Introduction
Diabetic nephropathy (DN) is the most common
complication induced by diabetes, which is one of the major reasons
for renal failure (1). Its morbidity
is increased gradually year by year, making it a global public
health challenge that severely damages human health (1). It is estimated by the World Trade
Organization that the number of diabetics in the world will rise to
0.37 billion by 2025, and 30% of these cases will develop DN
(2). The pathological
characteristics of DN primarily include glomerular hypertrophy and
hyperplasia, basilar membrane thickening, and increased
extracellular matrix. DN gradually develops into glomerular
sclerosis, interstitial fibrosis as well as loss of function
(3). Eventually, DN results in
chronic renal failure, which reduces quality of life and endangers
the patient's life (3). There are
multiple pathogeneses responsible for DN. However, these are
complicated processes that have not been completely illuminated yet
(4).
Uncoupling protein (UCP) is a type of proton channel
localized on the inner mitochondrial membrane (5). UCP belongs to the mitochondrial carrier
protein superfamily (5). It reduces
the electrochemical gradient on both sides of membrane, decreases
ATP production and leads to reduced production of reactive oxygen
species (ROS) in mitochondria (5).
These findings suggest the protective effects of UCP on tissues.
UCP2 is extensively distributed in multiple sites, including the
liver, brain, kidney, gland membrane, muscle, white adipose tissue,
retinal endothelial cells and pericytes (6). Previous findings have indicated that
UCP2 mRNA expression can be upregulated in the presence of elevated
intracellular ROS levels (6).
Furthermore, overexpression of UCP2 analogue or antioxidase can
reduce the level of ROS, which may prevent the occurrence of
complications associated with diabetes (7).
MicroRNAs (miRs) are endogenous, non-coding, small
RNA molecules that are ~20–24 nucleotides long (8). miRs are also extensively distributed
molecules that regulate gene expression (8). It is currently believed that miRs serve
important roles during the growth and development of organisms.
miRs can regulate genes at a post-transcription level. Thus, they
are involved in almost all physiological and pathological processes
in the body (9). These processes
include cell proliferation, differentiation, apoptosis and immune
inflammation (9). Previous research
in recent years has suggested that miRs serve important roles in
the genesis and development of DN through gene regulation,
suggesting miRs may be associated with the genesis and development
of DN (9,10). Although the understanding towards the
biological characteristics of miRs and their roles in DN has
improved (9,10), further studies are required.
Investigating the association of DN with miRs will provide a novel
viewpoint for understanding the genesis and development of DN. In
addition, this may also provide biomarkers for the early diagnosis
of DN, and novel targets for treatment and intervention. The
current study aimed to address the function of miR-214 on DN and
diabetes in proximal tubular cells.
Materials and methods
Animals
The present study was approved by the Taizhou
People's Hospital Ethics Committee (Taizhou, China). Male Sprague
Dawley rats (5–6 weeks old, 200–220 g) had unrestricted access to
food and water and were maintained in a controlled environment
(temperature, 22–23°C; humidity, 55–60%; and a 12-h light
(7:00)/dark (19:00) cycle). All rats were randomly assigned into a
sham (n=6) or a DN model (n=6) group. Animals in the sham group
were injected once with normal saline. The DN model rats were
injected once with 75 mg/kg streptozocin (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) (11). At
12 weeks after the induction of diabetes, mice were anesthetized
with 35 mg/kg of pentobarbital sodium (intraperitoneal,
Sigma-Aldrich; Merck KGaA) and sacrificed using decollation.
Hematoxylin and eosin staining
Kidney tissue samples were fixed with 4%
paraformaldehyde for 24 h at room temperature and processed using
paraffin at 55–60°C for 5–10 min. Paraffin-embedded tissue sections
(10 µm) were deparaffinized using xylene for 60 min at 60°C,
hydrated using ethyl alcohol at room temperature for 30 min and
stained with 0.5% hematoxylin for 10 min at room temperature,
washed with water for 5 min and 0.5% eosin for 1 min at room
temperature. Sections were observed using a light microscope
(magnification, ×200).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells or serum
following the induction of the model using an RNA extraction kit
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
cDNA was reverse transcribed using a Q-script kit (Quanta
Biosciences, Gaithersburg, MD, USA) and a TaqMan miR reverse
transcription kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.) at 37°C for 1 h followed by 82°C for 10 sec. qPCR was
performed using an ABI Prism 7500 Sequence Detection System
(Perkin-Elmer Inc., Waltham, MA, USA) and a standard SYBR Green PCR
kit (Toyobo Life Science, Osaka, Japan). The temperature protocol
was as follows: Initial denaturation at 95°C for 5 min; followed by
40 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for
30 sec and elongation at 72°C for 30 sec. Primer sequences were as
follows: miR-214, forward, 5′-AGCATAATACAGCAGGCACAGAC-3′ and
reverse, 5′-AAAGGTTGTTCTCCACTCTCTCAC-3′; U6, forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′. Results were analyzed using the
2−ΔΔCq method (12).
Gene expression profiling
Total RNA was hybridized to Affymetrix HG-U133 Plus
2.0 GeneChip arrays (Affymetrix; Thermo Fisher Scientific, Inc.).
Data were analyzed using the database for annotation, visualization
and integrated discovery and Qiagen Ingenuity Pathway Analysis
(Qiagen, Inc., Valencia, CA, USA).
Cell culture and transfection
Human HK-2 renal proximal tubular epithelial cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin. miR-214-5p (100 ng;
5′-GGCCTGGCTGGACAGAGTTG-3′) and negative control (100 ng;
5′-CCCCCCCCCCCCC-3′) were purchased from Sangon Biotech Co., Ltd.
(Shanghai, China). miR-214 mimics and mimic-negative control were
transfected with Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.). Following 48 h of transfection, cells were cultured in DMEM
supplemented with 35 mM glucose for 24 h for further analysis.
Cells were then treated with 2 nM MK 2206 dihydrochloride (Akt
inhibitor; MedChemExpress, Shanghai, China) or UCP2 inhibitor
(genipin; 50 µM; MedChemExpress, Monmouth Junction, NJ, USA) for 44
h and cultured in DMEM supplemented with 35 mM of glucose for 24 h
for further analysis.
ELISA kits
Following the induction of DN, supernatants of cells
were collected using centrifugation at 1,000 × g for 5 min at 4°C
and 10–50 µl of supernatant were used to analyze the levels of
oxidative stress [superoxide dismutase (SOD), A001-1-1;
malondialdehyde (MDA), A003-1; glutathione (GSH), A006-2;
GSH-peroxidase (PX), A005)] and ROS (E004) using ELISA kits
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The
fluorescence intensity was measured using a spectrofluorometer
(Fluorostar, BMG Labtech GmbH, Ortenberg, Germany) at 450 nm. Cells
were stained with dichloro-dihydro-fluorescein diacetate (10 µM)
for 20 min at 37°C and ROS levels were observed using a Zeiss
Axioplan 2 (magnification, ×200; Zeiss AG, Oberkochen,
Germany).
Western blot analysis
Cells were lysed using radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) for 15 min at 4°C and the protein content was then
determined using a BCA Protein Assay (Thermo Fisher Scientific,
Inc.). Equal amounts (50 µg) of protein were separated by SDS-PAGE
(10% gels) and blotted onto polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Blots were blocked
at room temperature with 5% non-fat dry milk in Tris-buffered
Saline with Tween 20 (TBST) for 1 h and incubated with UCP2 (89326;
1:2,000), phosphorylated (p)-Akt (4060; 1:2,000), p-mammalian
target of rapamycin (mTOR; 5536; 1:2,000) and GAPDH (5174; 1:5,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) overnight at
4°C. Membranes were exposed to anti-rabbit horseradish
peroxidase-conjugated secondary antibody (7074; 1:5,000, Cell
Signaling Technology, Inc.) for 1 h at 37°C, following several
washes with TBST. Membranes were detected using ECL Plus reagents
(GE Healthcare, Chicago, IL, USA) and quantified using the
Molecular Imager ChemiDoc XRS System and Quantity One 1-D analysis
software (Bio-Rad Laboratories, Inc.).
Luciferase activity
The 3′-untranslated region (UTR) of UCP2 was cloned
into luciferase reporter vector (CmiT000001- MT06; GeneCopoeia,
Inc., Rockville, MD, USA). The 3′UTR of UCP2 and miR-214 mimics
(100 ng) were co-transfected using Lipofectamine 2000 (Thermo
Fisher Scientific, Inc.). Cells were assayed using luciferase assay
kits (Promega Corporation, Madison, WI, USA) for 48 h after
transfection. Renilla luciferase activity was used for
normalization.
Statistical analysis
All data were expressed as the mean ± standard
deviation. The Student's t-test or one-way analysis of variance
with a Student-Newman-Keuls post hoc test was used to discern
individual differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
miR-214 expression in rats with
DN
Hematoxylin and eosin staining revealed that the
glomerulus was damaged and the volume of the glomerulus was
markedly reduced in rats with DN compared with the Sham group
(Fig. 1A). Furthermore, there were
significantly decreased levels of glutathione peroxidase (GSH-PX),
glutathione (GSH) and superoxide dismutase (SOD), but significantly
increased levels of malondialdehyde (MDA) in rats with DN compared
with the Sham group (Fig. 1B-E). As
indicated in Fig. 1F and G, miR-214
expression was significantly decreased in rats with DN compared
with the Sham group. These results suggested that miR-214 may
regulate the progression of DN.
 | Figure 1.miR-214 expression in rats with DN.
Hematoxylin and eosin staining for (A) glomerulus (magnification,
×200). ELISA was used to determine (B) MDA, (C) SOD, (D) GSH and
(E) GSH-PX levels. (F) GeneChip analysis. (G) Reverse
transcription-quantitative polymerase chain reaction was used to
determine miR-214 expression detection. **P<0.01 vs. Sham group.
miR, microRNA; DN, diabetic nephropathy; Sham, control group; MDA,
malondialdehyde; GSH-PX, glutathione peroxidase; GSH, glutathione;
SOD, superoxide dismutase. |
Overexpression of miR-214 decreases
oxidative stress in HK-2 cell
As indicated in Fig.
2, miR-214 overexpression significantly increased the levels of
GSH-PX, GSH and SOD, and significantly reduced the levels of MDA in
the in vitro model of DN compared with the control group.
Furthermore, miR-214 overexpression also significantly reduced ROS
levels in DN in vitro when compared with the control group
(Fig. 2E and F). The results
suggested that miR-214 could modulate oxidative stress in DN.
 | Figure 2.Overexpression of miR-214 increases
oxidative stress in HK-2 renal proximal tubular epithelial cells.
(A) Reverse transcription-quantitative polymerase chain reaction
was used to determine miR-214 expression. ELISA was used to
determine (B) MDA, (C) SOD, (D) GSH and (E) GSH-PX. (F) ROS levels
and (G) ROS probe staining (dichloro-dihydro-fluorescein diacetate;
magnification, ×200). **P<0.01 vs. control group. miR-214,
overexpression of miR-214 group; Control, control negative group;
miR-214, microRNA-214; MDA, malondialdehyde; GSH-PX, glutathione
peroxidase; GSH, glutathione; SOD, superoxide dismutase; ROS,
reactive oxygen species. |
Overexpression of miR-214 regulates
the Akt/mTOR signaling pathway by UCP2 in HK-2 cell
miR-214 targeted the 3′-untranslated region of UCP2,
and luciferase activity was significantly increased in the
overexpressed miR-214 group compared with the control group
(Fig. 3). As indicated in Fig. 3C-F, miR-214 overexpression
significantly increased the protein expression levels of UCP2,
p-Akt and p-mTOR in DN in vitro when compared with the
control group. These findings indicated that miR-214 regulated the
progression of DN through the Akt/mTOR signaling pathway by
UCP2.
UCP2 inhibitor attenuates the effects
of miR-214 upregulation on oxidative stress in DN in HK-2 cell
The function of UCP2 in DN via miR-214 was assessed
in the present study. Results indicated that UCP2 inhibitor,
genipin, significantly inhibited UCP2 expression in DN via miR-214
overexpression. As indicated in Fig.
4A-D, UCP2 inhibitor significantly suppressed the protein
expression levels of UCP2, p-Akt and p-mTOR in miR-214-upregulated
DN cells compared with miR-214 upregulation alone. In addition,
UCP2 inhibitor significantly reduced the levels of GSH-PX, GSH and
SOD, and significantly increased those of MDA and ROS in
miR-214-upregulated DN cells compared with miR-214 upregulation
alone (Fig. 4E-J). The results
suggested that UCP2 also serves a role in miR-214-regulated
oxidative stress in DN.
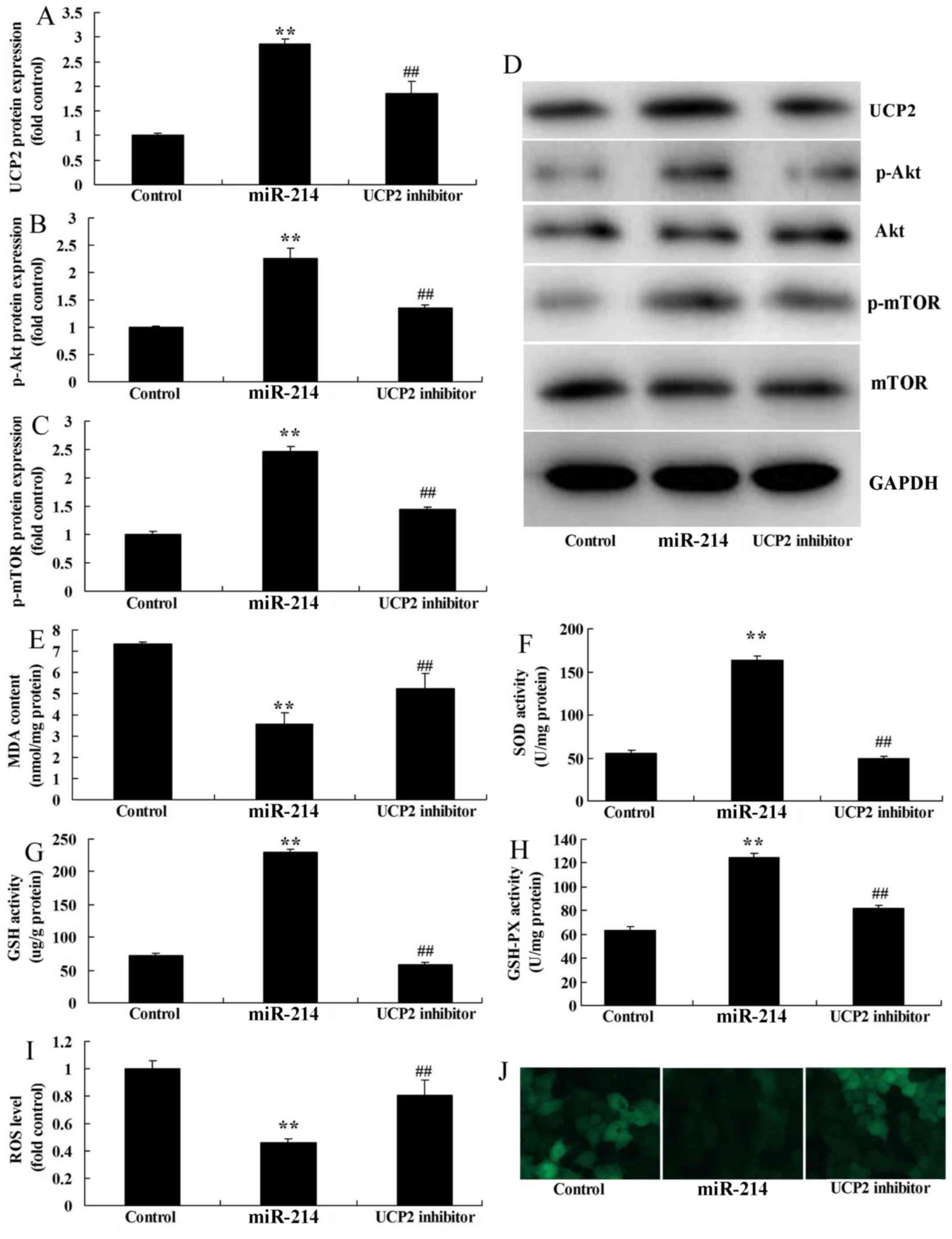 | Figure 4.UCP2 inhibitor reduces the effects of
miR-214 upregulation on oxidative stress in an in vitro DN
model. (A) UCP2, (B) p-Akt and (C) p-mTOR protein expression levels
were determined using statistical analysis and (D) western blot
analysis. ELISA was used to determine (E) MDA, (F) SOD, (G) GSH,
(H) GSH-PX levels and (I) ROS levels and (J) ROS probe staining
(dichloro-dihydro-fluorescein diacetate; magnification, ×200).
**P<0.01 vs. control group; ##P<0.01 vs. miR-214
group. miR-214, overexpression of miR-214 group; Control, control
negative group; UCP2 inhibitor, overexpression of miR-214 and UCP2
inhibitor group; miR-214, microRNA-214; UCP2, uncoupling protein 2;
mTOR, mammalian target of rapamycin; p, phosphorylated; MDA,
malondialdehyde; GSH-PX, glutathione peroxidase; GSH, glutathione;
SOD, superoxide dismutase; ROS, reactive oxygen species. |
Akt inhibitor reduces the effects of
miR-214 upregulation on oxidative stress in DN in in HK-2 cell
The role of Akt in the function of miR-214 on
inflammation in DN was investigated. As indicated in Fig. 5A-C, Akt inhibitor (2 nM MK 2206
dihydrochloride) significantly suppressed the protein expression
levels of p-Akt and p-mTOR in miR-214-upregulated DN cells compared
with miR-214 upregulation alone. Akt inhibitor significantly
reduced the levels of GSH-PX, GSH and SOD, and significantly
elevated those of MDA in miR-214-upregulated DN cells compared with
miR-214 upregulation alone (Fig.
5D-G). These results suggested that miR-214 regulates the
UCP2/ROS/Akt/mTOR signaling pathway to suppress oxidative stress in
diabetic nephropathy.
 | Figure 5.Akt inhibitor reduces the effects of
miR-214 upregulation on oxidative stress in an in vitro DN
model. (A) p-Akt and (B) p-mTOR protein expression using
statistical analysis. (C) Western blot analysis for p-Akt and
p-mTOR protein expression. ELISA was used to determine (D) MDA, (E)
SOD, (F) GSH and (G) GSH-PX. **P<0.01 vs. control group;
##P<0.01 vs. miR-214 group. miR-214, overexpression
of miR-214 group; Control, control negative group; Akt inhibitor,
overexpression of miR-214 and Akt inhibitor group; miR-214,
microRNA-214; mTOR, mammalian target of rapamycin; p,
phosphorylated; MDA, malondialdehyde; GSH-PX, glutathione
peroxidase; GSH, glutathione; SOD, superoxide dismutase. |
Discussion
Type 2 diabetes is a common and frequently-occurring
endocrine/metabolic disease (13).
People's living standards have increasingly improved over time
(13). However, unhealthy diets,
work pressures, lack of physical exercise and accelerated aging of
the population have resulted in increased diabetes-associated
morbidity (13). Diabetic
retinopathy is the microvascular complication of diabetes, which
may result in visual impairment and blindness (3). Thus, the morbidity and rate of
blindness of DN are increasing accordingly (3). To the best of our knowledge, the
present study is the first to indicate that miR-214 expression
levels in peripheral blood were decreased in rats with DN. Notably.
Costantino et al (14)
revealed that miR-214 is associated with hyperglycemic memory in
diabetic mice.
Previous results have indicated that UCP2 can
stimulate B cells to secrete insulin (5). Results of experiments on a rat UCP2
model indicated that overexpression of UCP2 led to reduced
intracellular ATP content (5). In
addition, overexpression of UCP2 decreased the sensitivity of
glucose on B cells, which therefore reduced insulin secretion
(15). It was also identified in
UCP2 gene knockout mice that increased ATP content can be observed
in B cells in vivo or in vitro (15). This also increases the sensitivity of
glucose to stimulate insulin secretion (15). As demonstrated in previous study,
elevated UCP2 activity or overexpression of UCP2 in B cells may
affect insulin secretion (7,15). The correlation of UCP2 gene
polymorphisms with diabetes has become a new research focus
(7). The current study suggested
that miR-214 overexpression induced UCP2 protein expression in the
in vitro DN model. Furthermore, Yu et al (16) indicated that miR-214 is a putative
tumor suppressor by targeting UCP2 in hepatocellular carcinoma.
Oxidative stress markers are a series of biochemical
substances that reflect oxidative stress levels in vivo
(17). There are various types of
oxidative stress markers. Of them, ROS, reactive nitrogen species
and lipid peroxide are commonly used in diabetes detection
(17). Determination of oxidative
stress markers serves an important role in revealing correlations
of diabetes genesis and development with oxidative stress (17). In the present study, it was observed
that miR-214 overexpression decreased oxidative stress in the in
vitro DN model. Notably, Gao et al (18) revealed that miR-214 protects
erythroid cells against oxidative stress by targeting activating
transcription factor 4 and enhancer of zeste homolog 2 (EZH2).
These results demonstrated that miR-214 overexpression decreased
oxidative stress in DN.
The inflammation-associated p38 mitogen-activated
protein kinase (MAPK) signaling pathway is associated with renal
tissue injury during the development of DN (19). Multiple factors, including
hyperglycemia, abnormal hemodynamics, oxidative stress and
pre-inflammatory factors, can activate the downstream p38 MAPK
signaling pathway (20). The
activated p38 MAPK signal pathway can further induce activation of
downstream inflammatory cells. Furthermore, it promotes the
expression of inflammatory mediators, and increases the production
of inflammatory factors (20).
Finally, the p38 MAPK signaling pathway leads to inflammatory
injury of renal tissues. The intervention of the p38 MAPK signaling
pathway is achieved through multiple approaches.
Akt is the downstream signaling molecule of
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) (21). Notably, the PI3K/Akt signaling
pathway serves an important role in insulin signal transmission
(22). Such signaling systems have
an important role in biological processes (22). These include metabolism, cell growth,
proliferation, survival and migration, as well as cytoskeletal
reconstruction, inflammation, cell apoptosis, membrane transport
and secretion (23). Furthermore,
the PI3K/Akt signaling pathway can promote cell survival,
differentiation and growth (23).
Studies have indicated that the PI3K/Akt signaling pathway is
associated with vascular smooth muscle contraction,
endothelium-mediated relaxation and blood pressure (24). The PI3K/Akt signaling pathway is also
extensively involved in the formation of atherosclerosis,
pathological angiogenesis, inflammatory cell aggregation and
vascular smooth muscle cell dysfunction (23). Additionally, the PI3K/Akt signaling
pathway can promote vasoconstriction, induce vascular remodeling
and accelerate the genesis and development of atherosclerosis
(25). It was identified that
miR-214 overexpression induced the Akt/mTOR signaling pathway in an
in vitro DN model in the present study. Das et al
(26) revealed that miR-214 reduces
insulin like growth factor 1 receptor expression and downstream
mTOR complex 1 signaling in renal carcinoma cells. These results
demonstrated that miR-214 suppressed oxidative stress in DN via
UCP2 in the ROS/Akt/mTOR signaling pathway.
Fig. 6 revealed a
schematic diagram of the suggested potential mechanism involved in
the function of miR-214 on oxidative stress in DN. In conclusion,
the present study demonstrated for the first time that miR-214 may
regulate diabetes through the insulin and ROS/Akt/mTOR signaling
pathways in proximal tubular cells by UCP2. Collectively, these
results suggest that miR-214 has protective effects against
oxidative stress in DN, which could be used in clinical
applications.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SY designed the experiment. XF, YL, BX, YM and HW
performed the experiments. SY analyzed the data and prepared the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Taizhou
People's Hospital Ethics Committee (Taizhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lindhardt M, Persson F, Currie G, Pontillo
C, Beige J, Delles C, von der Leyen H, Mischak H, Navis G, Noutsou
M, et al: Proteomic prediction and Renin angiotensin aldosterone
system Inhibition prevention Of early diabetic nephRopathy in TYpe
2 diabetic patients with normoalbuminuria (PRIORITY): Essential
study design and rationale of a randomised clinical multicentre
trial. BMJ Open. 6:e0103102016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakamura T, Sato E, Amaha M, Kawagoe Y,
Maeda S and Yamagishi S: Addition of aliskiren to angiotensin II
receptor blockers ameliorates renal tubular injury and reduces
intima media thickness of carotid artery in patients with diabetic
nephropathy. Int J Cardiol. 155:294–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koya D, Haneda M, Inomata S, Suzuki Y,
Suzuki D, Makino H, Shikata K, Murakami Y, Tomino Y, Yamada K, et
al: Long-term effect of modification of dietary protein intake on
the progression of diabetic nephropathy: A randomised controlled
trial. Diabetologia. 52:2037–2045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abe M, Higuchi T, Moriuchi M, Okamura M,
Tei R, Nagura C, Takashima H, Kikuchi F, Tomita H and Okada K:
Efficacy and safety of saxagliptin, a dipeptidyl peptidase-4
inhibitor, in hemodialysis patients with diabetic nephropathy: A
randomized open-label prospective trial. Diabetes Res Clin Pract.
116:244–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Friederich-Persson M, Aslam S, Nordquist
L, Welch WJ, Wilcox CS and Palm F: Acute knockdown of uncoupling
protein-2 increases uncoupling via the adenine nucleotide
transporter and decreases oxidative stress in diabetic kidneys.
PLoS One. 7:e396352012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qiu W, Zhou Y, Jiang L, Fang L, Chen L, Su
W, Tan R, Zhang CY, Han X and Yang J: Genipin inhibits
mitochondrial uncoupling protein 2 expression and ameliorates
podocyte injury in diabetic mice. PLoS One. 7:e413912012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Souza BM, Michels M, Sortica DA, Bouças
AP, Rheinheimer J, Buffon MP, Bauer AC, Canani LH and Crispim D:
Polymorphisms of the UCP2 gene are associated with glomerular
filtration rate in type 2 diabetic patients and with decreased UCP2
gene expression in human kidney. PLoS One. 10:e01329382015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nassirpour R, Raj D, Townsend R and
Argyropoulos C: MicroRNA biomarkers in clinical renal disease: From
diabetic nephropathy renal transplantation and beyond. Food Chem
Toxicol. 98:73–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SY and Choi ME: Urinary biomarkers for
early diabetic nephropathy: Beyond albuminuria. Pediatr Nephrol.
30:1063–1075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
von Brandenstein M, Pandarakalam JJ, Kroon
L, Loeser H, Herden J, Braun G, Wendland K, Dienes HP, Engelmann U
and Fries JW: MicroRNA 15a, inversely correlated to PKCalpha, is a
potential marker to differentiate between benign and malignant
renal tumors in biopsy and urine samples. Am J Pathol.
180:1787–1797. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamada H, Itoh M, Hiratsuka I and
Hashimoto S: Circulating microRNAs in autoimmune thyroid diseases.
Clin Endocrinol (Oxf). 81:276–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Araki H, Kuwagata S, Soumura M, Yamahara
K, Morita Y, Kume S, Isshiki K, Araki S, Kashiwagi A, Maegawa H and
Uzu T: Safety and efficacy of skin patches containing loxoprofen
sodium in diabetic patients with overt nephropathy. Clin Exp
Nephrol. 18:487–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Costantino S, Paneni F, Lüscher TF and
Cosentino F: MicroRNA profiling unveils hyperglycaemic memory in
the diabetic heart. Eur Heart J. 37:572–576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Persson MF, Franzén S, Catrina SB, Dallner
G, Hansell P, Brismar K and Palm F: Coenzyme Q10 prevents
GDP-sensitive mitochondrial uncoupling, glomerular hyperfiltration
and proteinuria in kidneys from db/db mice as a model of type 2
diabetes. Diabetologia. 55:1535–1543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu G, Wang J, Xu K and Dong J: Dynamic
regulation of uncoupling protein 2 expression by microRNA-214 in
hepatocellular carcinoma. Biosci Rep. 36:e003352016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Huang R, Zhang L, Li S, Luo J, Gu
Y, Chen Z, Zheng Q, Chao T, Zheng W, et al: A severe
atherosclerosis mouse model on the resistant NOD background. Dis
Model Mech. 11:dmm0338522018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao M, Liu Y, Chen Y, Yin C, Chen JJ and
Liu S: miR-214 protects erythroid cells against oxidative stress by
targeting ATF4 and EZH2. Free Radic Biol Med. 92:39–49. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang T, Chen SS, Chen R, Yu DM and Yu P:
Reduced beta 2 glycoprotein I improve diabetic nephropathy via
inhibiting TGF-β1-p38 MAPK pathway. Int J Clin Exp Med.
8:6852–6865. 2015.PubMed/NCBI
|
|
20
|
Lu HJ, Tzeng TF, Liou SS, Da Lin S, Wu MC
and Liu IM: Polysaccharides from Liriopes Radix ameliorate
streptozotocin-induced type I diabetic nephropathy via regulating
NF-κB and p38 MAPK signaling pathways. BMC Complement Altern Med.
14:1562014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu F, Wang Y, Cui W, Yuan H, Sun J, Wu M,
Guo Q, Kong L, Wu H and Miao L: Resveratrol prevention of diabetic
nephropathy is associated with the suppression of renal
inflammation and mesangial cell proliferation: Possible roles of
Akt/NF-κB pathway. Int J Endocrinol. 2014:2893272014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song Z, Guo Y, Zhou M and Zhang X: The
PI3K/p-Akt signaling pathway participates in calcitriol
ameliorating podocyte injury in DN rats. Metabolism. 63:1324–1333.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang G, Lv J, Li T, Huai G, Li X, Xiang
S, Wang L, Qin Z, Pang J, Zou B and Wang Y: Notoginsenoside R1
ameliorates podocyte injury in rats with diabetic nephropathy by
activating the PI3K/Akt signaling pathway. Int J Mol Med.
38:1179–1189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji X, Li C, Ou Y, Li N, Yuan K, Yang G,
Chen X, Yang Z, Liu B, Cheung WW, et al: Andrographolide
ameliorates diabetic nephropathy by attenuating
hyperglycemia-mediated renal oxidative stress and inflammation via
Akt/NF-κB pathway. Mol Cell Endocrinol. 437:268–279. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Das F, Dey N, Bera A, Kasinath BS,
Ghosh-Choudhury N and Choudhury GG: MicroRNA-214 reduces
insulin-like growth factor-1 (IGF-1) receptor expression and
downstream mTORC1 signaling in renal carcinoma cells. J Biol Chem.
291:14662–14676. 2016. View Article : Google Scholar : PubMed/NCBI
|




















