeng Y, Sun
Y, Luo S and Sun L: Effects of sevoflurane on NF-кB and TNF-α
expression in renal ischemia-reperfusion diabetic rats. Inflamm
Res. 66:901–910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raup-Konsavage WM, Gao T, Cooper TK,
Morris SJ Jr, Reeves WB and Awad AS: Arginase-2 mediates renal
ischemia-reperfusion injury. Am J Physiol Renal Physiol.
313:F522–F534. 2017. View Article : Google Scholar : PubMed/NCBI
|




















