Introduction
Hypertension extensively participates in the damage
to the body, with a ‘three-high’ characteristics, namely, high
incidence rate, high disability rate and high death rate (1). The incidence rate of hypertension is
increasing, and patients with the disease are getting younger along
with the constant development of global economy, extension of life
expectancy and the aging society, as well as the accelerated pace
of people's life and work and the changed dietary patterns.
Therefore, how to prevent and treat hypertension in a better way
has become one of the important medical problems at present
(2,3). Hypertension can cause damage to various
target organs in the body (including heart, brain and kidneys)
(4). Long-term and sustained
hypertension can lead to increased cardiac load, easily resulting
in myocardial morphological and structural changes such as cardiac
hypertrophy, interstitial fibrosis, ventricular dilatation and
cardiomyocyte apoptosis (5). A study
has demonstrated that cardiomyocyte apoptosis, as a cytological
basis and initiator of left ventricular remodeling of hypertension,
plays a regulatory role in the whole ventricular remodeling
(6). Caspases are initiators and
executioners of cell apoptosis, and in particular, caspase-3 is
essential for the apoptosis of myocardial cells (7). Reducing or eliminating the
hypertension-induced damage to the myocardial cells has great
clinical significance in preventing and treating pathological
changes in the heart with high blood pressure. Currently, there are
few studies or reports on the association of cardiomyocyte
apoptosis with the duration and severity of hypertension. As one of
the important model organisms, the rat is recognized as the closest
to human in essential hypertension. This study analyzed the
cardiomyocyte apoptosis of rats with hypertension and explored the
correlation between cardiomyocyte apoptosis and duration of
hypertension, severity of hypertension as well as caspase-3
expression, which provides a theoretical basis for the prevention
and treatment of cardiac pathological changes of patients with
hypertension.
Materials and methods
Experimental materials
Sixty normal adult Sprague-Dawley (SD) rats,
weighing ~200 g, were purchased from Beijing Huafukang Bioscience
Co., Ltd. (Beijing, China) and were selected and raised at room
temperature, with humidity of 50–60% and free access to food and
water. The rats were maintained in a 12:12-h light/dark cycle.
Major experimental instruments and reagents included centrifuge
[Eppendorf (Shanghai) International Trade Co., Ltd., Shanghai,
China], microtome (Leica Microsystems, Wetzlar, Germany), visible
spectrophotometer [Eppendorf (Shanghai) International Trade Co.,
Ltd.], microscope (JEOL Ltd., Tokyo, Japan), terminal
dexynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)
assay kit (Roche, Basel, Switzerland) and caspase-3 enzyme-linked
immunosorbent assay (ELISA) kit (Shanghai Yuanye Bio-Technology
Co., Ltd., Shangai, China).
The study was approved by the Ethics Committee of
Affiliated Hospital of Jining Medical University (Jining,
China).
Methods
Model preparation and grouping
The rats were fasted for 12 h before transverse
aortic constriction (TAC), but they were allowed to drink water
freely. Then 4% chloral hydrate (300 mg/kg) was injected
intraperitoneally, and no signs of peritonitis were observed. A
longitudinal incision was made at the lower left costal margin and
0.5 cm away from the middle line of abdomen after anesthesia, and
the abdominal aorta was bluntly dissected after entry into the
abdominal cavity. In the observation group, a 7-gauge injection
needle (with a blunt tip) was placed along the vessel course of the
rat, and the abdominal aorta was ligated. After that, the needle
was withdrawn to form stenosis of the abdominal aorta. In the
control group, however, only the abdominal aorta of the rat was
dissected, and TAC was not performed. Then the incision was sutured
and dressed, and the rats were administered with penicillin after
operation to prevent infection and keep them warm. After the rats
were fully awake, they were sent back to the cage for breeding, and
the experiment was conducted at 7, 14 and 28 days after operation,
respectively.
Measurement of blood pressure of the
rats
A non-invasive blood pressure measurement and
analysis system (Shanghai Alcott Biotech Co., Ltd., Shanghai,
China) was utilized to measure the caudal arterial blood pressure
values of each group of rats for 3 consecutive times at 7, 14 and
28 days after TAC, respectively, and the average values were
calculated. The systolic blood pressure (SBP) and diastolic blood
pressure (DBP) of the ventricles were recorded, and the mean
arterial pressure (MAP) was calculated using the following formula:
MAP = DBP + [1/3 (SBP - DBP)].
Collection of myocardium
specimens
The rats were sacrificed at 7, 14 and 28 days after
TAC, respectively. The rats were anesthetized with 3% pentobarbital
sodium (35 mg/kg) by intraperitoneal injection. After thoracotomy,
the heart was taken out after being rinsed with 0.9% sodium
chloride solution, and the maximum transverse diameter of the
coronal plane was cut off. Next, a portion of the heart was fixed
in paraformaldehyde overnight, sliced to 3-mm-thick sections with a
microtome, embedded in paraffin and stored at 4°C for TUNEL assay
and analysis. The other portion of the heart was placed in liquid
nitrogen for 1 h and then preserved in a refrigerator at −80°C for
ELISA.
Measurement of caspase-3
The preserved myocardial tissues (~60 mg) of
different groups of rats were fetched separately and mashed in a
mortar containing an appropriate amount of 0.9% sodium chloride
solution. Then the tissues were centrifuged at 2,650 × g/min for 15
min, and the supernatant (100 µl) was extracted. The ELISA was
performed to detect the content of caspase-3 in the myocardial
tissues in strict accordance with the manufacturer's instructions.
Next, the optical density (OD) was measured at the wavelength of
450 nm using the spectrophotometer, and the concentration of
caspase-3 was calculated.
TUNEL staining
The tissues embedded in paraffin in different groups
were sliced to sections (with a thickness of 3 µm) via the
microtome. After routine deparaffinization, 50 µl 3% hydrogen
peroxide solution was added and incubated at 20°C for 10 min, so as
to block the activity of endogenous peroxidase, followed by rinsing
with phosphate-buffered saline (PBS) 3 times. The reaction mixture
of TUNEL was prepared at 4°C away from light: 20 µl Reagent A
(components: terminal deoxynucleotidyl transferase of
Escherichia coli recombined with bovine thymosin) + 180 µl
Reagent B (components: mixture of nucleotides). Then the sections
were added into 50 µl reaction mixture of TUNEL and incubated at
37°C for 60 min, followed by washing with PBS 3 times. Next, 50 µl
converter-peroxidase (POD) was added and incubated at 37°C for 30
min, followed by rinsing with PBS 3 times. Reagents A, B and C in
the Dolichos biflorus agglutinin (DBA) kit were added into
the sections for color development for 10 min. After that, the
sections were washed with PBS 3 times, then counterstained with
hematoxylin for 10 sec and mounted in neutral balsam. The apoptotic
cells stained yellowish brown were observed and counted under the
microscope.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (IBM Corp., Armonk, NY, USA) was utilized to process
data. Measurement data were presented as mean ± standard deviation
(mean ± SD), and t-test was performed. Pearson's correlation
coefficients were applied to analyze the correlations. P<0.05
indicates that the difference was statistically significant.
Results
Comparison of MAP changes in
hypertensive rats at different time-points after TAC
The MAPs in subgroups 7, 14 and 28 days of the
observation group (148.15±7.73, 149.07±7.84 and 151.36±7.25) were
significantly higher than those in the corresponding subgroups of
the control group (121.76±6.42, 119.35±6.63 and 123.74±6.54)
(P<0.05) (Table I).
 | Table I.Changes in MAP in hypertensive rats at
different time-points (mean ± SD, mmHg). |
Table I.
Changes in MAP in hypertensive rats at
different time-points (mean ± SD, mmHg).
| Days | Control group | Observation
group | t | P-value |
|---|
| 7 | 121.76±6.42 | 148.15±7.73 | 8.305 | <0.001 |
| 14 | 119.35±6.63 | 149.07±7.84 | 9.153 | <0.001 |
| 28 | 123.74±6.54 | 151.36±7.25 | 8.945 | <0.001 |
Comparison of myocardial caspase-3
expression in hypertensive rats at different time-points
The 7-, 14- and 28-day subgroup of the observation
group had remarkably elevated myocardial caspase-3 expression
levels (5.15±0.73, 10.36±1.18 and 15.67±1.43) compared with the
subgroups of the control group (3.76±0.42, 6.24±1.03 and 9.38±1.29)
(P<0.05) (Table II).
 | Table II.Myocardial caspase-3 levels in
hypertensive rats at different time-points (mean ± SD, pmol/l). |
Table II.
Myocardial caspase-3 levels in
hypertensive rats at different time-points (mean ± SD, pmol/l).
| Days | Control group | Observation
group | t | P-value |
|---|
| 7 | 3.76±0.42 |
5.15±0.73 |
5.219 | <0.001 |
| 14 | 6.24±1.03 | 10.36±1.18 |
8.318 | <0.001 |
| 28 | 9.38±1.29 | 15.67±1.43 | 10.328 | <0.001 |
Comparison of cardiomyocyte apoptosis
in hypertensive rats
There was a small quantity of apoptotic cells
observed in the 7-day subgroups of both the observation and control
groups, and a certain number of apoptotic cells were visible in the
remaining subgroups of the observation and control groups.
Moreover, apoptosis rates of myocardial cells in the three
subgroups of the observation group were obviously higher than those
of the control group (P<0.05) (Table III).
 | Table III.Apoptosis rates of myocardial cells in
hypertensive rats at different time-points (mean ± SD, %). |
Table III.
Apoptosis rates of myocardial cells in
hypertensive rats at different time-points (mean ± SD, %).
| Days | Control group | Observation
group | t | P-value |
|---|
| 7 | 4.15±0.85 | 7.76±0.72 | 10.248 | <0.001 |
| 14 | 12.34±1.38 | 16.75±1.54 |
6.744 | <0.001 |
| 28 | 27.27±1.45 | 31.53±1.64 |
6.154 | <0.001 |
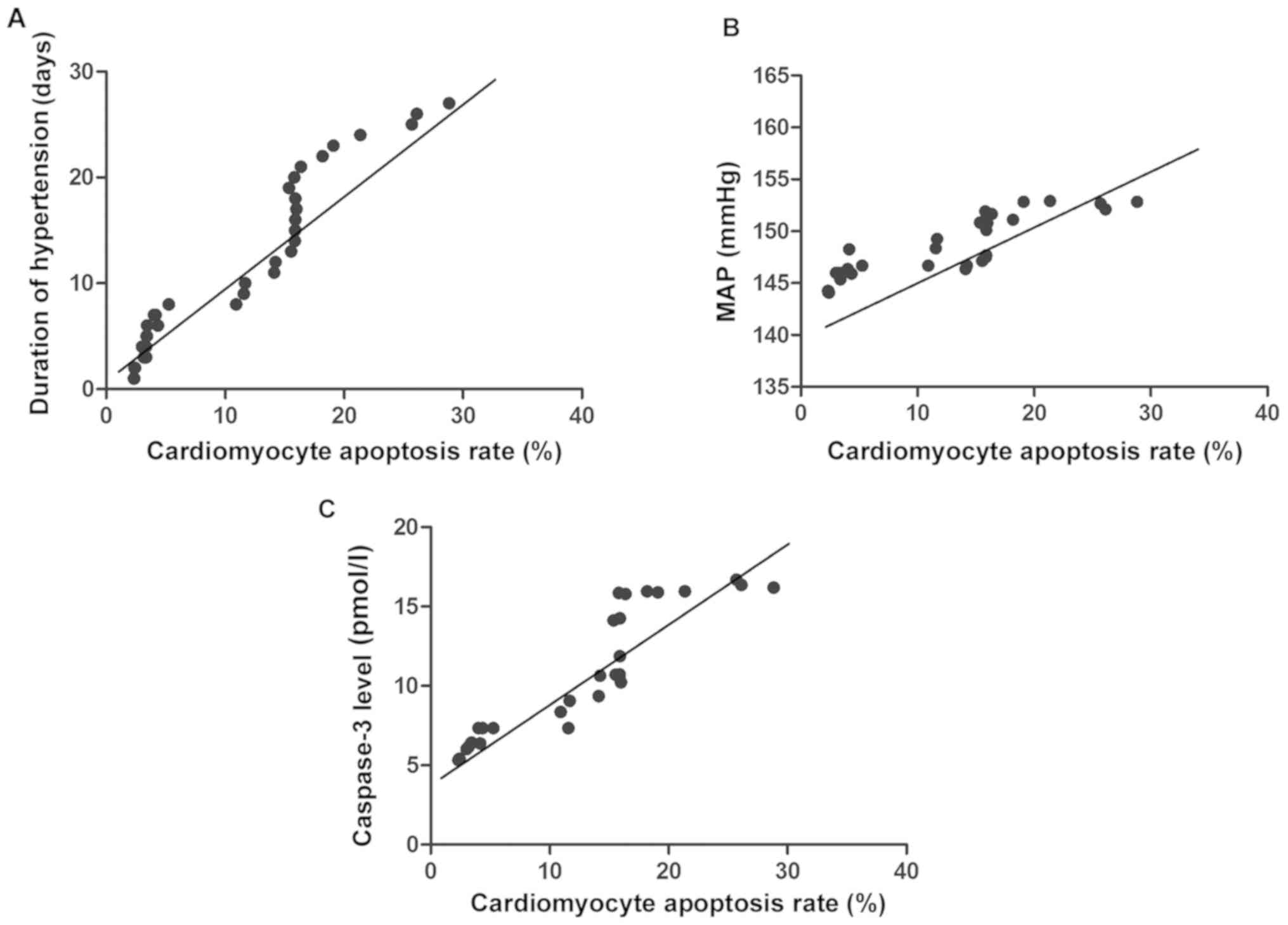
Analysis of correlation of
cardiomyocyte apoptosis with duration of hypertension, severity of
hypertension and caspase-3
Pearson's correlation coefficient analyses indicated
that the cardiomyocyte apoptosis rate of hypertensive rats was
positively correlated with the duration of hypertension, severity
of hypertension and caspase-3 expression (P<0.05) (Table IV and Fig. 1).
 | Table IV.Analysis of correlation of
cardiomyocyte apoptosis with duration and severity of hypertension
and caspase-3. |
Table IV.
Analysis of correlation of
cardiomyocyte apoptosis with duration and severity of hypertension
and caspase-3.
|
| Correlation with
SBP |
|---|
|
|
|
|---|
| Items | r | P-value |
|---|
| Duration of
hypertension | 0.413 | 0.014 |
| Severity of
hypertension | 0.407 | 0.008 |
| Caspase-3
expression | 0.426 | 0.013 |
Discussion
Hypertension is a kind of progressive cardiovascular
syndrome, which is generally divided into essential type and
secondary type (8). In developed
countries, the incidence rate of the disease is as high as 20%, and
it may be triggered by various causes which are mainly
environmental and social factors, including infection, toxin
effect, medicine, diet, psychological pressure, urbanization and
socioeconomic status (9).
Hypertension can damage multiple target organs, especially
different levels of the heart (cardiac tissues and cells), leading
to functional and structural changes in the heart and blood vessels
(10,11). In the state of hypertension, the
neuro-endocrine abnormality occurs. The cardiac cavity is enlarged
and the left ventricle is thickened when the cardiac load is
increased, which is known as ventricular remodeling (12). The ventricular remodeling can induce
a variety of cardiovascular diseases (such as heart failure, acute
myocardial infarction, sudden cardiac death and arrhythmia)
(13), and the mechanism is complex
and diversified. In related studies, it is stated that ventricular
remodeling of hypertension occurs and develops on the basis of
cardiomyocyte apoptosis which is associated with various factors
(including ischemia and hypoxia, activation of neurohormonal
factors, mechanical strain and oxidative stress) (14).
Cell apoptosis refers to the proactive process of
programmed cell death triggered by the combined actions of
extracellular environment and cellular factors under certain
circumstances where the body maintains the stability of internal
environment following its own procedures (15). There are usually three pathways for
cell apoptosis, namely, death receptor pathway, mitochondrial
pathway and endoplasmic reticulum pathway (16). Imbalance of endoplasmic reticulum
stability occurs due to the influence of some factors, which causes
reactions at the cellular level, i.e., endoplasmic reticulum stress
(ERS), thereby leading to physiological disorders in body (17). Endowed with double functions, ERS not
only mediates resistance to apoptosis but also promotes apoptotic
response, thus participating in the apoptosis of myocardial cells
(18). The TUNEL staining in this
study manifested that a small number of apoptotic cells existed in
the 7-day subgroups of both the observation and control groups, and
the quantities of apoptotic cells in the 14- and 28-day subgroups
of the observation and control groups were gradually increased.
Furthermore, the apoptosis rates of myocardial cells in the 7-, 14-
and 28-day subgroups of the observation group were remarkably
higher than those in the control group (P<0.05). It was revealed
in the analysis through Pearson's correlation coefficients that the
cardiomyocyte apoptosis rate of hypertensive rats had a positive
correlation with the duration of hypertension, severity of
hypertension and caspase-3 expression. It could demonstrate that
ERS becomes excessively long and strong as the duration of
hypertension is extended and the degree of hypertension is
exacerbated. Therefore, the homeostasis of ERS in the myocardial
tissues is broken, the ERS-mediated protective response is
weakened, and the pro-survival effect of the myocardial cells
converts into a pro-apoptotic effect. Accordingly, when the
severity and duration of hypertension increase constantly and
exceed certain limits, the apoptotic proteases are activated in the
endoplasmic reticulum, and the apoptosis rate of myocardial cells
is elevated and increasingly severe.
Caspases, as initiators and executioners of cell
apoptosis, are generally divided into two major categories:
promoter caspase (such as caspase-2, −8 and −9) and effector
caspase (including caspase-3, −6 and −7), of which caspase-3 is the
most critical apoptotic protease playing a key executive role
downstream of cascade connection and has a decisive role in the
process of cell death (19,20). The results of this research revealed
that the 7-, 14- and 28-day subgroup of the observation group had
remarkably higher myocardial caspase-3 levels than the
corresponding subgroups of the control group. Pearson's correlation
coefficient analysis manifested that the cardiomyocyte apoptosis
rate of hypertensive rats had a positive correlation with caspase-3
level (P<0.05). It is likely because the caspase-3 is inactive
in normal state, but the ERS is induced as the blood pressure is
rising. In consequence, caspase in the cytoplasm and caspase-9 are
activated, and then caspase-3 on the membrane of endoplasmic
reticulum is activated, leading to degradation of deoxyribonucleic
acid (DNA) repair enzyme, destruction of nuclear protein and
skeleton protein as well as cell apoptosis. With the upregulation
of caspase-3 expression, the number of TUNEL positive cells is
increasing, and the apoptosis rate of myocardial cells is on the
rise. This is consistent with the study results of Morishima et
al (21).
In conclusion, the constantly increased level and
extended duration of hypertension aggravates the cardiomyocyte
apoptosis in hypertensive rats, and the activation of caspase-3 is
an important factor for apoptosis of myocardial cells, whose
upregulated expression can promote apoptosis.
Acknowledgements
We would like to thank the National Natural Science
Foundation of China for supporting this study.
Funding
This study was supported by National Natural Science
Foundation of China (Dysfunctional autophagic-lysosomal system in
degenerative aortic stenosis 81400291).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW drafted the manuscript and was responsible for
model preparation and grouping. YC collected myocardium specimens.
NL and SP were mainly devoted to TUNEL staining. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Affiliated Hospital of Jining Medical University (Jining,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nkeh-Chungag BN, Sekokotla AM,
Sewani-Rusike C, Namugowa A and Iputo JE: Prevalence of
hypertension and pre-hypertension in 13–17 year old adolescents
living in Mthatha - South Africa: A cross-sectional study. Cent Eur
J Public Health. 23:59–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baszczuk A, Kopczynski Z and Thielemann A:
Endothelial dysfunction in patients with primary hypertension and
hyperhomocysteinemia. Postepy Hig Med Dosw. 68:91–100. 2014.(In
Polish). View Article : Google Scholar
|
|
3
|
Lin BM, Curhan SG, Wang M, Eavey R,
Stankovic KM and Curhan GC: Hypertension, diuretic use, and risk of
hearing loss. Am J Med. 129:416–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumura K, Arima H, Tominaga M, Ohtsubo
T, Sasaguri T, Fujii K, Fukuhara M, Uezono K, Morinaga Y, Ohta Y,
et al: COMFORT Investigators: Effect of losartan on serum uric acid
in hypertension treated with a diuretic: The COMFORT study. Clin
Exp Hypertens. 37:192–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ozturk N, Olgar Y, Aslan M and Ozdemir S:
Effects of magnesium supplementation on electrophysiological
remodeling of cardiac myocytes in L-NAME induced hypertensive rats.
J Bioenerg Biomembr. 48:425–436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peters EL, Offringa C, Kos D, Van der
Laarse WJ and Jaspers RT: Regulation of myoglobin in hypertrophied
rat cardiomyocytes in experimental pulmonary hypertension. Pflugers
Arch. 468:1697–1707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu M, Sun L, Jiang B, Tan S, Liu K and
Xiao X: [Effect of nucleolin on cardiac cell apoptosis in Type 2
diabetic cardiomyopathy mice]. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
42:241–245. 2017.(In Chinese). PubMed/NCBI
|
|
8
|
Missault LH, Duprez DA, Brandt AA, de
Buyzere ML, Adang LT and Clement DL: Exercise performance and
diastolic filling in essential hypertension. Blood Press.
2:284–288. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gradman AH, Basile JN, Carter BL and
Bakris GL;: American Society of Hypertension Writing Group:
Combination therapy in hypertension. J Clin Hypertens (Greenwich).
13:146–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davey R and Raina A: Hemodynamic
monitoring in heart failure and pulmonary hypertension: From analog
tracings to the digital age. World J Transplant. 6:542–547. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hao C, Kang C, Xue J, Shi K, Lv H and Li
Z: Effects of blood pressure and sex on heart-vessel coupling in
essential hypertension. Turk J Med Sci. 46:680–685. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cavasin MA, Stenmark KR and McKinsey TA:
Emerging roles for histone deacetylases in pulmonary hypertension
and right ventricular remodeling (2013 Grover Conference series).
Pulm Circ. 5:63–72. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hamirani YS, Kundu BK, Zhong M, McBride A,
Li Y, Davogustto GE, Taegtmeyer H and Bourque JM: Noninvasive
detection of early metabolic left ventricular remodeling in
systemic hypertension. Cardiology. 133:157–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iglarz M, Landskroner K, Bauer Y,
Vercauteren M, Rey M, Renault B, Studer R, Vezzali E, Freti D,
Hadana H, et al: Comparison of macitentan and bosentan on right
ventricular remodeling in a rat model of non-vasoreactive pulmonary
hypertension. J Cardiovasc Pharmacol. 66:457–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Holdenrieder S, Stieber P, Förg T, Kühl M,
Schulz L, Busch M, Schalhorn A and Seidel D: Apoptosis in serum of
patients with solid tumours. Anticancer Res 19A. 2721–2724.
1999.
|
|
16
|
Sõti C, Sreedhar AS and Csermely P:
Apoptosis, necrosis and cellular senescence: Chaperone occupancy as
a potential switch. Aging Cell. 2:39–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou J, Gan X, Wang Y, Zhang X, Ding X,
Chen L, Du J, Luo Q, Wang T, Shen J, et al: Toxoplasma gondii
prevalent in China induce weaker apoptosis of neural stem cells
C17.2 via endoplasmic reticulum stress (ERS) signaling pathways.
Parasit Vectors. 8:732015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun XY, Qin HJ, Zhang Z, Xu Y, Yang XC,
Zhao DM, Li XN and Sun LK: Valproate attenuates diabetic
nephropathy through inhibition of endoplasmic reticulum
stress-induced apoptosis. Mol Med Rep. 13:661–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Büssing A, Vervecken W, Wagner M, Wagner
B, Pfüller U and Schietzel M: Expression of mitochondrial Apo2.7
molecules and caspase-3 activation in human lymphocytes treated
with the ribosome-inhibiting mistletoe lectins and the cell
membrane permeabilizing viscotoxins. Cytometry. 37:133–139. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang JD, Takahara S, Nonomura N, Ichimaru
N, Toki K, Azuma H, Matsumiya K, Okuyama A and Suzuki S: Early
induction of apoptosis in androgen-independent prostate cancer cell
line by FTY720 requires caspase-3 activation. Prostate. 40:50–55.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent
activation of caspase-9 by caspase-12. J Biol Chem.
277:34287–34294. 2002. View Article : Google Scholar : PubMed/NCBI
|