Introduction
Transjugular intrahepatic portosystemic stent shunt
(TIPS) is an effective treatment for esophageal variceal hemorrhage
caused by portal hypertension (1,2). It
has been demonstrated that compared with medical treatment and
endoscopic treatment, the early use of a TIPS in patients with
hemorrhages improves the survival rate significantly. However, the
TIPS technique was previously difficult to apply due to the low
long-term patency rate of the bare metal stents and the cost of
re-intervention (3–5). An estimated 80% of the world’s TIPS
are now created with Viatorr stent grafts and numerous
retrospective, prospective and randomized trials comparing their
efficacy with those of bare stents have identified that long-term
patency rates have increased significantly (6–9).
however, it remains necessary to investigate new and more
convenient stent grafts. Studies have revealed that stent
thrombosis, pseudointimal hyperplasia, ingrowth of liver tissue
into the shunt and hemodynamic disorders caused by stent
angulations result in postoperative shunt stenosis and obstruction
(10). Theoretically, the
structure of a covered stent such as the FLUENCY expanded,
polytetrafluoroethylene (PTFE)-covered stent may avoid stent
dysfunction, including bile leakage, thrombosis and abnormal
pseudointimal proliferation (11–14).
Although the use of covered stents in TIPS has been rare, it is
becoming increasingly valued. Therefore the efficacy of FLUENCY
expanded, PTFE-covered stents was explored in the present
study.
Materials and methods
General materials
The FLUENCY PTFE-coated self-expanding nitinol stent
manufactured by BARD (Murray Hill, NJ, USA) was used for 114
patients in TIPS surgery, including 77 males and 37 females with an
average age of 54±14 years. The patients all suffered from hepatic
cirrhosis decompensation with portal hypertension. There were 92
cases of pure esophageal variceal disruption hemorrhage, 8 of pure
refractory cirrhotic ascites and 14 of esophageal variceal
disruption hemorrhage with refractory ascites. According to the
Child-Pugh Liver function class, there were 29 cases of class A
liver function, 68 of class B and 34 of class C. Written and
informed consent was obtained from every patient and the study was
approved by the ethics review board of Guiyang Medical College
(Guiyang, China).
Preoperative preparation
Cardiopulmonary, liver and coagulation functions
were analyzed prior to TIPS to exclude surgical contraindications.
Hypoproteinemia and coagulation disorders were corrected. Enhanced
CT scans of the abdomen and other images were examined to observe
the location and anatomical associations of the portal vein and its
branches, while excluding the thrombosis of the portal vein and
inferior vena cava and portal vein cavernous transformation. In
cases without enhanced CT scans, indirect portal venography was
performed prior to the TIPS procedure.
Operative technique
The right-internal jugular vein was selected and the
right or left branch was punctured through the right-hepatic or
hepatic vein in order to measure the portosystemic pressure
gradient (PSG) and select a balloon catheter to expand the
portosystemic shunt. When the PSG decreased to <1.176 cmHg, the
covered stent was implanted in accordance with the corresponding
diameter. The parenchymal section and the hepatic vein side of the
shunt required complete coverage of the stents. The varicose
gastric coronary vein was embolized intraoperatively. Stents with a
diameter of 8 mm and length of 60 mm were implanted. Bare metal
stents were implanted into 15 patients and covered stents of the
same diameter were also implanted simultaneously in the hepatic
vein side.
Postoperative treatment
The patients were required to avoid dietary protein
for 2 weeks to maintain smooth stools. Low-molecular weight heparin
was administered via subcutaneous injection according to the
coagulation conditions. Antiplatelet therapy with 75 mg clopidogrel
was administered orally each day. Coagulation was monitored during
the medication period.
Follow-up
During the follow-up, recurrent bleeding, ascites
and complications were observed. At 7 days, 1, 3 and 6 months and 1
year following TIPS implantation, Doppler ultrasound was performed
to examine the shunt. After that time, liver Doppler ultrasound
examination was performed every 6 months. Follow-up lasted until
March 30, 2012. When recurrent gastrointestinal bleeding occurred
or ultrasound examination revealed shunt dysfunction, portography
was performed and intervention applied with the use of balloon
dilation and bare metal stent implantation to support the narrow
shunt. Loss of follow-up, mortality and the emergence of shunt
dysfunction were all classified as follow-up termination.
Statistical analysis
Shunt patency rates were assessed using a
Kaplan-Meier survival curve. The portal venous pressure and mean
pressure gradient were assessed with the paired t-test. P<0.01
was considered to indicate a statistically significant
difference.
Results
Clinical efficacy
All patients achieved a 100% technical success. The
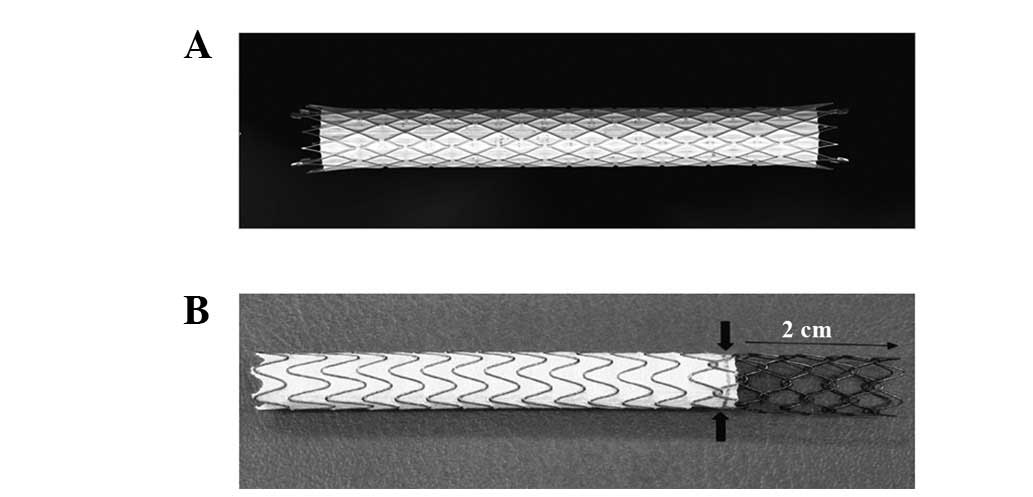
covered stents (Fig. 1A) in the
114 cases were 8 mm in diameter. Bare metal stents of the same
diameter were implanted in 15 cases simultaneously. The mean portal
venous pressure dropped from 2.499±0.588 cmHg to 1.764±0.294
cmHg.
The varicose gastric coronary vein was also
embolized in the procedure so that 37 of the 40 patients stopped
bleeding within 24 h after emergency surgery. Seven days after
surgery, three patients succumbed to various causes: one patient
with alcoholic cirrhosis had continuous heavy bleeding 24 h after
surgery and succumbed to disseminated intravascular coagulation
(DIC) and multiple organ failure within 48 h; one patient stopped
bleeding but succumbed to multiple organ failure on the third day
after the surgery; and one patient had intraoperative splenic vein
occlusion so that the coronary vein embolization could not be
performed. The patient had 24 h of bleeding, and succumbed to DIC
and multiple organ failure within 72 h. Eight cases of pure
refractory ascites subsided significantly after two days. Two of
the 14 patients with gastrointestinal bleeding and massive ascites
succumbed after one week. Of the remaining 12 cases, ascites were
significantly reduced in eight cases after two weeks and ascites
were reduced slightly in one other case.
One patient with pure refractory ascites exhibited
symptoms of hypovolemic shock 3 h after surgery with significant
abdominal distension. Diagnostic peritoneal puncture revealed a
bloody fluid that was not solidified. Laparotomy surgery was
performed 4 h after TIPS and revealed a petechia on the top right
of the hepatoduodenal ligament with a visible break of 2-mm
diameter. Varicose veins on the right side of the hepatic portal
were ruptured and bleeding and ascites subsided after one month of
treatment. Besides this, operation-associated complications were
not observed. Hepatic encephalopathy occurred in 23 patients after
one month, of which two cases were stage IV and improved following
medical treatment, while the remaining 21 cases exhibited stage I
or II hepatic encephalopathy, dizziness, drowsiness, confusion and
abnormal behaviors. All the symptoms disappeared following medical
treatment.
With the exception of cases of lost follow-up and
mortality from of other causes, 19 cases of recurrent bleeding were
observed between postoperative days 3 and 1597, and three patients
succumbed within seven days. A further 16 cases were confirmed as
shunt occlusion or stenosis using liver Doppler ultrasound or
portal venography. Of these 16 cases, five were thrombosis due to
stenosis of the hepatic vein, three were thrombosis caused by
portal vein blockage due to the cover of the stent and eight were
thrombosis due to the cover of the stent overlay on the portal vein
wall, resulting from the small angle between the stent portal end
and portal vein wall. In two of the 16 patients, TIPS was performed
again in the left branch of the portal vein via hepatic vein
puncture, while in 12 cases the occluded portal vein was opened and
expanded by a shunt balloon, with a bare metal stent implanted
temporarily to support the hepatic or portal vein side. The
remaining two cases failed to be treated due to widely occurring
thrombosis in the portal vein.
Shunt patency
In the present study, the one-year cumulative
patency rate was 86.7% and the two-year patency rate was 75.2%
(Fig. 2). These were slightly
higher than the patency rate of the dedicated TIPS Viatorr stent
reported previously (15) and
significantly higher than previously reported bare-metal stent
patency rates (16–17). There was a significant difference
between the covered and bare stents but no significant difference
was observed between the covered and Viatorr stents.
Discussion
In the present study, the one-year cumulative
patency rate was slightly higher than the patency rates of
dedicated TIPS stents revealed in previous studies and was
significantly higher than previously reported bare metal stent
patency rates (15–17). The covered stent was almost
entirely covered with a PTFE membrane, leaving exposed sections
only at each end with lengths of 2 mm (Fig. 1A), in order to facilitate the
placement of tantalum markers. However, the dedicated Viatorr stent
has a 2 cm uncovered section on the portal vein side, which is
separated by metal rings and a gold marker (Fig. 1B). Due to the great tissue
compatibility of PTFE, it did not stimulate thrombosis, prevented
bile leakage and provided a good matrix for neointimal coverage of
the stent surface. Thus the rate of shunt patency was improved.
The treatment of bleeding in the present study was
also satisfactory, with 37 out of 40 cases of acute bleeding being
effectively controlled. However the occurrence of hepatic
encephalopathy was not avoided or resolved by the TIPS technique.
On the basis of effectively reducing the portal pressure and PSG, a
small-diameter stent would theoretically reduce the occurrence and
extent of hepatic encephalopathy. Compared with the bare metal
stents reported previously, although the patency rate in the
present study was improved, the incidence of hepatic encephalopathy
was not reduced. Early in the present study, a 10-mm stent was
adopted to control the portal vein pressure. Afterwards 8-mm stents
were selected since a reduction of the shunt volume may
theoretically control the incidence of hepatic encephalopathy.
However, a comparative study of the incidences of hepatic
encephalopathy between various diameter shunts was not performed
due to the limited number of cases. Saxon et al(18) considered the 8-mm stent to be the
best choice for both the prevention of hepatic encephalopathy and
appropriate diversion but it is unknown whether it is suitable for
the physiological characteristics of Asian populations or hepatitic
cirrhosis. Whether ≤7-mm stents are more suitable in Asian
populations for reducing the incidence of hepatic encephalopathy
while maintaining a stable shunt volume, requires larger samples of
clinical case observations and studies.
In practical applications, fully-covered FLUENCY
stents have the following two benefits: i) simplified surgical
procedures; and ii) fully-covered structures which reduce the
incidence of portal vein hemodynamic disorder. However,
fully-covered structures and the woven metal structure also have
certain problems. Firstly, when the stent is too deep in the portal
vein, the stent cover may overlay the portal vein which may lead to
portal vein thrombosis and shunt dysfunction. Three cases of
obstruction of the portal vein branch occurred in the present
study, resulting in significant increases in PSG. The Viatorr stent
is effective for avoiding this situation and the structure of bare
metal stents in the portal vein side enables the covered section to
maintain a certain distance, thus preventing the ‘cap’ and reducing
the risk of cover obstruction of the portal vein branch. Therefore,
further study of the association between two stents is
necessary.
References
|
1
|
Syed MI, Karsan H, Ferral H, et al:
Transjugular intrahepatic porto-systemic shunt in the elderly:
Palliation for complications of portal hypertension. World J
Hepatol. 4:35–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Theophilidou E, Waraich N, Raza T and
Agarwal PK: Liver metastases, a rare cause of portal hypertension
and stoma bleeding. Brief review of literature. Int J Surg Case
Rep. 3:173–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pua U and Punamiya S: Transjugular
intrahepatic portosystemic shunt occlusion via modified Pringle
maneuver for radiofrequency ablation of nearby tumor. J Vasc Interv
Radiol. 23:563–565. 2012. View Article : Google Scholar
|
|
4
|
Rosemurgy AS, Frohman HA, Teta AF, et al:
Prosthetic H-Graft portacaval shunts vs transjugular intrahepatic
portasystemic stent shunts: 18-year follow-up of a randomized
trial. J Am Coll Surg. 214:445–453. 2012.PubMed/NCBI
|
|
5
|
Riggio O, Nardelli S, Moscucci F, et al:
Hepatic encephalopathy after transjugular intrahepatic
portosystemic shunt. Clin Liver Dis. 16:133–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haskal ZJ, Davis A, McAllister A and Furth
EE: PTFE encapsulated endovascular stent-graft for transjugular
intrahepatic portosystemic shunts: experimental evaluation.
Radiology. 205:682–688. 1997. View Article : Google Scholar
|
|
7
|
ter Borg PC, Hollemans M, Van Buuren HR,
et al: Transjugular intrahepatic portosystemic shunts: long-term
patency and clinical results in a patient cohort observed for 3–9
years. Radiology. 231:537–545. 2004.PubMed/NCBI
|
|
8
|
Narayan RL, Vaishnava P and Kim M: Radial
artery perforation during transradial catheterization managed with
a coronary polytetrafluoroethylene-covered stent graft. J Invasive
Cardiol. 24:185–187. 2012.
|
|
9
|
Artifon EL, Coelho F, Frazao M, et al: A
prospective randomized study comparing partially covered metal
stent versus plastic multistent in the endoscopic management of
patients with postoperative benign bile duct strictures: a
follow-up above 5 years. Rev Gastroenterol Peru. 32:26–31.
2012.
|
|
10
|
Moszura T, Zubrzycka M, Michalak KW, et
al: Acute and late obstruction of a modified Blalock-Taussig shunt:
a two-center experience in different catheter-based methods of
treatment. Interact Cardiovasc Thorac Surg. 10:727–731. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fogarassy G, Apró D and Veress G:
Successful sealing of a coronary artery perforation with a
mesh-covered stent. J Invasive Cardiol. 24:E80–E83. 2012.PubMed/NCBI
|
|
12
|
García-Pagán JC, Caca K, Bureau C, et al:
Early use of TIPS in patients with cirrhosis and variceal bleeding.
N Engl J Med. 362:2370–2379. 2010.
|
|
13
|
Escorsell A and Bosch J: Self-expandable
metal stents in the treatment of acute esophageal variceal
bleeding. Gastroenterol Res Pract. 2011:9109862011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thabut D, Rudler M and Lebrec D: Early
TIPS with covered stents in high-risk patients with cirrhosis
presenting with variceal bleeding: are we ready to dive into the
deep end of the pool? J Hepatol. 55:1148–1149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hausegger KA, Karnel F, Georgieva B, et
al: Transjugular intrahepatic portosystemic shunt creation with the
Viatorr expanded polytetrafluoroethylene-covered stent-graft. J
Vasc Interv Radiol. 15:239–248. 2004. View Article : Google Scholar
|
|
16
|
Haskal ZJ, Pentecost MJ, Soulen MC, et al:
Transjugular intrahepatic portosystemic shunt stenosis and
revision: early and midterm results. AJR Am J Roentgenol.
163:439–444. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lind CD, Malisch TW, Chong WK, et al:
Incidence of shunt occlusion or stenosis following transjugular
intrahepatic porto-systemic shunt placement. Gastroenterology.
106:1277–1283. 1994.PubMed/NCBI
|
|
18
|
Saxon RR: A new era for transjugular
intrahepatic portosystemic shunts? J Vasc Interv Radiol.
15:217–219. 2004. View Article : Google Scholar : PubMed/NCBI
|