Introduction
Lunatomalacia, one of the main causes of wrist pain,
is a disease characterized by cystic change, fragmentation and
progressive collapse of the lunate (1–3).
Lunatomalacia was first reported by Peste in 1843. In 1910, Robert
Kienböck formulated a classic description of its clinical and X-ray
manifestations, for which it was also named Kienböck’s disease
(4). More than 100 years have
passed since the first report on lunatomalacia; however, the origin
of this disease remains unclear and no theory satisfactorily
explains its original factors and pathological process. To date, a
number of factors have been thought to correlate with the
pathogenesis of lunatomalacia. These factors include vascular
factors, ulnar minus variation, morphological changes of the lunate
and blood hypercoagulable states due to systemic factors, a
decrease in arterial perfusion and venous blood stasis, application
of corticosteroids, sickle red blood cell disease, cerebral palsy
and septicemic embolism (5–8).
These factors require different treatment methods. For early-phase
treatment of lunatomalacia, non-operative approaches and bed rest
are mainly taken. For aggressive-phase treatment, various surgical
procedures may be adopted, each having its own advantages and
disadvantages (9–11). Although different surgical routes
offer multiple choices for lunatomalacia treatment, no criteria
currently exist for determination of a preferred method of
treatment (2). With the exception
of conservative treatments, all the methods to treat the
lunatomalacia by surgical procedures are substitution therapies, in
which one trauma is adopted for another. They are not specifically
based on the origin of lunatomalacia or its pathological
changes.
To date, studies on animal models of lunatomalacia
remain rare. Accordingly, this study aimed to provide an
experimental basis for the clinical treatment of lunatomalacia by
creating dog models of lunatomalacia through liquid nitrogen
freezing.
Materials and methods
Animals
A total of 12 adult healthy crossbred dogs weighing
15–20 kg were selected. The surgical procedures were approved by
the Institute of Animal Ethics of Tongji Medical College.
Sample perfusion
The blood supply sources for the lunate were
identified using the emulsion perfusion method. The upper
extremities were taken from fresh dog cadavers and 1 liter heparin
saline (at a ratio of 12,500 units heparin to 200 ml physiological
saline) was injected into the axillary artery until a colorless
liquid was observed to flow out from the veins. A red emulsion (red
ink and white emulsion, 1:8) was then injected into the axillary
artery until a red liquid was observed to flow out from the
proximal veins. The samples were placed in a refrigerator for 24 h
and then taken out.
Applied anatomy: constitution of carpal
bones
The carpal bones of dogs are composed of two bones
in the proximal row and four in the distal row. The radial carpal
bone in the proximal row is the lunate (or scapholunatum). Dogs are
often used as animal models for studies on lunate or scaphoid
diseases due to the similarity of their anatomical position and the
biomechanic relationships in the constitution of the carpal bones
to those of humans.
Distribution of blood vessels
The axillary artery was traced until the elbow,
where it diverged into the radial and ulnar arteries at the
proximal end of the forearm. The radial and ulnar arteries spread
off in branches at the wrist to form volar and dorsal nets. Tiny
branches from the nets extended into the lunate. No blood vessels
were observed on the articular surface of the lunate.
Model preparation
Skin preparation was performed on a unilateral upper
extremity. After administration of an intravenous anesthetic with
3% pentobarbital, an S-shaped incision was made on the dorsum of
the radial carpal joint in a sterile condition to expose the wrist
joint completely. The joint capsule was cut open and the lunate was
isolated. The volar tissues were stored. A two-ply sterilized
rubber tissue was used to separate the lunate from its surrounding
tissues. The lunate was frozen in liquid nitrogen for 10 min and
then warmed to room temperature. After three cycles of freezing and
warming, the joint capsule, ligaments and skin were sutured and
then fixed with plaster. The dogs were fed on normal food and the
raising conditions were kept the same as those before surgery.
After 3 weeks, the plaster was dismantled and the stitches were
removed. The animals were observed at 3, 6 and 12 weeks after
surgery. At each observation time point, four dogs were
anesthetized and studied iconographically. After the dogs were
sacrificed, their bilateral lunates were removed for gross and
histomorphological examinations.
Observational indices and methods:
general conditions
The weight, mental state, coat color, gait and wound
of the dogs were observed.
X-ray detection
Lateral radiographs of the bilateral lunates were
obtained at 3, 6 and 12 weeks after surgery. The experimental
lunate was compared with the control at each observation time point
to determine the changes in the density of the lunate and bone
destruction following surgery. The results were also analyzed based
on the Lichtman staging criteria (12).
Computed tomography (CT) scanning
Bilateral lunates were scanned by CT and
reconstructed three-dimensionally to observe changes in the
morphology, sclerotin and density of the lunate following
surgery.
Magnetic resonance imaging (MRI)
detection
MRI detection was performed using an eight-channel
wire coil to observe changes in the signals and morphology of the
lunate following surgery.
Visual observation
The shapes, sizes, articular surfaces, color and
densities of the bilateral lunates were observed and compared
visually.
Histomorphological observation
The lunate was cut open along the coronal plane,
fixed with 10% formaldehyde solution, decalcified with 5% nitric
acid, dehydrated gradiently with routine ethanol and then embedded
with paraffin. Sections were cut and stained with hematoxylin and
eosin.
Results
General observation
Following surgery, the dogs presented a negative
mental state. The appetite, weight and glossiness of the coat of
the dogs decreased. Furthermore, subcutaneous fat was observed.
Four weeks after surgery, they began to support their weight using
the forelimb on the side utilized for preparing the model.
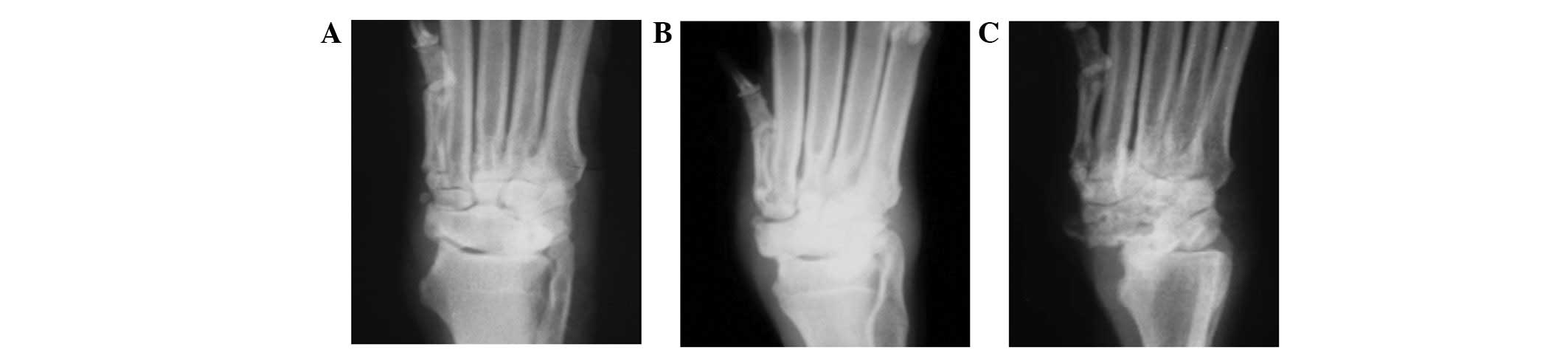
X-ray detection
No marked changes in sclerotin were identified at 3
and 6 weeks after surgery. However, abnormalities were observed at
12 weeks after surgery. The density of the lunate became uneven,
the capsular space flattened and the lunate presented an irregular
shape with an unsmooth articular surface (Fig. 1).
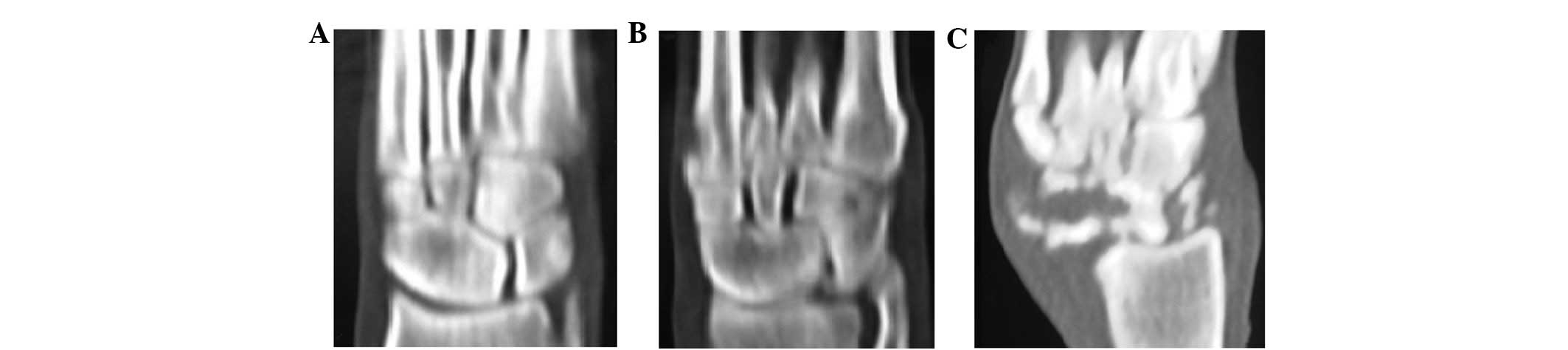
CT scanning
No obvious abnormalities were detected by CT
three-dimensional reconstruction at 3 or 6 weeks after surgery.
Damaged articular surface and discontinuous bone cortex were
apparent at 12 weeks after surgery. The structure of the trabecular
bones disappeared and hypointense foci of irregular morphology
appeared in the pulp chamber (Fig.
2).
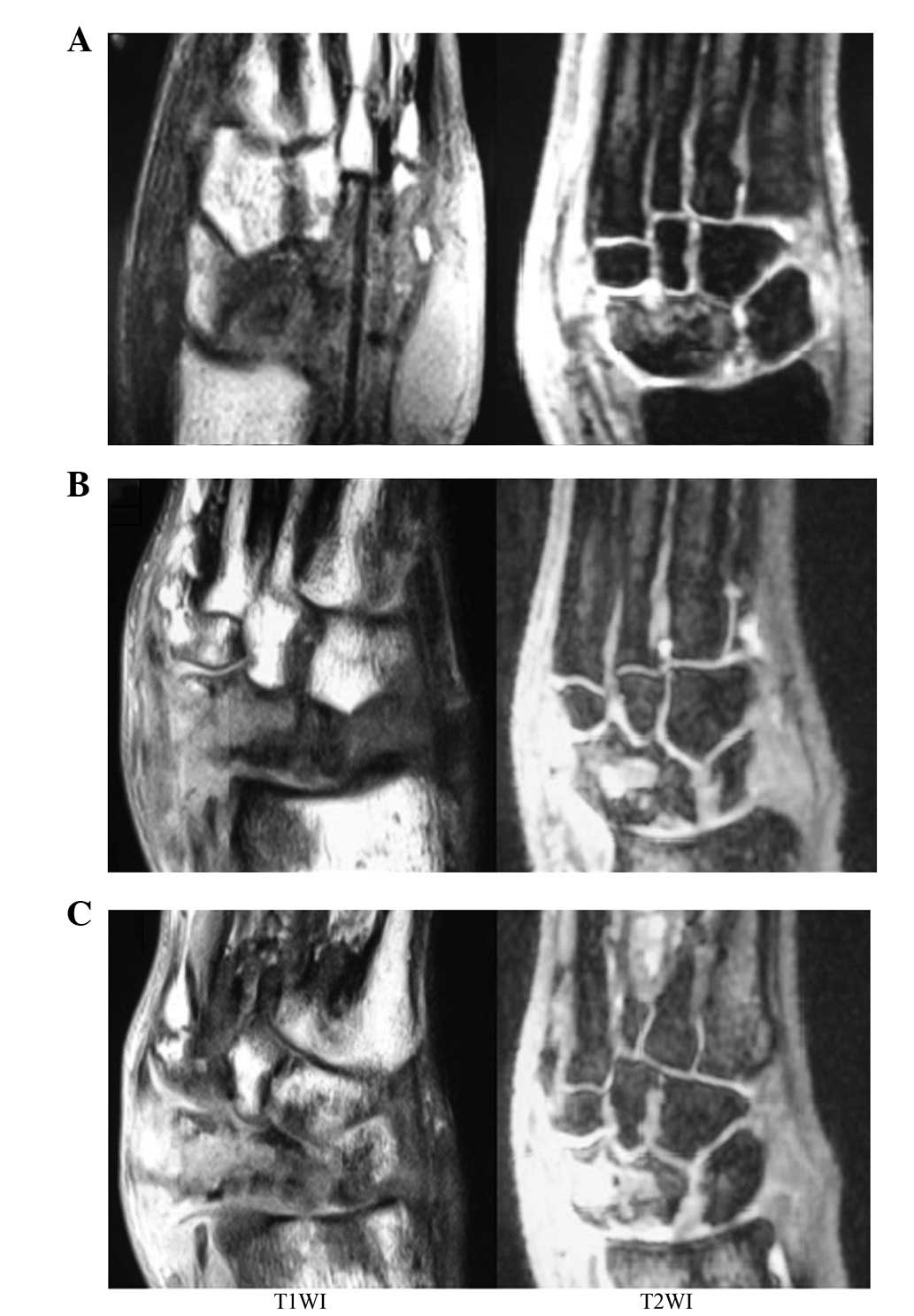
MRI detection
At 3 weeks after surgery, hypointense focus-like
images on T1-weighted images (T1WI) and
disseminated punctate hyperintense images on T2WI were
observed. At 6 weeks after surgery, changes in the hypointense and
hyperintense images were detected. At 12 weeks after surgery, large
areas of hypointensity and hyperintensity were captured on
T1WI and T2WI, respectively. Areas of diffuse
punctate hypointensity were embedded in the large area of
hyperintensity (Fig. 3).
Histomorphological observation: gross
sample observation
At 6 weeks after surgery, the lunate presented
destroyed sclerotin on the surface, a smaller volume compared with
the control, a dull articular surface without luster and a marked
decrease in the cancellous bone density. At 12 weeks after surgery,
a large area of dead bones with crisp texture was detected on the
coronal section of the lunate. Furthermore, a number of cancellous
bones were observed under the cartilage surface, as a result of
osteosclerosis.
Observation under a light microscope
At 3 weeks after surgery, the trabecular bones
appeared thinner, a number of bone lacunas became vacant and vessel
density decreased. The marrow cavity bled and neither osteoblasts
nor osteoclasts were detected. At 6 weeks after surgery, the
trabecular bones became sparse and fractured, calcium salt was lost
and a large area of osteocytes disappeared. More than 50% of the
bone lacunas became vacant and inflammatory cell infiltration was
observed. At 12 weeks after surgery, the trabecular bones
fractured, all bone lacunas were empty and fibroplasia was
identified around the trabecular bones. By contrast, the
corresponding healthy lunate presented normal bone tissues, in
which abundant trabecular bones, full lacunas and abundant
capillary vessels were observed (Fig.
4).
Discussion
The current methods used for the preparation of
models of osteonecrosis include the following: i) hormone
induction: Zhao et al used hormones and endotoxins to induce
femur head necrosis in rabbits (13). They found that satisfactory
necrosis was achieved within a short time (7). ii) Blood vessel ligation: Nishino
et al prepared models with osteonecrosis through artificial
dislocation of the hip joint and ligations of the medial and
lateral femoral circumflex arteries and veins (14). These authors identified that
dislocation of the joint or ligations of the blood vessels alone do
not lead to osteonecrosis, and osteonecrosis only occurs when blood
flow is at least 20% lower than that in the healthy control. iii)
Osteotomy: Gong et al established models with femur head
ischemic necrosis through osteotomy and apparent osteonecrosis was
obtained two months after surgery (15). iv) Liquid nitrogen freezing: Yang
et al established models with femur head necrosis via liquid
nitrogen imbibition (16). These
authors identified that the whole femur head presented complete
necrosis with empty bone lacunas and necrotic tissue fragments
filling the pulp chamber. Aside from the aforementioned techniques,
methods used for the preparation of models of osteonecrosis also
include local cell inactivation, the microwave method and acid base
destruction.
Bone absorption and formation are closely associated
with the roles of blood vessels in the bone (17). During bone growth and repair, bone
formation is initiated and supported by blood vessels. Osteoblasts
differentiate and proliferate around the vessel, arrange along the
vascular endothelium and then excrete osteoids in a direction away
from the vessel. When the osteoblasts mature and develop into
osteocytes, the newly formed bones deposit around the vessel. In
the current study, liquid nitrogen freezing, isolation of the
lunate from its surrounding tissues and destruction of the blood
supply for the lunate were adopted, to prepare models of
lunatomalacia. Sparseness and disruption of the trabecular bones,
loss of calcium salt, disappearance of a large area of osteocytes
and infiltration of inflammatory cells were found at 6 weeks after
surgery. Systemic or local intravascular coagulation is the last
common pathway in the pathogenetic process of osteonecrosis caused
by various factors (16). Liquid
nitrogen freezing instantly leads to vasospasm and embolism in the
lunate due to the small volume of the lunate and extenuation of the
blood vessel supply running in it. As a result, vascular
permeability is enhanced, vascular endothelial cells are damaged
and intravascular coagulation is disseminated, thereby causing
hemorrhage and ischemic reperfusion injury after rewarming
(18,19). The vascular injury in the lunate
combined with the isolation of the lunate from its surrounding soft
tissues inhibited the spontaneous repair reactions of the
lunate.
According to the improved Lichtman staging criteria
formulated by Allan et al(8), Kienböck’s disease is divided into
four stages. At stage I, pain, loss of strength and motion
limitation at the wrist may occur. No abnormality is detected by
X-ray in the majority of cases but a number of abnormal changes may
be identified by MRI. At stage II, the bone density and fragments
in the lunate increase; however, its volume, shape and anatomic
associations with adjacent bones do not change. At stage III, the
lunate begins to collapse. At stage IIIa, the location and
correspondence of the wrist bones are normal. At stage IIIb, the
joint space between the lunate and scaphoid widens. Palmar flexion
of the scaphoid, an angle between the scaphoid and radius of
>60° and ulnar deviation of the triangular bone are observed.
Finally, at stage IV, the lunate collapses and fragments, the
capitate bone shifts proximally, osteoarthritis occurs between the
intercarpal joints, joint surfaces become coarse and uneven, joint
spaces narrow, osteophytes are formed and the bones are hardened
and degenerated cystically. In the present study, hypointense and
disseminated hyperintense images were captured on T1WI
and T2WI, respectively, at 3 weeks after surgery.
However, such changes were not detected by X-ray or CT at the same
time point. These results indicate that MRI possesses a
hyper-sensitivity to early lunatomalacia. At 12 weeks after
surgery, a hyperintense area in which areas of punctate
hypointensity were embedded was observed on T2WI. The
hyperintense area represented watery changes caused by inflammatory
and repair reactions and the hypointense images in the area were
necrotic bone chips.
Lunatomalacia is an independent clinical disease.
Thus far, little is known about the cause of this disease. Factors
including nutrient artery injury of the lunate, venous embolism,
ulnar variation, morphologic abnormality of the lunate, rheumatoid
arthritis, sicklemia, cerebral palsy, application of non-steroidal
drugs and mechanical injuries are thought to be correlated with the
incidence of lunatomalacia. Lunatomalacia is currently accepted as
the consequence of the combined actions of various factors and its
essence is bone avascular necrosis, in which anatomical variation
may be the causative factor and acute and chronic injuries and
inflammation may be its primary factor (20,21).
To date, studies on animal models with lunatomalacia
have rarely been reported. Various methods have been used for model
preparation. These methods have attempted to simulate the possible
causes and pathological processes of osteonecrosis to provide an
experimental basis for further studies on this disease. Sunagawa
et al successfully prepared dog models with discontinuity
between the scaphoid and lunate by resorting to artificial
scapholunatum fractures and liquid nitrogen freezing (7). However, none of these methods reflect
the pathological process of osteonecrosis completely. Therefore,
the selection of proper experimental models for different purposes
is of great practical significance. In this study, animal models of
osteonecrosis were prepared by the destruction of blood supply to
the lunate and liquid nitrogen freezing. Compared with other
methods, this method has a number of advantages. First, it achieves
a high success rate with reliable osteonecrosis and a short
pathological process. Second, it is easy and convenient to perform.
Third, it duplicates the primary pathological process of
lunatomalacia, although not completely. Finally, no residue of
drugs or chemical materials is left and the basic properties of the
bone are maintained. Based on the advantages of this method in
model preparation, the present study is expected to provide a good
platform for further remedial studies on lunatomalacia.
References
|
1.
|
Jones JP Jr: Fat embolism, intravascular
coagulation and osteonecrosis. Clin Orthop Relat Res. 292:294–308.
1993.PubMed/NCBI
|
|
2.
|
Nakamura T, Matsumoto T, Nishino M, Tomita
K and Kadoya M: Early magnetic resonance imaging and histologic
findings in a model of femoral head necrosis. Clin Orthop Relat
Res. 334:68–72. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ledoux P, Lamblin D, Wuilbaut A and
Schuind F: A finite-element analysis of Kienbock’s disease. J Hand
Surg Eur Vol. 33:286–291. 2008.
|
|
4.
|
Kienböck R: Uber traumaische malazie des
mondbeins und ihre folgezustande: Entartungs for men und
kompressionsfrakturen. Fortschritte Rontgenstrahlen. 16:77–103.
1910.(In German).
|
|
5.
|
Gelberman RH, Bauman TD, Menon J and
Akeson WH: The vascularity of the lunate bone and Kienböck’s
disease. J Hand Surg Am. 5:272–278. 1980.PubMed/NCBI
|
|
6.
|
Kristensen SS and Søballe K: Kienböck’s
disease - the influence of arthrosis on ulnar variance
measurements. J Hand Surg Br. 12:301–305. 1987.
|
|
7.
|
Sunagawa T, Bishop AT and Muramatsu K:
Role of conventional and vascularized bone grafts in scaphoid
nonunion with avascular necrosis: A canine experimental study. J
Hand Surg Am. 25:849–859. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Allan CH, Joshi A and Lichtman DM:
Kienbock’s disease: diagnosis and treatment. J Am Acad Orthop Surg.
9:128–136. 2001.
|
|
9.
|
Razemon JP: Pathological study of
Kienboeck’s disease. Ann Chir Main. 1:240–242. 1982.(In
French).
|
|
10.
|
Mirabello SC, Rosenthal DL and Smith RJ:
Correlation of clinical and radiographic findings in Kienböck’s
disease. J Hand Surg Am. 21:1049–1054. 1987.
|
|
11.
|
Kmano M, Koshimune M, Toyama M and Kazuki
K: Palmar plating system for Colles’ fractures - a preliminary
report. J Hand Surg Am. 30:750–755. 1991.PubMed/NCBI
|
|
12.
|
Lichtman DM and Degnan GG: Staging and its
use in the determination of treatment modalities for Kienböck’s
disease. Hand Clin. 9:409–416. 1993.PubMed/NCBI
|
|
13.
|
Zhao J, Lu S, Xue Z and Deng Q: Establish
and evaluate animal models of steriod-induced osteonecrosis of the
femoral head. Chin J Bone Tumor & Bone Disease. 4:220–225.
2007.(In Chinese).
|
|
14.
|
Nishino M, Matsumoto T, Nakamura T and
Tomita K: Pathological and hemodynamic study in a new model of
femoral head necrosis following traumatic dislocation. Arch Orthop
Trauma Surg. 116:259–262. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gong X and Lu L: The development of
Treating of Lunatomalacia. Foreign Medical Sciences (Section of
Orthopaedics). 25:272–274. 2005.(In Chinese).
|
|
16.
|
Yang S, Yang C, Xu W, Li J and Zhang Y:
Avascular necrosisi of the Femoral Head Prouced in Rabbits by
Freezing. Orthopedic J China (Chin). 8:103–106. 2001.(In
Chinese).
|
|
17.
|
Beredjiklian PK: Kienböck’s disease. J
Hand Surg Am. 34:167–175. 2009.
|
|
18.
|
Goldfarb CA, Hsu J, Gelberman RH and Boyer
MI: The Lichtman classification for Kienböck’s disease: an
assessment of reliability. J Hand Surg Am. 28:74–80. 2003.
|
|
19.
|
Bain GI and Begg M: Arthroscopic
assessment and classification of Kienbock’s disease. Tech Hand Up
Extrem Surg. 10:8–13. 2006.
|
|
20.
|
Keith PP, Nuttall D and Trail I: Long-term
outcome of nonsurgically managed Kienböck’s disease. J Hand Surg
Am. 29:63–67. 2004.
|
|
21.
|
Moran SL, Cooney WP, Berger RA, Bishop AT
and Shin AY: The use of the 4+5 extensor compartmental vascularized
bone graft for the treatment of Kienböck’s disease. J Hand Surg Am.
30:50–58. 2005.
|