Introduction
The pathogenesis of lung cancer and the criteria
that regulate its progression are under investigation. Pulmonary
lesions, such as small nodules with focal ground-glass opacity
(GGO), have been increasingly detected due to the widespread use of
computed tomography (CT) scanning. Histologically, these lesions
can be classified as atypical adenomatous hyperplasia (AAH),
bronchioalveolar carcinoma (BAC) or adenocarcinoma (AC). Several
studies have suggested that AAH, frequently found in tissue
surrounding lung AC, may be a forerunner in the development of AC;
moreover, the more recent discovery of lung nodules manifesting as
GGOs further supports a stepwise process in the development of
pulmonary AC (1–3). However, the genetic relationship
between AC and the associated foci of AAH is not yet well defined.
In particular, it is not clear whether multiple foci of AAH and AC
in the same patients are clonally related or are independent
neoplastic foci (4). Several
studies performed loss of heterozygosity (LOH), fluorescence in
situ hybridization (FISH), microarrays and immunohistochemistry
analyses and demonstrated an increasing genetic complexity
associated with lung cancer progression (4–7) but,
to the best of our knowledge, no cytogenetic study showing a clear
clonal relationship among AC, BAC and AAH has been reported thus
far.
We report the case of a patient histologically
diagnosed with AC in the superior right lobe and BAC in the
inferior lobe, previously identified as a pure GGO nodule by a CT
scan. AAH was diagnosed in the middle lobe, considered to be normal
at CT scan and during surgery. The cytogenetic studies performed on
biopsies from the three lobes allowed the identification of
different chromosome rearrangements with clonal evolution that
supports the hypothesis of a complex multi-step carcinogenesis in
which lung AC develops from AAH through BAC.
Case report
A 54-year-old female who never smoked was diagnosed
with a lung tumor in the upper right lobe by a CT scan. A
transthoracic fine-needle aspiration of the lesion was performed,
followed by histological diagnosis of AC with a BAC component. The
CT scan also showed a pure GGO lesion in the lower lobe, while the
middle lobe appeared to be normal. The patient underwent
pneumonectomy and samples from the 3 lung lobes were sent to the
cytogenetic laboratory. Cytogenetic analyses were performed on
spontaneous metaphases obtained by the direct method and short-term
cultures as previously reported (8). The normal appearing middle lobe
showed high spontaneous replication activity after 24 h of
incubation, with 9 of 9 cells presenting a normal karyotype. The
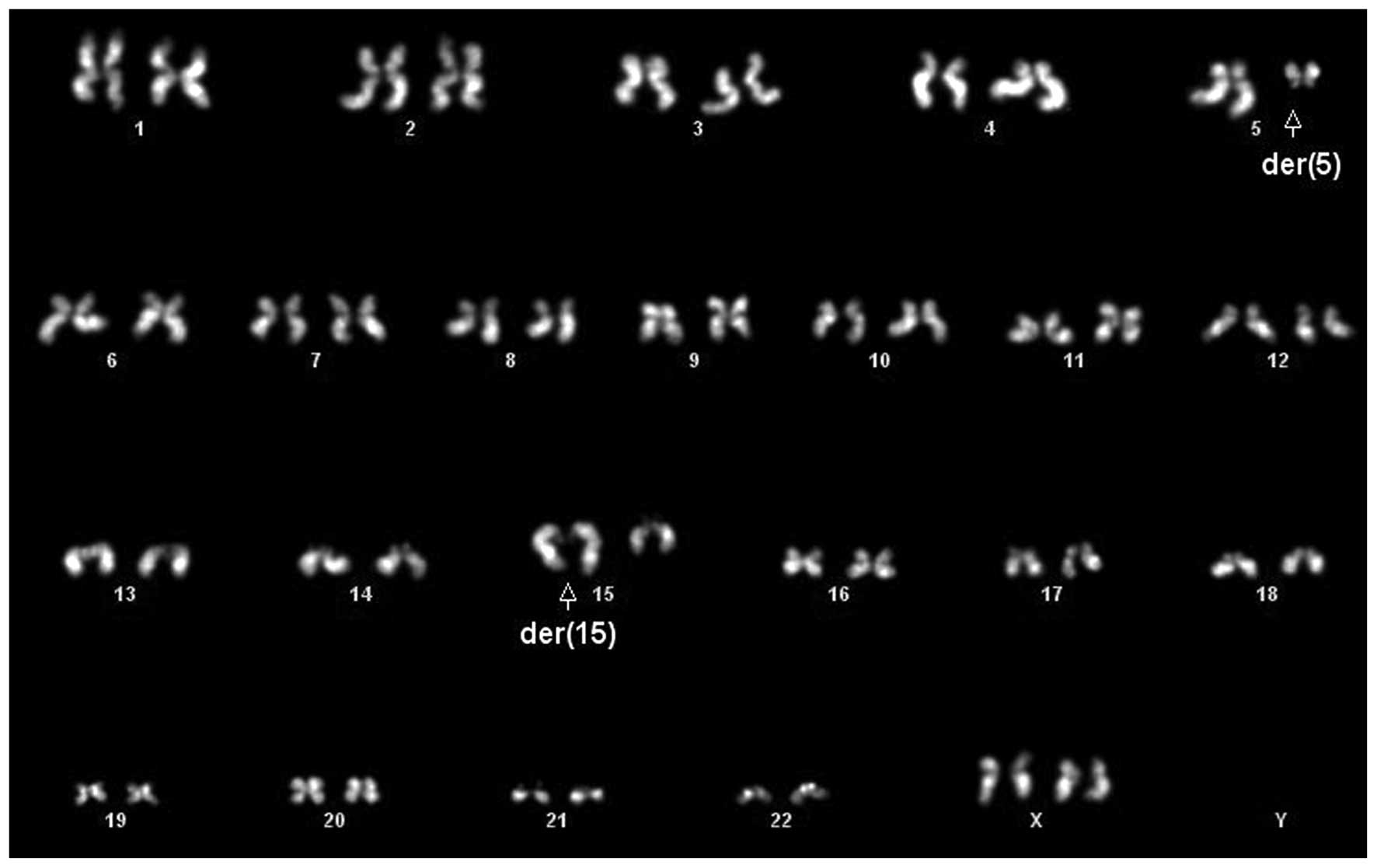
GGO lesion had 4 cells with a t(5;15)(q13;q25-26) as a single
anomaly (Fig. 1), 2 cells with the
t(5;15) translocation with complex rearrangements, including a
derivative chromosome 1 with unknown additional material on the
short arm, and 4 cells with a normal karyotype. In the AC, the same
rearrangements present in the GGO were observed in 9 of 9
metaphases, 2 of which included the t(5;15). Most of the metaphases
showing complex rearrangements were incomplete and in these cases
we defined a composite karyotype following the ISCN recommendations
based on the recurrent abnormalities observed (9): 44∼46,X,del(X) (p11.2),der(1)add(1)(p32),t(5;15)(q13;q25-26) [cp9].
The karyotypes obtained from all samples after
short-term cultures (5–7 days) were normal, indicating that the
tumor cytogenetic profile is rapidly obscured in short-term
cultures due to a selective advantage of karyotypically normal
cells, as previously reported (8).
Since a fusion gene between echinoderm
microtubule-associated protein-like 4 (EML4) and anaplastic
lymphoma kinase (ALK) has been identified in a subset of
non-small cell lung cancer patients who never smoked (10), FISH was performed on our specimens
using the ALK (2p23) break probe and ALK/EML4 t(2;2);
inv(2) Fusion Probe (Poseidon™, a
gift from Kreatech Diagnostics, The Netherlands). A normal pattern
was observed. This case report was part of a research project
approved by the local ethics committee (authorization No. 850), and
informed patient consent was obtained.
Discussion
The molecular drivers that determine histology in
lung cancer remain largely unknown and it is difficult to identify
a valid parameter of tumor aggressiveness that may be used as a
prognostic factor. The hypothesis of a multi-step carcinogenic
process has recently been supported by the observations of Min
et al (11) in a patient
over a 10-year follow-up period, in whom CT and PET imaging
findings showed the progression from a focal pure GGO nodule
(presumed to be AAH or BAC) to an invasive AC.
The cytogenetic findings in our case support this
hypothesis. The observation of active replication with spontaneous
metaphases obtained after a few hours of incubation in the biopsy
from the middle lobe suggests that in the normal appearing tissue
the cell cycle control was lost. It is well known that cancer is a
disease of hyper-proliferation predisposing to chromosome
instability and this may have led to the first rearrangement we
identified in the sample from the GGO, the translocation
t(5;15)(q13;q25-26). Notably, the breakpoint at band q13 in the
long arm of chromosome 5 is the same as we observed in a
constitutional pericentric inversion previously reported in a
patient with a pure GGO lesion (12). This band contains at least 4 genes
(CCNB1, CDK7, CENPH, RAD17) encoding important regulators of the
cell cycle that could be disrupted by the chromosome rearrangement
(13). Moreover, a genome-wide
association study reported that the chromosome 15q25.1 region,
which includes three nicotinic cholinergic receptor genes (CHRNA5,
CHRNB4, CHRN) and cell proliferation gene (PSMA4), is associated
with lung cancer risk in Caucasian individuals irrespective of
smoking status or propensity to smoke tobacco (14). In our case, the clone with the
complex karyotype which was present in a few cells from the GGO and
in all the cells from the AC probably had a strong proliferative
advantage on the clones harboring the t(5;15). This is in agreement
with the well-known observation that the complexity of chromosomal
aberrations in cancer is correlated with the aggressiveness of the
disease.
Common gene variants involved in lung cancer have
been recently identified through large, collaborative, genome-wide
association studies. Three loci markedly associated with lung
cancer susceptibility have been reported: 5p15, 6p21 and 15q25,
where genes that regulate acetylcholine niconitic receptors and
telomerase production are located (15). In the present case, two of these
three relevant regions were involved in chromosome rearrangements
that may either cause gene inactivation or dysregulation,
supporting their crucial role in the disease.
To the best of our knowledge, this is the first
study to report a clonal relationship among AC, BAC and AAH,
present simultaneously in different lobes of the same lung. This
case suggests that the entire lung was somehow prone to the
neoplastic transformation, possibly primed by cells with high
proliferative activity such as those present in the middle lobe
affected by AAH. Genetic studies of multiple lesions present in the
same lung should be performed in order to verify this
hypothesis.
Acknowledgements
The authors wish to thank Dr Robert
Nicholls for the critical reading of the manuscript.
References
|
1
|
Kitamura H, Kameda Y, Ito T and Hayashi H:
Atypical adenomatous hyperplasia of the lung. Implications for the
pathogenesis of peripheral lung adenocarcinoma. Am J Clin Pathol.
111:610–622. 1999.PubMed/NCBI
|
|
2
|
Henschke CI, Yankelevitz DF, Mirtcheva R,
McGuinness G, McCauley D and Miettinen OS; ELCAP Group: CT
screening for lung cancer: frequency and significance of part-solid
and nonsolid nodules. AJR Am J Roentgenol. 178:1053–1057. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakata M, Saeki H, Takata I, Segawa Y,
Mogami H, Mandai K and Eguchi K: Focal ground-glass opacity
detected by low-dose helical CT. Chest. 121:1464–1467. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morandi L, Asioli S, Cavazza A, Pession A
and Damiani S: Genetic relationship among atypical adenomatous
hyperplasia, bronchioloalveolar carcinoma and adenocarcinoma of the
lung. Lung Cancer. 56:35–42. 2007. View Article : Google Scholar
|
|
5
|
Kerr KM, Carey FA, King G and Lamb D:
Atypical alveolar hyperplasia: relationship with pulmonary
adenocarcinoma, p53, and c-erbB-2 expression. J Pathol.
174:249–256. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomida S, Yatabe Y, Yanagisawa K,
Mitsudomi T and Takahashi T: Throwing new light on lung cancer
pathogenesis: updates on three recent topics. Cancer Sci. 96:63–68.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sano T, Kitayama Y, Igarashi H, Suzuki M,
Tanioka F, Chida K, Okudela K and Sugimura H: Chromosomal numerical
abnormalities in early stage lung adenocarcinoma. Pathol Int.
56:117–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bettio D, Rizzi N, Giardino D, Persani L,
Pecori-Giraldi F, Losa M and Larizza L: Cytogenetic study of
pituitary adenomas. Cancer Genet Cytogenet. 98:131–136. 1997.
View Article : Google Scholar
|
|
9
|
ISCN 2009: An international system for
human cytogenetic nomenclature. Shaffer LG, Slovak ML and Campbell
LJ: Karger; Basel: pp. 1–138. 2009
|
|
10
|
Mano H: Non-solid oncogenes in solid
tumors: EML4-ALK fusion genes in lung cancer. Cancer Sci.
99:2349–2355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Min JH, Lee HY, Lee KS, Han J, Park K, Ahn
MJ and Lee SJ: Stepwise evolution from a focal pure pulmonary
ground-glass opacity nodule into an invasive lung adenocarcinoma:
An observation for more than 10 years. Lung Cancer. 69:123–126.
2010.PubMed/NCBI
|
|
12
|
Bettio D, Venci A, Cariboni U, Di Rocco M
and Infante M: Fluorescent in situ hybridization (FISH) in the
differential diagnosis of ground-glass opacities in the lung. Lung
Cancer. 71:319–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
UCSC Genome Browser (database online).
Santa Cruz: University of California, Genome Bioinformatics Group;
2003. Updated February, 2009. Available at: http://genome.ucsc.edu/cgi-bin/hgGatewayuri.
Accessed September 22, 2011.
|
|
14
|
Hung RJ, McKay JD, Gaborieau V, Boffetta
P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N,
Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L,
Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G,
Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F,
Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F,
Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S,
Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P,
Trichopoulos D, Holcátová I, Merletti F, Kjaerheim K, Agudo A,
Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A,
Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D,
Matsuda F, Blanche H, Gut I, Heath S, Lathrop M and Brennan P: A
susceptibility locus for lung cancer maps to nicotinic
acetylcholine receptor subunit genes on 15q25. Nature. 452:633–637.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brennan P, Hainaut P and Boffetta P:
Genetics of lung-cancer susceptibility. Lancet Oncol. 12:399–408.
2011. View Article : Google Scholar
|