Introduction
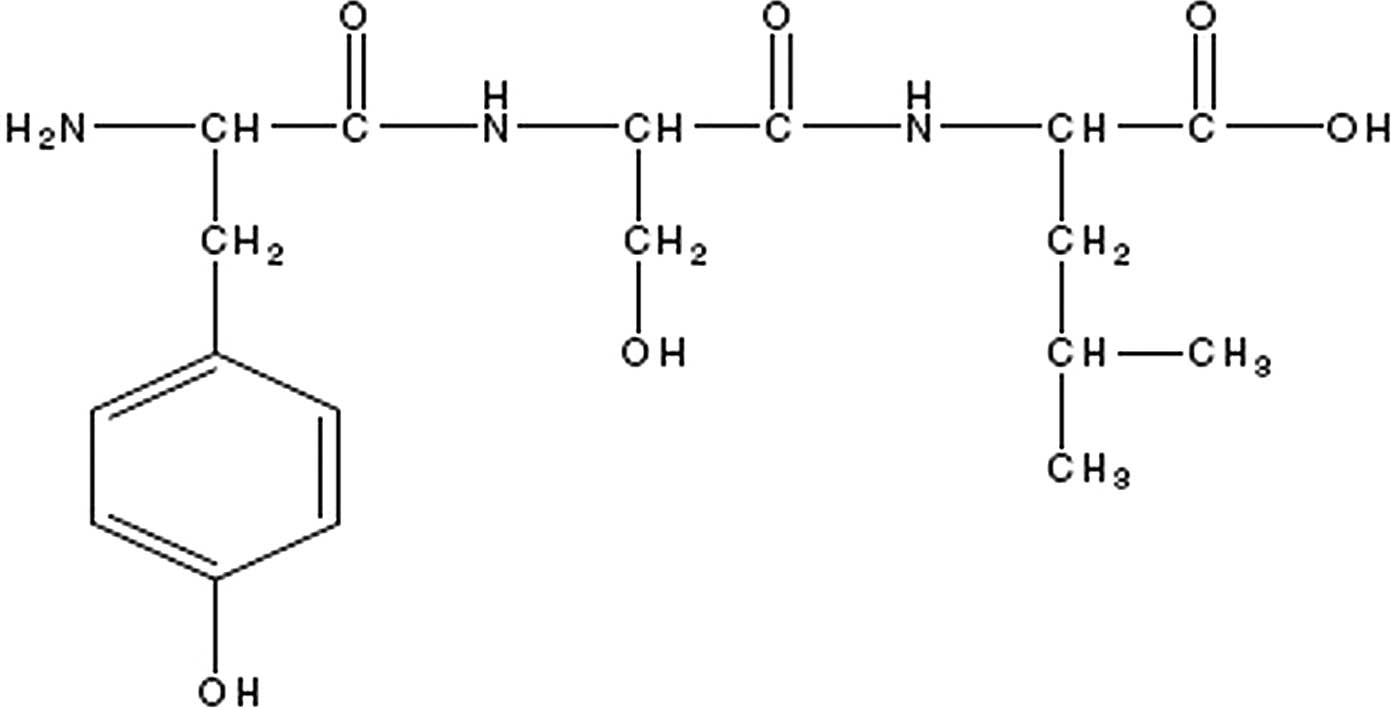
Tyroserleutide (YSL) is a tripeptide compound
extracted from the spleen of pigs. It consists of three natural
amino acids, L-tyrosine, L-serine and L-leucine. Its chemical
structure is shown in Fig. 1. YSL
has exhibited potent antitumor activities in human tumor xenografts
and tumor cell lines (1,2).
However, the exact mechanism by which YSL exerts its
antitumor activity is not yet fully understood. In our previous
study, we observed that YSL induced aptosis and necrosis in
BEL-7402 human hepatocellular carcinoma cells in vitro and
compromised the organelles of the cancer cells by causing
mitochondrial swelling, dissolution and endoplasmic reticulum
cisternae expansion (3,4). These observations prompted us to
investigate the subcellular location of YSL at the cellular level,
with the aim of identifying the pharmacological target implicated
in or responsible for YSL-induced apoptosis.
Due to its crucial role in cell apoptosis, the
mitochondria have emerged as a novel pharmacological target for
anticancer chemotherapy (5,6). A
number of anticancer chemotherapeutic drugs that act on
mitochondrial targets are under investigation. For example, Bcl-2
ligand HA-14, a small molecule inhibitor of the Bcl-2 family
protein, is capable of inducing tumor regression (7). Another mitochondriotoxic lipophilic
cation, F16, has been reported to trigger apoptosis and necrosis of
carcinoma cells (8). This provides
a rationale for investigating the possibility of the mitochondria
as the antitumor target of YSL.
In this study, we focus on establishing the
subcellular location of YSL in hepatocellular carcinoma cells and
the effect of YSL on the isolated mitochondria. Based on these
data, we aimed to identify the pharmacological target of YSL and to
examine the exact mechanism by which YSL exerts its antitumor
activity.
Materials and methods
Cell culture
BEL-7402, a human hepatocellular carcinoma
epithelial cell line (Chinese Medical Academy of Science, Beijing,
China), was grown in RPMI-1640 medium (Gibco Invitrogen Corp.,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Hyclone Corp., South Logan, UT, USA), 75 μg/ml penicillin and 100
μg/ml streptomycin at 37°C, 5% CO2.
YSL fluorescent labeling
YSL (Shenzhen Kangzhe Pharmaceutical Co., Ltd.,
Shenzhen, China) was reacted with
[5-(and-6)-carboxytetramethylrhodamine, succinimidyl ester
(TAMRASE; Biotium, Hayward, CA, USA)] at 4°C overnight. The
bioconjugate was purified by sephadex G-15 chromatographic column
and 20% polyacrylamide gel electrophoresis. Details of the
preparation of the fluorescent conjugate are described in our
previous study (9).
Confocal microscopy
Human hepatocellular carcinoma cells
(1×105/ml) were grown on the cover glass for 24 h, then
treated with 26.2 μM fluorescent labeled YSL for 1 h. After being
washed with D-Hank’s solution (Sigma-Aldrich Corp., Shanghai,
China), the cells were observed under confocal microscopy (Radiance
2000; Bio-Rad Microscience Corp., Hemel Hempstead, Hertfordshire,
UK) using a x60 oil objective lens to examine the subcellular
location of YSL. A Bioptech FCS2 chamber (Bioptech Corp., Butler,
PA, USA) maintained at 37°C was used to examine live cells grown on
glass coverslips. To visualize the subcellular compartments,
Hoechst 33258 was used (2 μg/ ml; Invitrogen Corp.) as a nuclei
marker and Mitotracker green FM (200 nM; Life Technologies Corp.,
Grand Island, NY, USA) as a mitochondrial marker. The lasers that
excited the fluorescent analogue of YSL, nuclei marker and
mitochondrial marker were blue diode 405 nm, Aron 488 nm and Green
HeNe 543 nm, respectively, and the fluorescent signal was collected
using the appropriate filters.
Isolation of cell mitochondria
The BEL-7402 human hepatocellular carcinoma cells
(2×107) were washed three times with PBS (Sigma-Aldrich
Corp.) and centrifuged at 2,500 rpm for 10 min. The supernatant was
discarded and the cell pellets were collected for mitochondrial
isolation. The mitochondria were isolated using a Mitochondria
Isolation kit for Cultured Cells (Pierce Biotechnology, Rockford,
IL, USA). The protein concentration of the mitochondria was
determined with BCA protein assay reagent (Pierce Biotechnology).
The isolated mitochondria were then added to the buffer, which
contained 70 mM sucrose, 230 mM mannitol (Kemiou Chemical Reagent
Corp., Tianjin, China), 3 mM HEPES (Sigma-Aldrich Corp.), 2 mM
Tris-phosphate (Kemiou Chemical Reagent Corp.), 5 mM succinate
(Kemiou Chemical Reagent Corp.) and 1 μM rotenone (Sigma-Aldrich
Corp.).
Mitochondrial potential for isolated
mitochondria (10)
The mitochondrial potential of isolated mitochondria
of BEL-7402 cells was qualitatively assessed using
tetramethylrhodamine methyl ester (TMRM; Sigma-Aldrich Corp.)
fluorescence intensity. TMRM, a lipophilic cation, accumulates
selectively in the mitochondria according to the mitochondrial
membrane potential (Δψm) (11).
The high TMRM concentration will quench the mitochondria
fluorescence, When mitochondria depolarize, TMRM will be released
into the cytosol, and the fluorescence signal increases (12). Mitochondria (0.35 mg/ml) were added
to medium containing TMRM (1.0 μM) and 100 μM YSL, and the
potential was assessed by reversion of quenching of the
fluorescence intensity (excitation/emission 550/575 nm) using a
fluorescent spectrophotometer (F-4500; Hitachi Corp., Japan).
Mitochondrial swelling for isolated
mitochondria (13)
Mitochondria (0.5 mg/ml) were incubated with 100 μM
YSL at 37°C for 1 h. The absorbance at 540 nm was recorded using a
spectrophotometer (F-4500; Shimadzu Corp., Kyoto, Japan) every 10
min. Mitochondrial swelling was measured by decrease in absorbance
at 540 nm.
Results
YSL localizes to mitochondria in BEL-7402
hepatocellular carcinoma cells
First, the YSL fluorescent analogue was used to
determine the subcellular distribution of YSL in BEL-7402
hepatocellular carcinoma cells. As shown in Fig. 2, YSL primarily located in the
cytoplasm was enriched in a definite area. To determine the
subcellular compartment in which YSL accumulated, the cells were
stained with nuclear and mitochondrial dye. As shown in Fig. 3, YSL was not observed in the
nucleus, as demonstrated by a lack of colocalization with
cell-permeant DNA stain Hoechst 33258. However, YSL had a high
degree of colocalization with mitotracker green FM, a specific
mitochondrial probe, indicating that YSL mainly accumulated at the
mitochondria of the BEL-7402 cells.
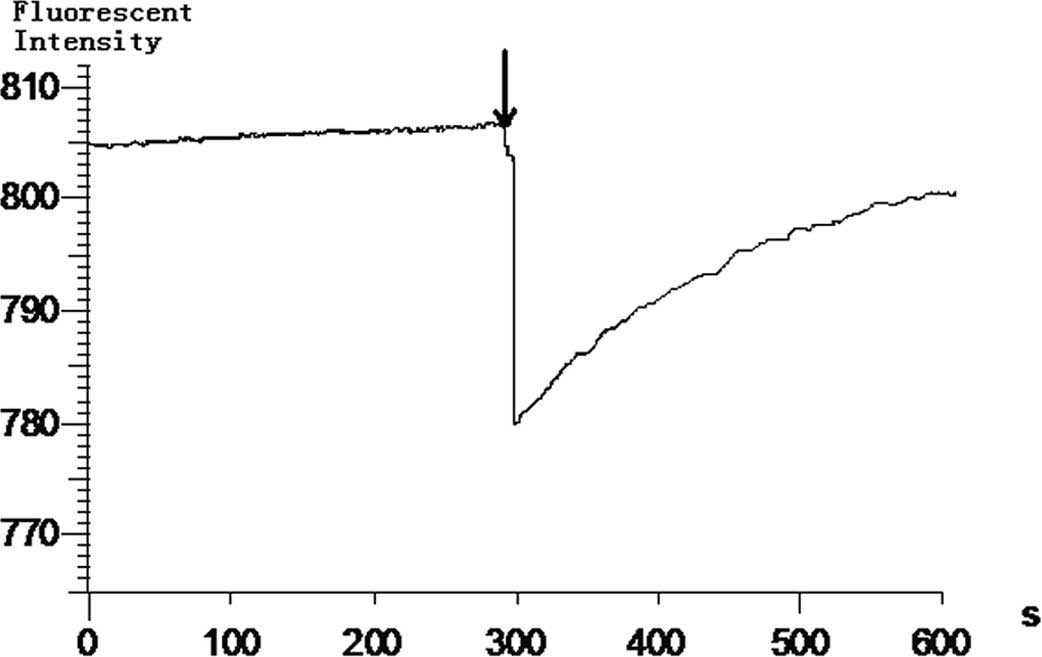
YSL decreases the isolated mitochondrial
potential of BEL-7402 cells
Although we found that YSL damages the mitochondria
of cancer cells, there is no direct evidence that YSL directly
affects the isolated mitochondria. To demonstrate the effect of YSL
on isolated mitochondrial potential, we treated the isolated
mitochondria with a TMRM mitochondrial potential-sensitive probe to
observe the reversion of the fluorescent signal quenching, which
represented the decrease in mitochondrial potential. The results
indicate that YSL decreases the potential of the isolated
mitochondria. When the mitochondria were added to the TMRM
solution, the fluorescent signal was quenched. The fluorescence
intensity was decreased from 805 to 780. The fluorescence intensity
then rose again and continued to rise. When the mitochondria were
added to the TMRM solution for 600 sec, the fluorescence intensity
rose to 800; i.e., 95% of the original intensity (Fig. 4). These data indicate that the
effect of YSL on isolated mitochondria is indicated by the loss of
mitochondrial membrane potential, which in this study appears as an
increase in TMRM fluorescence intensity.
YSL causes swelling of the isolated
mitochondria of BEL-7402 cells
Decrease in the Δψm is reminiscent of the opening of
the mitochondrial permeability transition (MPT) pore, a key
phenomenon in cell death by apoptosis and necrosis. The extensive
and prolonged opening of the pore responsible of MPT causes the
dissipation of the Δψm as well as swelling of the mitochondrial
matrix (14). Since YSL induces
the disruption of Δψm, we investigated whether YSL provoked Δψm
changes dependent on MPT pore opening. The early involvement of the
MPT pore opening in YSL may have direct effects on isolated
mitochondria. Since the mitochondrial size can be monitored by the
changing of the absorbance at 540 nm, we applied spectrophotometry
to analyze the mitochondrial size on isolated mitochondria purified
from BEL-7402 cancer cells. The results indicated that YSL causes
the isolated mitochondria of BEL-7402 cells swelling. After 100 μM
YSL was incubated with isolated mitochondria for 5 min, the
absorbance at 540 nm was decreased from 1.301 to 1.186. As the
incubation time reached 60 min, the absorbance decreased to 1.091,
which indicates that the mitochondria size increased and began to
swell (Fig. 5).
Discussion
This study examined a novel facet of the subcellular
distribution of the tripeptide YSL, which possesses a marked
antitumor effect. In our previous study, we observed that YSL acted
as a potent inducer of apoptosis to kill the BEL-7402
hepatocellular carcinoma cells. However, the exact target of the
antitumor effect of YSL has yet to be elucidated. YSL belongs to a
family of antitumor polypeptides. Due to their marked antitumor
effect and lower toxicity, polypeptides with antitumor effects have
attracted the attention of investigators studying tumor therapy
(15,16). Although the chemical composition of
these peptides is similar, their pharmaceutical targets are quite
different. Several peptides react with the receptor, which is
located at the cell membrane, to stimulate the downstream pathway
to kill the cells (17,18). Certain drugs act directly on the
intracellular organelles to produce an antitumor effect (19,20).
Therefore, variations in the subcellular distribution of the
antitumor polypeptide drug in the target cells will determine the
various targets of particular antitumor drugs. Thus, we were
prompted to investigate the subcellular distribution of YSL to
further investigate the target of the antitumor effect.
In our study, we synthesised a fluorescent analogue
of YSL using a fluorescence stain [5-(and
-6)-carboxytetramethylrhodamine, succinimidyl ester] to trace YSL
in BEL-7402 human hepatocellular carcinoma cells (9). The results revealed that YSL was
primarily located at the cytoplasm, whereas the YSL distribution
was absent from the cell membrane. These findings indicate that the
target of YSL may not be at the membrane of the cells, but in the
cancer cells. Since YSL, which consists of tyrosine, serine and
leucine, is a water-soluble peptide, it may be passively
transported into the tumor cells.
To continue tracing YSL when it enters the tumor
cells, we set up a colocalization method. The fluorescent analogue
of YSL integrated with other two organelles: A fluorescent probe
was used to discover the exact location of YSL in cancer cells
under laser scanning confocal microscopy. From the extent of the
merging of the YSL fluorescence with the cell organelles, we
speculated on the kind of organelle at which YSL was located.
Mounting evidence has revealed that YSL induces the
apoptosis of cancer cells affecting various cellular components,
including mitochondria, endoplasmic reticulum, histone, protein
kinases and phosphates (1–4). Our study identifies the mitochondria
as a new pro-apoptotic target of YSL. In the investigation of the
location of YSL at the mitochondria, we found that YSL colocalizes
with the mitochondria. Indeed, according to our findings, YSL is
likely to induce the apoptosis of cancer cells by directly
targeting the mitochondria.
As mentioned in the Introduction, the mitochondrion
has become a new pharmaceutical target in tumor therapy. Since
mitochondria play a significant role in cell apoptosis, drugs that
compromise the structure or function of mitochondria will provide
opportunities to kill cancer cells if they can be specifically
delivered to the tumor site (21,22).
In our previous study on YSL-induced cancer cell apoptosis, we
found that YSL can cause mitochondrial dysfunction (data not
shown). Despite the significance of the mitochondria for the
induction of YSL-mediated apoptosis, the results did not explain
the mechanism by which YSL activated the mitochondrial pathway.
Therefore, we tested this hypothesis on isolated mitochondria. In
this study, we found that when YSL acted on isolated mitochondria,
it collapsed the Δψ and caused mitochondrial swelling. The
dissipation of the Δψ as well as swelling of the mitochondrial
matrix were the results of the extensive and prolonged opening of
the pore responsible for MPT (23). Therefore, we speculate that the
anticancer effect of YSL may involve the opening of the MPT.
Further research should be conducted to verify this conclusion.
On theoretical grounds, an agent that directly
targets the mitochondria acts on more downstream levels of
apoptosis control and may be advantageous for treating cancers, in
which such signal transducing systems are interrupted. Therefore,
the identification of YSL and its analogue, which directly affects
mitochondria, is of substantial clinical interest.
At present, a number of observations have indicated
that the antitumor effects of polypeptides involve multiple
pathways and multiple targets (24). This study is limited to the
subcellular location and the correlation between the direct effect
of mitochondria and the antitumor effect of YSL. We will
investigate the anticancer mechanism of YSL to clarify the real
target of YSL.
In conclusion, we have demonstrated that
mitochondria may be the subcellular location of YSL, and YSL
directly causes the dissipation of the Δψ as well as mitochondrial
swelling. These preliminary findings will contribute to further
investigation of the real target of YSL and be beneficial in
clinical cancer treatment and the development of the other relevant
new drugs.
Acknowledgements
This study was supported by grants
from the National High Technology Research and Development Program
of China (2005AA2Z3D40) (863 Program).
References
|
1.
|
Z YaoR LuJ JiaThe effect of tripeptide
tyroserleutide (YSL) on animal models of
hepatocarcinomaPeptides2711671172200610.1016/j.peptides.2005.02.02616129512
|
|
2.
|
R LuJ JiaL BaoExperimental study of the
inhibition of human hepatocarcinoma Bel7402 cells by the tripeptide
tyroserleutide (YSL)Cancer Chemother
Pharmacol57248256200610.1007/s00280-005-0046-z16028100
|
|
3.
|
Z FuR LuG LiTyroserleutide tripeptide
affects calcium homeostasis of human hepatocarcinoma BEL-7402
cellsSci China C Life Sci48523530200510.1360/062004-13216315604
|
|
4.
|
L ZhaoQ ZhaoR LuEffects of tyroserleutide
on gene expression of calmodulin and PI3K in hepatocellular
carcinomaJ Cell Biochem103471478200810.1002/jcb.2140917546603
|
|
5.
|
J YaoZ JiangW DuanInvolvement of
mitochondrial pathway in triptolide-induced cytotoxicity in human
normal liver L-02 cellsBiol Pharm
Bull31592597200810.1248/bpb.31.59218379047
|
|
6.
|
P CostantiniE JacototD DecaudinG
KroemerMitochondrion as a novel target of anticancer chemotherapyJ
Natl Cancer Inst9210421053200010.1093/jnci/92.13.104210880547
|
|
7.
|
BC TurnerT EvesY RefaeliSmall-molecule
inhibitors of Bcl-2 family proteins are able to induce tumor
regression in a mouse model of pre-B cell acute lymphocytic
lymphomaDNA Cell Biol27133142200810.1089/dna.2007.067518163880
|
|
8.
|
VR FantinP LederF16, a mitochondriotoxic
compound, triggers apoptosis or necrosis depending on the genetic
background of the target carcinoma cellsCancer
Res1329336200410.1158/0008-5472.CAN-03-089914729642
|
|
9.
|
X JianZ FuYL ZhangSynthesis of
tyroserleutide fluorescent analogue and its application on the
target research of antitumor therapyProg Biochem
Biophys35116111672008
|
|
10.
|
RC Scaduto JrLW GrotyohannMeasurement of
mitochondrial membrane potential using fluorescent rhodamine
derivativesBiophys
J76469477199910.1016/S0006-3495(99)77214-09876159
|
|
11.
|
B EhrenbergV MontanaMD WeiJP WuskellLM
LoewMembrane potential can be determined in individual cells from
the nernstian distribution of cationic dyesBiophys
J53785794198810.1016/S0006-3495(88)83158-83390520
|
|
12.
|
P KorgeJI GoldhaberJN WeissPhenylarsine
oxide induces mitochondrial permeability transition,
hypercontracture and cardiac cell deathAm J Physiol Heart Circ
Physiol280H2203H2213200111299223
|
|
13.
|
K ZhaoGM ZhaoD WuY SoongAV BirkPW
SchillerHH SzetoCell-permeable peptide antioxidants targeted to
inner mitochondrial membrane inhibit mitochondrial swelling,
oxidative cell death, and reperfusion injuryJ Biol
Chem2793468234690200410.1074/jbc.M402999200
|
|
14.
|
JJ LemastersAL NieminenT QianThe
mitochondrial permeability transition in cell death: a common
mechanism in necrosis, apoptosis and autophagyBiochim Biophys
Acta1366177196199810.1016/S0005-2728(98)00112-19714796
|
|
15.
|
N DiasC BailyDrugs targeting mitochondrial
function to control tumor cell growthBiochem
Pharmacol70112200510.1016/j.bcp.2005.03.02115907809
|
|
16.
|
KM DebatinD PoncetG KroemerChemotherapy:
targeting the mitochondrial cell death
pathwayOncogene2187868803200210.1038/sj.onc.120603912483532
|
|
17.
|
HC HarshaA JimenoH MolinaActivated
epidermal growth factor receptor as a novel target in pancreatic
cancer therapyJ Proteome
Res746514658200810.1021/pr800139r18821783
|
|
18.
|
PO CheneJO FuchsJ BohnA small synthetic
peptide which inhibits the p53-hdm2 interaction stimulates the p53
pathway in tumor cell linesJ Mol
Biol299245253200010.1006/jmbi.2000.373810860736
|
|
19.
|
LC PapadopoulouAS TsiftsoqlouMitochondrial
cytochrome c oxidase as a target site for daunomycin in K-562 cells
and heart tissueCancer Res531072107819938382552
|
|
20.
|
V FogalL ZhangS KrajewskiE
RuoslahtiMitochondrial/ cell-surface protein p32/gC1qR as a
molecular target in tumor cells and tumor stromaCancer
Res6872107218200810.1158/0008-5472.CAN-07-675218757437
|
|
21.
|
R RotemA HeyfetsO FingrutD BlicksteinM
ShaklaiE FlescherJasmonates: novel anticancer agents acting
directly and selectively on human cancer cell mitochondriaCancer
Res6519841993200510.1158/0008-5472.CAN-04-309115753398
|
|
22.
|
JC LaiW TanL BenimetskayaP MillerM
ColombiniCA SteinA pharmacological target of G3139 in melanoma
cells may be the mitochondrial
VDACPNAS10374947499200610.1073/pnas.060221710316648253
|
|
23.
|
Y TsujimotoS ShimizuRole of the
mitochondrial membrane permeability transition in cell
deathApoptosis12835840200710.1007/s10495-006-0525-717136322
|
|
24.
|
S FaivreC DelbaldoK VeraSafety,
pharmacokinetic, and antitumor activity of SU11248, a novel oral
multitarget tyrosine kinase inhibitor, in patients with cancerJ
Clin Oncol2445200610.1200/JCO.2005.02.219416314617
|