Introduction
Cases of arteriovenous fistula include congenital
and acquired arteriovenous fistulae. Acquired arteriovenous fistula
is common in traumas and iatrogenic surgery, including acupuncture,
moxibustion and femoral artery puncture (1–5).
Puerile congenital arteriovenous fistula is rare in the clinic and
it may occur at any site of body, including coronary arteriovenous,
dural arteriovenous, spinal peripheral arteriovenous and pulmonary
arteriovenous fistulae (6,7). Additionally, reports on puerile
congenital deep femoral arteriovenous fistula are rare and its
treatment methods mainly depend on surgery (8). With the development of intervention
technology in China in the past 20 years, interventional
embolization is increasingly applied in the treatment of
arteriovenous fistula (9–11). However, there are still no reports
on interventional embolization as a treatment of puerile congenital
deep femoral arteriovenous fistula. In this study, we used
interventional embolization to treat 9 cases of puerile congenital
deep femoral arteriovenous fistulae to obtain improved treatment
efficiency.
Materials and methods
General data
This study was conducted in accordance with the
declaration of Helsinki. This study was conducted with approval
from the Ethics Committee of the Guangzhou Women and Children's
Medical Center, and written informed consent was obtained from all
participants. A total of 9 patients, 6 male and 3 female, were
included in this study. Patient ages ranged from 4 to 15 years and
their mean age was 10.7 years. Body weight ranged from 16 to 49 kg
and the mean body weight was 24.7 kg. Additionally, no patients had
explicit trauma history. In 3 cases, the lesions were located in
the left thigh and in 6 cases the lesions were located in the right
thigh. For all 9 patients, fistula skin temperature had increased
and the skin temperature at the affected side was higher (maximum
increase, 1.5°C) compared to that at the the same site on the
healthy side. Seven patients presented local skin hyperhidrosis; 2
patients presented red spots on the skin surface; 5 patients
presented palpable vibratory sense of lesions; 3 patients presented
apparent pulsatory sense; 3 patients suffered from superficial
varicose veins on the root of the thigh (caused by standing); 3
patients suffered from local thickening of the thigh, including 1
patients who suffered from local thickening of the thigh
accompanied with mild atrophy of the calf muscle; 2 patients
presented affected limb elongation, by a maximum of 1.5 cm; 5
patients often felt limb numbness, swelling or pain and in 2
patients these symptoms were occasionally accompanied by pelvic
cavity and lower back pains. In addition, all 9 patients had no
clear palpitation after activity and no skin ulcers or gangrene
occurred in the affected limb. Color Doppler B-ultrasound
examination was conducted for all 9 patients to ensure correct
diagnosis of arteriovenous fistula. Selective angiography via
femoral artery approach was also conducted. The angiography results
revealed that the femoral vein developed in advance of the deep
femoral artery. The orificium fistulae were located at the proximal
end of the deep femoral artery or the distal end of its branch.
According to Vollmar typing, 6 cases were type I, 2 cases were type
II and 1 case was type III.
Treatment methods
The femoral artery at the healthy side was punctured
with a 4F pediatric puncture sheath kit (Terumo Company, Tokyo,
Japan) by the Seldinger technique. After guide wires were
exchanged, a 4F catheter (Terumo Company) was placed into the
common iliac artery at the affected limb and radiography was
carried out. After determining the location of the fistula, a 2.7F
microcatheter (Terumo Company) was inserted into the orificium
fistula and its location was repeatedly confirmed by radiography.
Subsequently, embolism materials, including spring steel rings or
absolute ethyl alcohol, were injected into the vessel. Occlusion of
the fistulae branches was performed by locally puncturing lesions.
After 15 min, the femoral angiography was conducted again. If there
were still residual orificium fistulae, fistulae branches were
continuously occluded by the same method until orificium fistulae
were completely closed. One month after surgery, a re-examination
was conducted. If necessary, the interventional embolization
therapy was conducted again.
Results
Angiography
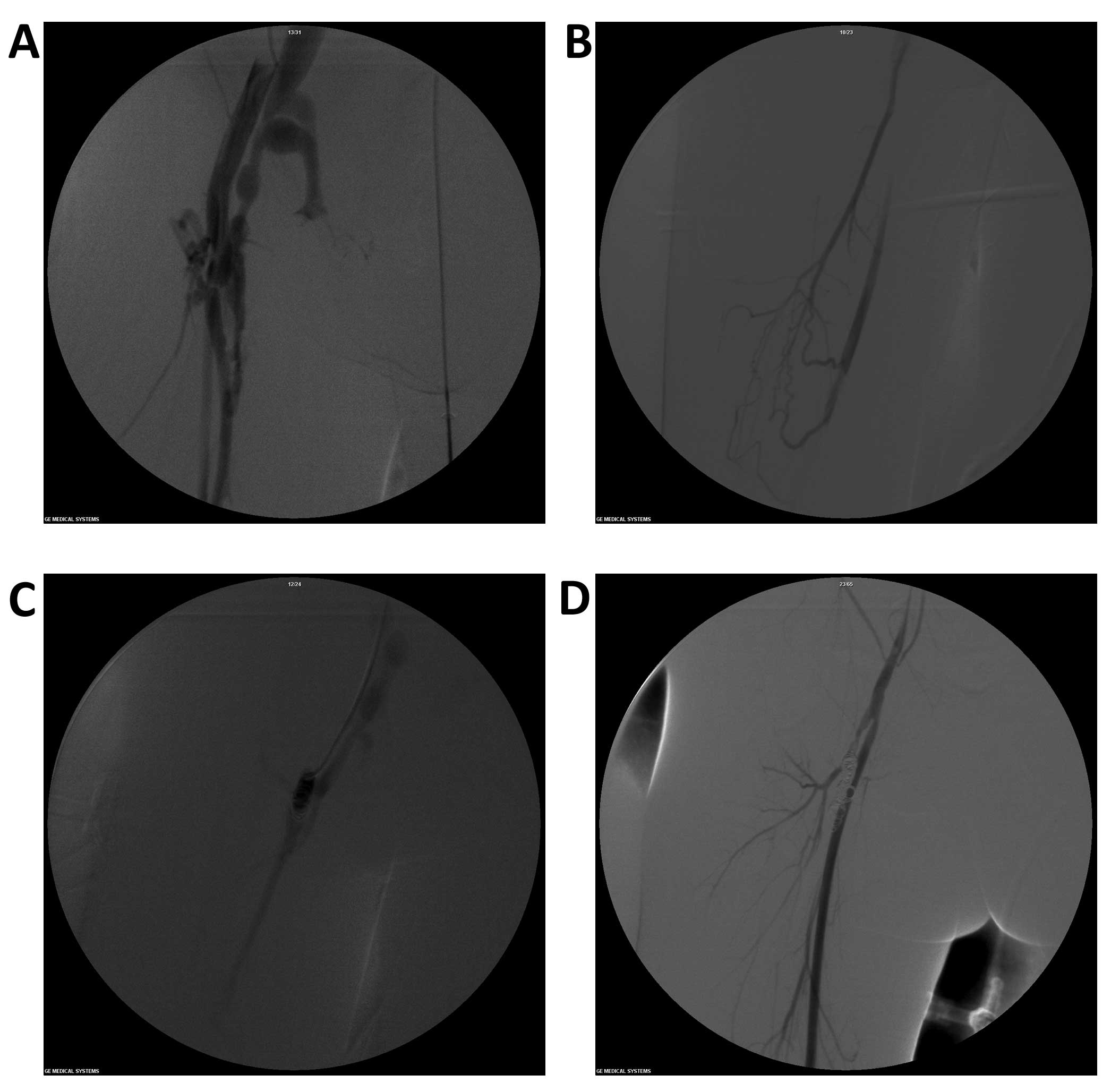
Angiography clearly showed the state of the deep
femoral artery and its branches, as well as the orificium fistulae
(Fig. 1A). Among the 9 cases of
congenital arteriovenous fistula (CAVF) in this study, 4 cases
presented a single orificium fistula and 5 cases presented a number
of orificium fistulae. The main orificium fistulae were located in
the trunk of the deep femoral artery, 1–3 cm away from the deep
femoral artery opening. The branches of the orificium fistulae of
the deep femoral artery were finer (Fig. 1B). The proximal arteries and veins
of all 9 cases of orificium fistulae presented varying extents of
dilation and distortion. Among them, the proximal deep femoral
arteries in 6 cases of orificium fistulae presented tumor-like
dilation accompanied with deep femoral artery branch dilation. The
distal arteries and veins in 4 cases of orificium fistulae were
almost normal. The distal veins in 2 cases of orificium fistulae
presented slow blood backflow and reverse flow and the backflow in
the vein in 1 case flowed towards the pelvic cavity.
Operative techniques and fistula
occlusion
A total of 11 interventional embolization treatments
were conducted for 9 patients and a total of 47 spring steel rings
were released. Technical surgery success rate was 100% (Fig. 1C and D) and no ectopic embolism of
spring steel ring occurred. The intraoperative immediate fistula
occlusion rate reached 100%. One month after surgery, angiography
re-examination was conducted again and the fistula occlusion rate
was 88.9% (8/9 cases). Among them, numerous tiny orificium fistulae
were visible in the branches of the deep femoral artery in 1 case.
A total of three successive interventional embolizations were
conducted for the patient.
Improvement of clinical symptoms and
follow-up
Compared with before treatment, skin temperature at
the affected side in 9 patients was reduced. Six cases were almost
normal; pulsatory or vibratory sense in 7 cases disappeared; 1 case
presented palpable mild fremitus and the orificium fistulae
location was unclear; the affected limb in 1 case was occasionally
accompanied with pain, and superficial varicosis on the thigh of 1
case was still visible; however, it was milder than before surgery.
During hospitalization, no surgical complications, including
cutaneous necrosis occurred. Within the postoperative period of 6
months to 2 years, 8 cases reached the clinical criteria for being
cured. In addition, although 1 case still experienced CAVF
symptoms, the disease condition did not progress more clearly
compared to before surgery.
Discussion
CAVF lesions are occasionally present at birth;
however, after inquiring about disease history, we noted that
patients had no clinical symptoms. Due to this, CAVF does not
attract attention and therefore has a longer latency. Usually there
are clear manifestations in the school age or adolescent period,
which cause concern. Of the 9 patients in this study, symptoms in 3
cases appeared in the school age period and symptoms in 6 cases
appeared during the adolescent period, which is possibly associated
with endocrine hormone stimulation, over-activity or trauma
(although puerile patients had no clear trauma history) in this
stage (12).
Although a number of classification methods are
available for CAVF, the Vollmar classification method is the most
common in the clinic. In 1976, Vollmar divided CAVF into three
types according to the morphology: type I (trunk-like arteriovenous
fistula), traffic branches on the horizontal direction among
peripheral arteriovenous trunks; type II (tumor-like arteriovenous
fistula), a number of fine traffic branches on the horizontal
direction among peripheral arteriovenous involving trunks and local
soft tissues and skeletons; and type III (mixed mode), trunk- and
tumor-like multiple arteriovenous traffic branches. The Vollmar
classification method represents the occurrence and development
process of CAVF and its complexity. In this study, 6 cases were
type I, with angiography clearly showing the orificium fistula
location and the progressive dilation and tortuosity of arteries
and veins at the proximal orificium fistula. The arteries and veins
at the distal orificium fistula were almost normal and the majority
presented palpable fremitus. Auscultation identified a ‘purring
thrill’ sound and a number of cases presented palpable pulsation.
Two cases were type II and angiography showed multiple branches of
the fistula sinus, increased arterial and venous collateral cycle
and an evident thickening of local soft tissue. Additionally, the
vibratory sense was wide; however, the location was unclear. One
case was type III and angiography showed tumor-like dilation at the
proximal orificium fistulae of the deep femoral artery accompanied
with deep femoral artery branch dilation. At the distal end,
several microfistulae were visible and back-flow veins were
tortuous. Additionally, partial venous valve function insufficiency
and distal venous blood stasis were visible and reverse blood flow
was occasionally visible. In the clinic, extended and thickened
limbs with superficial varicosis at the affected side were evident.
Pain symptoms in the lower extremities and pelvic cavity were also
more apparent.
Although CAVF is a benign lesion, it has the
biological behaviors of a malignant tumor. Therefore, the lesion
continuously develops, spreads and often involves adjacent tissues
and organs, inducing severe complications, including affected limb
swelling, thickening, pain, hyperhidrosis, pigmentation, festering,
necrosis and congestive heart failure. The cases in this study
showed no severe complications, including limb necrosis and
congestive heart failure, which may be associated with earlier
identification of the lesion and timely clinical treatment. A
number of studies have reported that after adulthood, CAVF presents
various severe complications, including the steal syndrome,
intractable ulcers and progressive heart failure (6,7). As
CAVF has no self-healing tendency, diagnosis and treatment must be
conducted as early as possible. However, clinical treatment is
difficult. From the perspective of hemodynamics, CAVF is an
abnormal communication between the high-pressure and
high-resistance arterial system and the low-pressure,
low-resistance and high-capacity venous system. Surgical ligation
is difficult but it completely clears lesions. Usually, the
ligation end is too far away from the orificium fistula or numerous
microfistulae are present. Surgery only ligates the main fistula
branches, while post-operative fine fistula branches may either
gradually dilate or the collateral artery may enter the fistula
cycle, inducing lesion recurrence. Particularly for complex CAVF,
treatment often begins with surgery or embolism and ends with
amputation (13).
CAVF is the result of long-term development of
vascular abnormalities. In contrast to traumatic or iatrogenic
arteriovenous fistula, the majority of CAVF, other than the main
arteriovenous fistula, usually present varying extents of minute
orificium fistulae at the main trunk branches. Therefore, a high
pressure injector is used and angiography examination clearly shows
the effects. In this study, 5 cases presented varying amounts of
microfistulae, while surgical ligation readily caused recurrence.
We used the spring steel rings plus absolute ethyl alcohol
embolization treatment method (14,15)
and obtained improved efficiency. The one-time interventional
surgery fistula occlusion rate in 8 cases reached 100%. Following
interventional surgery in 1 case, belonging to type III, orificium
fistulae formed again. After three interventional embolization
treatments, the disease conditions were better controlled. We
consider that the following should be noted for surgery: spring
steel rings create a permanent embolism. Before releasing spring
steel rings, it is necessary to ensure that no other fistula exists
at the distal end of this branched artery by repeating angiography.
Otherwise, it is possible to aggravate the disease condition and it
becomes impossible to conduct subsequent treatments as there is no
vascular channel. Additionally, before spring steel rings are
released, it is necessary to ensure the opening end of the catheter
is at the orificium fistula or in the sinus fistula, which may be
confirmed by radiography, i.e., conducting backflow vein
development of the orificium fistula following injection of a
contrast agent and then increasing pressure again. As a result, the
deep femoral artery back-flow developing at the proximal orificium
fistula is visible. To ensure the embolism level reaches the
orificium fistula grade, fistula cycling in the collateral branch
must be avoided, as this causes lesion recurrence. For the small
branch or residual orificium fistulae following the release of the
spring steel rings, it is possible to occlude residual lesions by
injecting absolute ethyl alcohol via an endovascular injection or
local puncture of the lesions. A larger orificium fistula usually
requires more spring steel rings. If the blood flow of the
orificium fistula is larger, it is possible to firstly fix spring
steel rings onto any branch near the orificium fistula by the
anchoring method (Fig. 2A and B).
On this base, spring steel rings are gradually and densely filled
and the high-flow rate fistula tract becomes a low-flow rate
fistula tract. Afterwards, residual mesh-like, microfistulae are
occluded by injecting absolute ethyl alcohol (Fig. 2C and D). However, absolute ethyl
alcohol injection may induce acute hemolysis, cutaneous necrosis
and pulmonary hypertension; therefore it may be necessary to
conduct pulmonary artery pressure monitoring. Additionally, it is
recommended that surgery is conducted by an experienced senior
interventional physician (16–18).
To conclude, puerile congenital deep femoral
arteriovenous fistula sustainably progresses, so it must be
diagnosed and treated as early as possible. Interventional
embolization has a number of advantages, including small trauma,
little complication and clear efficiency and is increasingly
applied in the clinic. Therefore, it may become the preferred
therapeutic regimen for puerile congenital deep femoral
arteriovenous fistula.
References
|
1.
|
Koshy CG, Keshava SN, Surendrababu NR,
Moses V, Stephen E and Agarwal S: Endovascular management of
posttraumatic arteriovenous fistula. Cardiovasc Intervent Radiol.
32:1042–1052. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yang DH, Hu HD, Zhang Q, Yang W and Duan
ZQ: The surgical treatment of post-traumatic arteriovenous
fistulas. Chin J General Surg. 17:652–653. 2002.
|
|
3.
|
Megremis SD, Christaki MA, Mourkoyiannis
NK, Papadopoulos GS and Tsilimigaki AM: Iatrogenic brachial
arteriovenous fistula in a child: color Doppler ultrasonographic
evaluation. J Ultrasound Med. 25:809–812. 2006.PubMed/NCBI
|
|
4.
|
Beck-Razi N, Soudack M and Gaitini DE:
Acquired arteriovenous fistula at an unusual site. AJR Am J
Roentgenol. 188:547–549. 2007. View Article : Google Scholar
|
|
5.
|
Zhu S, Liu Y, Song B and Liu D:
Spontaneous arteriovenous shunt after microsurgical operation. J
Plast Reconstr Aesthet Surg. 63:1569–1571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Chen XS, Ling T, Guan YB and Chen DL:
Diagnosis and management of congenital arteriovenous fistula in
children. Chin J Pediatric Surg. 24:317–318. 2003.
|
|
7.
|
Xie CH, Xia CS, Gong FQ, Zhou YB and Zhu
WH: Interventional occlusion of congenital vascular malformations.
World J Pediatr. 5:296–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Davidovic LB, Banzić I, Rich N, Dragaš M,
Cvetkovic SD and Dimic A: False traumatic aneurysms and
arteriovenous fistulas: retrospective analysis. World J Surg.
35:1378–1386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Pratesi G, Marek J, Fargion A, Pulli R,
Dorigo W and Pratesi C: Endovascular repair of a ruptured popliteal
artery aneurysm associated with popliteal arteriovenous fistula.
Eur J Vasc Endovasc Surg. 40:645–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Katada Y, Ouchi K, Wakita T and Nozaki M:
Liquid sclerotherapy for posttraumatic arteriovenous fistula of the
radialis indicis artery. J Vasc Surg. 52:1343–1345. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Kuo HF, Shih MC, Kao WP, et al:
Acupuncture-induced popliteal arteriovenous fistula successfully
treated with percutaneous endovascular intervention. J Med Sci.
26:158–162. 2010.
|
|
12.
|
Hassanein AH, Mulliken JB, Fishman SJ,
Alomari AI, Zurakowski D and Greene AK: Risk of progression during
childhood and adolescence. Ann Plast Surg. 68:198–201. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wu JP and Qiu FZ: Huang Jiasi Surgery. 6th
edition. People's Medical Publishing House; Beijing: pp. 848–850.
2000
|
|
14.
|
Jiang ZB, Li ZR, Shan H, et al:
Interventional treatment for hepatocellular carcinoma with
arterioportal fistulas: clinical effect analyses in 105 cases. Chin
J Radiol. 28:36–39. 2004.
|
|
15.
|
Guan SH, Shan H, Jiang ZB, et al:
Transmicrocatheter local injection of ethanol to treat
hepatocellular carcinoma with high flow arteriovenous shunts. Chin
J Radiol. 36:997–1000. 2002.
|
|
16.
|
Young SD, Kwang BK and Sung KC: How do we
treat arteriovenous malformations (tips and tricks)? Tech Vasc
Interventional Rad. 10:291–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Yakes WF, Rossi P and Odink H: How I do
it. Arteriovenous malformation management. Cardiovasc Intervent
Radiol. 19:65–71. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jin YB, Liu XX, Hu XJ, et al:
Superselective ethanol endovascular therapy under digital
subtraction angiography for craniofacial arteriovenous
malformations. Chin J Plastic Surg. 25:406–410. 2009.
|