Introduction
Oral cavity squamous cell carcinoma (OCSCC) accounts
for at least 90% of all oral malignancies. It is a multifactorial
condition with etiological links to a wide variety of external
causes of cancer, including alcohol, tobacco and betel nut use, and
certain viral infections. The high and increasing prevalence of
OCSCC in Taiwan has been attributed to the popularity of betel nut
chewing. It was estimated that, in 2006, more than 4,000 people in
Taiwan were diagnosed with oral cancer. This represents 5.49% of
all newly diagnosed malignancies. Despite advances in technology
and the implementation of multidisciplinary treatment programs,
only modest improvements in survival rates have been achieved, and
these are primarily due to earlier diagnosis, rather than improved
therapeutic interventions (1).
Moreover, the rate of recurrence of advanced tumors remains
relatively high. Salvage outcomes are unsatisfactory, although they
depend on the stage of the recurrent tumors (2). Investigation of OCSCC progression
from a genetic perspective has identified distinct patterns and
timings of genetic alterations (3). The most important prognostic factors
in OCSCC are those that form part of the grading system, including
tumor stage and lymph node status (4–6). The
identification of new prognostic factors linked to OCSCC initiation
and progression may aid in the development of new diagnostic tools
and treatment strategies.
Among the various molecular factors implicated in
carcinogenesis, telomere dysfunction has emerged as an early event
associated with genetic instability. Telomeres stabilize the ends
of chromosomes, protect them from end-to-end fusion and mediate
chromosome pairing during cell division (7–10).
Recently, telomere-associated proteins, such as telomeric
repeat-binding factor 1 (TRF1) and 2 (TRF2), have been identified
as putative modulators of telomerase activity and have been
suggested to play key roles in the maintenance of the telomere
function (8,9,11,12).
Several reports have indicated that the altered expression of TRF1
and TRF2 proteins is associated with tumor progression in various
human carcinomas, including lung, stomach, adrenal and pancreatic
cancer; the altered expression has also been identified in
malignant hematopoietic cells and colorectal pre-neoplastic lesions
(13–20). However, the relationship between
TRF1 and TRF2 and OCSCC remains unclear. The aim of the present
study was to examine TRF1 and TRF2 expression in OCSCC and to
determine its relationship with clinicopathological variables and
survival.
Materials and methods
Patients and tumor samples
The study population included 256 OCSCC patients who
underwent primary surgical resection without previous radiotherapy
and/or chemotherapy between October 1996 and August 2005.
Clinicopathological information for each subject, including gender,
age, tumor (T) stage, nodal (N) status, tumor node metastasis (TNM)
stage and overall survival, was obtained retrospectively from
clinical records and pathological reports. TNM status was
classified according to the 1997 American Joint Committee on Cancer
(AJCC) system. The study was approved by the Medical Ethics and
Human Clinical Trial Committee at Chang Gung Memorial Hospital,
Taipei, Taiwan. The patient group comprised 17 women and 239 men,
with an average age of 50.9 years (range, 26–87 years). Thirty-nine
patients were diagnosed with T1 tumors, 55 with T2, 64 with T3 and
98 with T4. A total of 153 patients had an N status of N0, 38 had
N1, 48 had N2b, 13 had N2c and 4 had N3. Thirty-four patients had
stage I tumors, 38 stage II, 61 stage III and 123 stage IV.
Immunoblot analysis
For tissue protein extraction, frozen samples
(adjacent non-tumor and tumor tissues) were homogenized in RIPA
lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5%
Na-deoxycholate and 0.1% SDS), and the protein concentrations were
quantified using a Bio-Rad Protein assay (Bio-Rad, Hercules, CA,
USA). Immunoblotting was performed according to standard
procedures. Anti-TRF1 and -TRF2 polyclonal antibodies and the
anti-GAPDH monoclonal antibody (Santa Cruz Biotechnology Santa
Cruz, CA, USA) were used. The bound primary antibody was detected
by incubation with HRP-conjugated secondary antibody (Bio/Can
Scientific, Mississauga, ON, Canada). Blots were developed using
the Western Lighting reagent, and protein bands were visualized
using X-ray film.
Immunohistochemical analysis
OCSCC and adjacent non-cancerous tissue samples were
identified by a pathologist based on diagnosis and microscopic
morphology. Tumor tissues were fixed in 10% buffered formalin,
embedded in paraffin and decalcified in 10% EDTA. Formalin-fixed
paraffin-embedded tissue was sectioned to a thickness of 4 μm, and
the sections were deparaffinized in xylene and rehydrated in a
graded series of ethanol (100, 90, 80 and 70%). The sections were
washed in phosphate-buffered saline (PBS) and treated with 3%
H2O2 for 30 min to block the endogenous
peroxidase activity. Antigens were retrieved by microwaving the
sections in 10 mM citrate buffer, pH 6.0. The sections were then
incubated with anti-TRF1 and -TRF2 antibodies (diluted 1:100) for 1
h, washed in PBS and incubated for 30 min with a horseradish
peroxidase-Fab polymer conjugate (PicTure™-Plus kit; Zymed, South
San Francisco, CA, USA). After the sections were washed in PBS, the
immunoreactive bands were visualized by incubation with
3,3′-diaminobenzidine for 5 min. As a negative control, the primary
antibody was omitted. Two pathologists blinded to the subjects’
clinical information independently evaluated the reactivity level
of the immunostained tissues in 15–20 high-power fields. Criteria
were developed for quantitating the immunoreactivity of the TRF1
and TRF2 staining in the adjacent non-tumor and tumor sections
using a score range of 0 to +3. A percentage value of 0 indicated
0–25% of the area stained; +1, 25–50%; +2, 50–75% and +3, >75%
stained. Similarly, the staining intensity was graded as +0, +1, +2
or +3. High expression of TRF1 and TRF2 was defined as ≥+2 in both
scoring methods. Low expression of TRF1 and TRF2 was defined as ≤+1
in both scoring methods.
Statistical analysis
Fisher’s exact test was used to evaluate the
correlation between TRF1 and TRF2 expression and various
clinicopathological variables, including gender, age, N status, T
stage and TNM stage. A p-value <0.05 was considered to indicate
statistical significance. TRF1 and TRF2 expression and the
clinicopathological variables were used in a Kaplan-Meier analysis
of survival, and statistical significance (p<0.05) was assessed
by the log-rank test. To determine the effects of specific
prognostic factors on survival, a multivariate analysis was
performed using Cox’s regression model.
Results
Down-regulation of TRF1 and TRF2
expression in OCSCC tissues
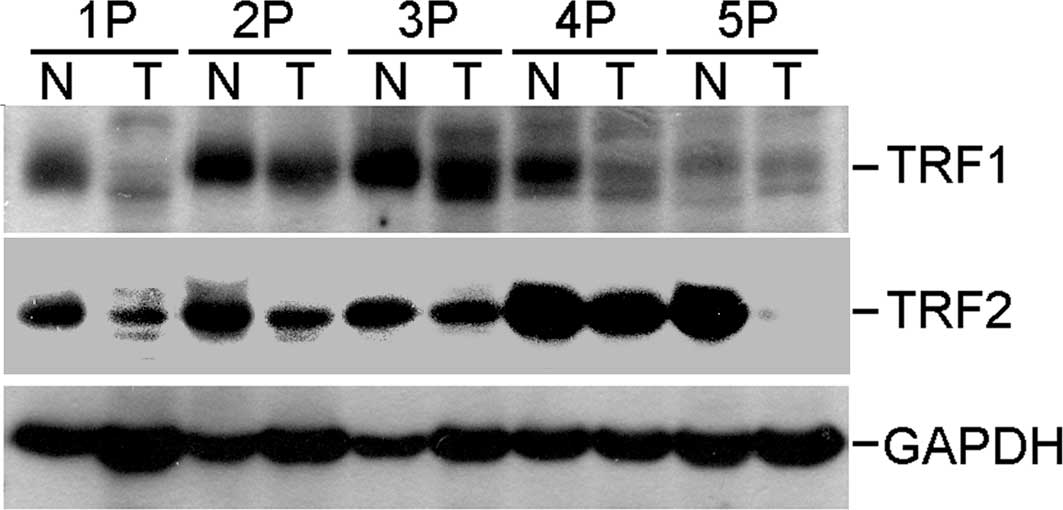
To investigate the potential roles of TRF1 and TRF2
in the pathogenesis of OCSCC, their expression was assessed in
representative and paired tumor and adjacent non-cancerous tissue
samples by Western blot analysis using anti-human TRF1 and TRF2
polyclonal antibodies. The protein expression levels of TRF1 and
TRF2 were lower in the tumor samples compared to those in the
paired non-cancerous tissues (Fig.
1).
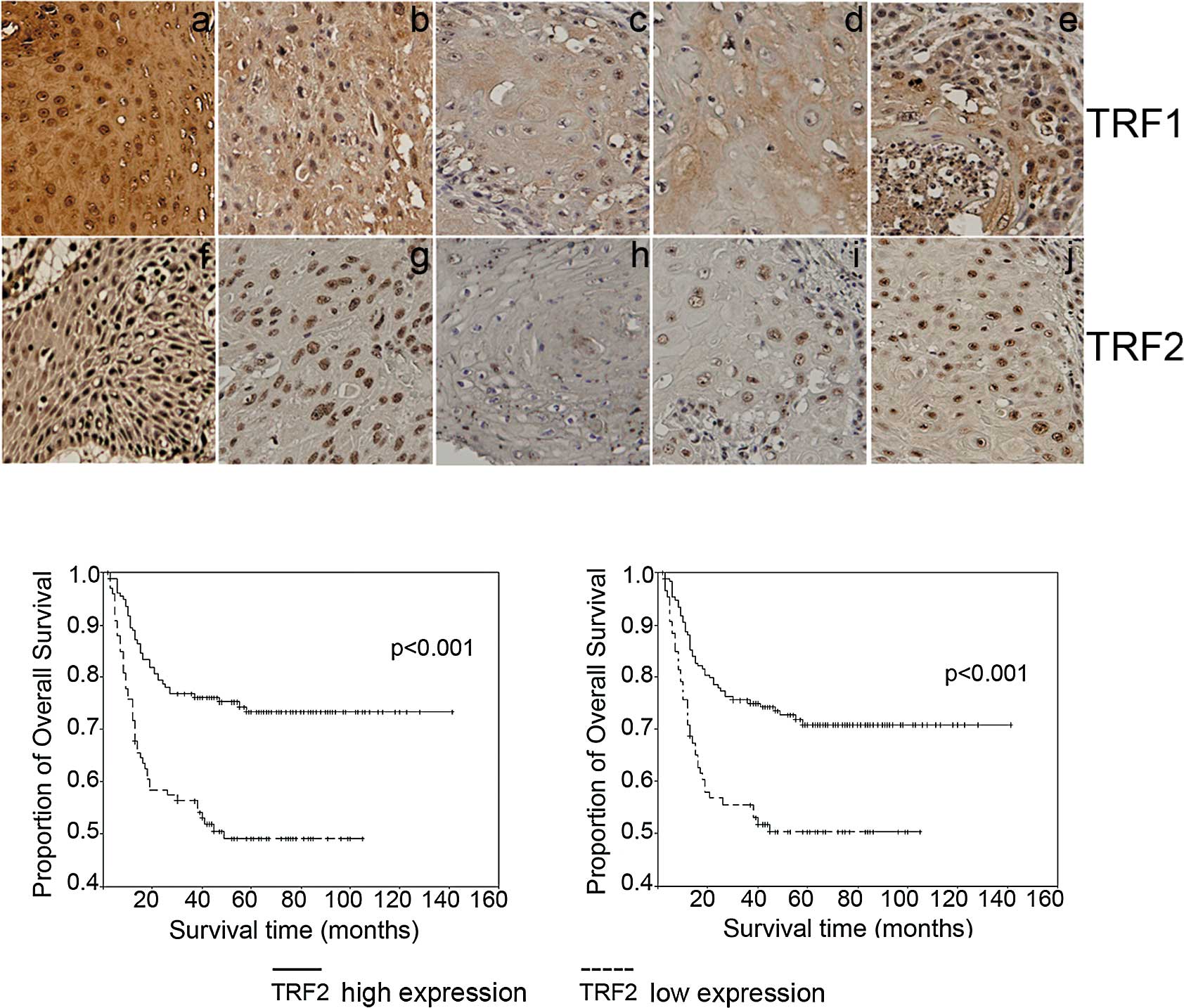
TRF1 and TRF2 expression in the tumor and adjacent
non-cancerous tissues from the 256 OCSCC patients was also examined
immunohistochemically. Representative results of TRF1 and TRF2
immunostaining are presented in Fig.
2A. Staining was stronger in the adjacent non-cancerous tissues
(Fig. 2A-a and -f) than the tumor
tissues (Fig. 2A-b and -c, TRF1;
Fig. 2A-g and -h, TRF2). Moreover,
TRF1 and TRF2 expression levels in the tumor samples were
negatively correlated with T stage (Fig. 2A-b and -c, TRF1; Fig. 2A-g and -h, TRF2) and N stage
(Fig. 2A-d and -e, TRF1; Fig. 2A-i and -j, TRF2). Notably, TRF1 and
TRF2 were focally expressed in the nuclei of both tumor and
non-cancerous cells (Fig. 2A-b and
-c, TRF1; Fig. 2A-g and -h,
TRF2).
To investigate whether the expression of TRF1 and
TRF2 is associated with various prognostic factors, including age,
gender and TNM pathologic classification, we classified the
patients into two groups based on the immunohistochemical analysis:
low (−/+) and high (++/+++) TRF1 or TRF2 expression. Low TRF1
expression was correlated with advanced T stage (p<0.001) and
advanced TNM stage (p<0.001). Low TRF2 expression was correlated
with advanced T stage (p<0.001) and advanced TNM stage
(p<0.001), as well as positive lymph node metastasis (p=0.022).
Neither TRF1 nor TRF2 expression was correlated with age or gender
(Table I). These findings suggest
that TRF1 and TRF2 expression levels may be linked to tumor
progression in OCSCC.
 | Table I.Correlation between the
clinicopathological features and expression of TRF1 and TRF2 in the
oral squamous cell carcinoma cases. |
Table I.
Correlation between the
clinicopathological features and expression of TRF1 and TRF2 in the
oral squamous cell carcinoma cases.
| Variables | No. of patients | TRF1 expression
| P-value | TRF2 expression
| P-value |
|---|
| Low | High | Low | High |
|---|
| Gender | | | | 0.452 | | | 0.434 |
| Male | 239 | 95 | 144 | | 83 | 156 | |
| Female | 17 | 5 | 12 | | 4 | 13 | |
| Age (years) | | | | 0.272 | | | 0.333 |
| <60 | 202 | 75 | 127 | | 72 | 130 | |
| ≥60 | 54 | 25 | 29 | | 15 | 39 | |
| Tumor stage | | | | <0.001a | | | <0.001a |
| T1 and T2 | 94 | 15 | 79 | | 11 | 83 | |
| T3 and T4 | 162 | 85 | 77 | | 76 | 86 | |
| Nodal stage | | | | 0.090 | | | 0.022a |
| Negative | 153 | 53 | 100 | | 43 | 110 | |
| Positive | 103 | 47 | 56 | | 44 | 59 | |
| TNM stage | | | | <0.001a | | | <0.001a |
| I and II | 66 | 5 | 61 | | 1 | 65 | |
| III and IV | 190 | 95 | 95 | | 86 | 104 | |
TRF1 and TRF2 expression and OCSCC
patient survival
In view of the finding that TRF1 and TRF2 expression
levels were associated with T stage, we investigated whether TRF1
and TRF2 expression was correlated with patient prognosis. As shown
in Fig. 2B, Kaplan-Meier overall
survival analysis revealed that the prognosis of patients with low
(−/+) tumor expression of TRF1 and TRF2 was significantly poorer
than that of patients displaying higher (++/+++) expression
(p<0.001). Univariate analysis revealed that advanced T stage
(p<0.001), positive N stage (p<0.001), advanced TNM stage
(p<0.001), low TRF1 expression and low TRF2 expression each
predicted a significantly worse prognosis for OCSCC patients
(Table II). The prognosis was not
associated with age or gender. Cox regression analysis revealed
that T stage (95% CI, 1.585–5.037; RR=2.826; p<0.001), N status
(95% CI, 1.966–4.681; RR=3.034; p<0.001) and TRF1 expression
(95% CI, 0.391–0.924; RR=0.601; p=0.02) were independent prognostic
factors for survival. These results clearly indicate that the
clinical prognosis for OCSCC patients is affected by the tumor
expression of TRF1 and TRF2, and suggest that TRF1 and TRF2 may be
good prognostic indicators in OCSCC.
 | Table II.Cumulative 5-year overall survival
rate according to clinicopathological features. |
Table II.
Cumulative 5-year overall survival
rate according to clinicopathological features.
| Variables | No. of
patients | Cumulative 5-year
overall survival rate (%) | P-value |
|---|
| Gender | | | 0.1360 |
| Male | 239 | 62.5 | |
| Female | 17 | 81.9 | |
| TRF1
expression | | | <0.0010a |
| Low | 100 | 73.3 | |
| High | 156 | 49.1 | |
| TRF2
expression | | | 0.0003a |
| Low | 87 | 70.1 | |
| High | 169 | 50.3 | |
| Age (years) | | | 0.1300 |
| <60 | 202 | 66.2 | |
| ≥60 | 54 | 55.1 | |
| Tumor stage | | | <0.0010a |
| T1 and T2 | 94 | 84.0 | |
| T3 and T4 | 162 | 56.5 | |
| Nodal stage | | | <0.0010a |
| Negative | 153 | 77.0 | |
| Positive | 103 | 44.7 | |
| TNM stage | | | <0.0010a |
| I and II | 66 | 92.4 | |
| III and IV | 190 | 53.9 | |
Discussion
To our knowledge, this is the first investigation of
TRF1 and TRF2 expression in primary OCSCC specimens from a large
cohort of patients. Our results indicate that the reduced
expression of TRF1 and TRF2 is associated with increased tumor
aggressiveness and poor prognosis in OCSCC patients. Low expression
of TRF1 and TRF2 and advanced T stage and N stage were correlated
with a poor prognosis. As it is difficult to determine a prognosis
for these patients, TRF1 and TRF2 staining of oral cancer cells may
be helpful in selecting an appropriate therapeutic strategy
following surgery. Our findings suggest that TRF1 is a good
prognostic indicator in OCSCC and a candidate molecular target for
oral cancer therapy.
Semi-quantitative RT-PCR analysis of TRF1 and
TRF2 mRNA expression in total RNA from tumor samples and
matched adjacent non-cancerous tissues from 5 OCSCC patients
demonstrated that TRF1 and TRF2 mRNA levels did not
differ significantly between the tumor and adjacent non-cancerous
tissues (data not shown). This observation suggests that altered
TRF1 and TRF2 protein expression during the development of OCSCC
may be realized post-transcriptionally.
Telomere-binding proteins have attracted increasing
interest due to their essential roles in regulating the length of
telomeric DNA tracts and protecting against chromosomal end-to-end
fusion (21). In cancer, telomeres
become dysfunctional due to the loss or alteration of
telomere-binding proteins involved in telomere maintenance or to
DNA damage caused by oxidative stress (22). The telomere-binding proteins TRF1
and TRF2 are crucial for the protection and maintenance of
telomeres in mammalian cells (8,23).
TRF1 and TRF2 contain a Myb-like helix-turn-helix domain in the
C-terminus of the protein, and a conserved central domain that is
responsible for the formation of homodimers (24). Previous studies have indicated that
TRF1 and TRF2 are down-regulated in malignant tissues (13,14,25–28).
To our knowledge, the present study is the first to report not only
that TRF1 and TRF2 are down-regulated in OCSCC, but also that their
expression levels are correlated with the clinical characteristics
of tumors. The correlation of TRF1 and TRF2 expression with
clinical T stage may be explained at the cellular level by the
roles of TRF1 and TRF2 in regulating the growth of cancer cells,
while the correlation with N stage may reflect the participation of
TRF1 and TRF2 in the control of metastasis. In contrast to our
results, other studies have revealed that TRF1 and TRF2 are
up-regulated in aggressive adenocarcinoma (29–32).
This apparent disparity may be the result of differences in the
tumors examined and in their microenvironments.
In the present study, decreased expression of TRF1
and TRF2 was detected in OCSCC patients based on Western blotting
and immunohistochemistry. TRF1 and TRF2 were strongly expressed in
the nucleus of adjacent non-cancerous tissues, and weakly expressed
in human OCSCC specimens. Additionally, expression of TRF1 and TRF2
was correlated with 5-year overall survival and clinical prognosis.
Notably, TRF1 expression was an independent prognostic indicator
for OCSCC in this cohort. These results indicate that TRF1 and TRF2
may be critical regulators of disease progression in OCSCC, making
them potential therapeutic targets. Future studies of the
physiological targets of TRF1 and TRF2 and their potential roles in
the pathogenesis of OCSCC may facilitate the development of novel
therapeutic strategies.
Acknowledgements
This study was supported by Chang Gung
Memorial Hospital, Taiwan (grant nos. CMRPG870411, CMRPG870412 and
CMRPG860513).
References
|
1.
|
Shah JP and Singh B: Why the lack of
progress for oral cancer? Lancet Oncol. 7:356–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Chien CY, Su CY, Chuang HC, et al:
Angiopoietin-1 and -2 expression in recurrent squamous cell
carcinoma of the oral cavity. J Surg Oncol. 97:273–277. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Chen PH, Ko YC, Yang YH, et al: Important
prognostic factors of long-term oropharyngeal carcinoma survivors
in Taiwan. Oral Oncol. 40:847–855. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Matsuo JM, Patel SG, Singh B, et al:
Clinical nodal stage is an independently significant predictor of
distant failure in patients with squamous cell carcinoma of the
larynx. Ann Surg. 238:412–421. 2003.PubMed/NCBI
|
|
5.
|
Chien CY, Su CY, Chuang HC, et al:
Comprehensive study on the prognostic role of osteopontin
expression in oral squamous cell carcinoma. Oral Oncol. 45:798–802.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Chen CH, Chien CY, Huang CC, et al:
Expression of FLJ10540 is correlated with aggressiveness of oral
cavity squamous cell carcinoma by stimulating cell migration and
invasion through increased FOXM1 and MMP-2 activity. Oncogene.
28:2723–2737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Vaziri H, Schachter F, Uchida I, et al:
Loss of telomeric DNA during aging of normal and trisomy 21 human
lymphocytes. Am J Hum Genet. 52:661–667. 1993.PubMed/NCBI
|
|
8.
|
Van Steensel B and de Lange T: Control of
telomere length by the human telomeric protein TRF1. Nature.
385:740–743. 1997.
|
|
9.
|
Smogorzewska A, van Steensel B, Bianchi A,
et al: Control of human telomere length by TRF1 and TRF2. Mol Cell
Biol. 20:1659–1668. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sfeir A, Kosiyatrakul ST, Hockemeyer D, et
al: Mammalian telomeres resemble fragile sites and require TRF1 for
efficient replication. Cell. 138:90–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Van Steensel B, Smogorzewska A and de
Lange T: TRF2 protects human telomeres from end-to-end fusions.
Cell. 92:401–413. 1998.PubMed/NCBI
|
|
12.
|
Ancelin K, Brunori M, Bauwens S, et al:
Targeting assay to study the cis functions of human telomeric
proteins: evidence for inhibition of telomerase by TRF1 and for
activation of telomere degradation by TRF2. Mol Cell Biol.
22:3474–3487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yamada K, Yagihashi A, Yamada M, et al:
Decreased gene expression for telomeric-repeat binding factors and
TIN2 in malignant hematopoietic cells. Anticancer Res.
22:1315–1320. 2002.PubMed/NCBI
|
|
14.
|
Yamada M, Tsuji N, Nakamura M, et al:
Down-regulation of TRF1, TRF2 and TIN2 genes is important to
maintain telomeric DNA for gastric cancers. Anticancer Res.
22:3303–3307. 2002.PubMed/NCBI
|
|
15.
|
Miyachi K, Fujita M, Tanaka N, Sasaki K
and Sunagawa M: Correlation between telomerase activity and
telomeric-repeat binding factors in gastric cancer. J Exp Clin
Cancer Res. 21:269–275. 2002.PubMed/NCBI
|
|
16.
|
Lin X, Gu J, Lu C, Spitz MR and Wu X:
Expression of telomere-associated genes as prognostic markers for
overall survival in patients with non-small cell lung cancer. Clin
Cancer Res. 12:5720–5725. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Raynaud CM, Jang SJ, Nuciforo P, et al:
Telomere shortening is correlated with the DNA damage response and
telomeric protein down-regulation in colorectal preneoplastic
lesions. Ann Oncol. 19:1875–1881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Frias C, Garcia-Aranda C, De Juan C, et
al: Telomere shortening is associated with poor prognosis and
telomerase activity correlates with DNA repair impairment in
non-small cell lung cancer. Lung Cancer. 60:416–425. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
d’Adda di Fagagna F, Reaper PM,
Clay-Farrace L, et al: A DNA damage checkpoint response in
telomere-initiated senescence. Nature. 426:194–198. 2003.PubMed/NCBI
|
|
20.
|
Yajima T, Yagihashi A, Kameshima H,
Kobayashi D, Hirata K and Watanabe N: Telomerase reverse
transcriptase and telomeric-repeat binding factor protein 1 as
regulators of telomerase activity in pancreatic cancer cells. Br J
Cancer. 85:752–757. 2001. View Article : Google Scholar
|
|
21.
|
Salhab M, Jiang WG, Newbold RF and Mokbel
K: The expression of gene transcripts of telomere-associated genes
in human breast cancer: correlation with clinico-pathological
parameters and clinical outcome. Breast Cancer Res Treat.
109:35–46. 2008. View Article : Google Scholar
|
|
22.
|
Feldser DM, Hackett JA and Greider CW:
Telomere dysfunction and the initiation of genome instability. Nat
Rev Cancer. 3:623–627. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Chong L, van Steensel B, Broccoli D, et
al: A human telomeric protein. Science. 270:1663–1667. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Broccoli D, Smogorzewska A, Chong L and de
Lange T: Human telomeres contain two distinct Myb-related proteins,
TRF1 and TRF2. Nat Genet. 17:231–235. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Saito K, Yagihashi A, Nasu S, Izawa Y, et
al: Gene expression for suppressors of telomerase activity
(telomeric-repeat binding factors) in breast cancer. Jpn J Cancer
Res. 93:253–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Yamada K, Yajima T, Yagihashi A, et al:
Role of human telomerase reverse transcriptase and telomeric-repeat
binding factor proteins 1 and 2 in human hematopoietic cells. Jpn J
Cancer Res. 91:1278–1284. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
La Torre D, de Divitiis O, Conti A, et al:
Expression of telomeric repeat binding factor-1 in astroglial brain
tumors. Neurosurgery. 56:802–810. 2005.PubMed/NCBI
|
|
28.
|
Kishi S, Wulf G, Nakamura M and Lu KP:
Telomeric protein Pin2/TRF1 induces mitotic entry and apoptosis in
cells with short telomeres and is down-regulated in human breast
tumors. Oncogene. 20:1497–1508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Oh BK, Kim YJ, Park C and Park YN:
Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is
related to telomere shortening during human multistep
hepatocarcinogenesis. Am J Pathol. 166:73–80. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Klapper W, Qian W, Schulte C and
Parwaresch R: DNA damage transiently increases TRF2 mRNA expression
and telomerase activity. Leukemia. 17:2007–2015. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Klapper W, Krams M, Qian W, Janssen D and
Parwaresch R: Telomerase activity in B-cell non-Hodgkin lymphomas
is regulated by hTERT transcription and correlated with
telomere-binding protein expression but uncoupled from
proliferation. Br J Cancer. 89:713–719. 2003. View Article : Google Scholar
|
|
32.
|
Nakanishi K, Kawai T, Kumaki F, et al:
Expression of mRNAs for telomeric repeat binding factor (TRF)-1 and
TRF2 in atypical adenomatous hyperplasia and adenocarcinoma of the
lung. Clin Cancer Res. 9:1105–1111. 2003.PubMed/NCBI
|