Introduction
In the wound-healing process, dermal substitutes
play a guiding and supporting role during the cell and blood vessel
recovery process. As a dermal regeneration template, they also
promote the guided regeneration function of the tissue, reduce scar
hyperplasia and improve the quality of wound-healing (1). In 1995, Livesey et al
(2) discovered the acellular
dermal matrix (ADM) by using physical and chemical processes to
remove allogeneic cell components from the skin. ADM combines well
with the wound and promotes the ingrowth of fibroblasts on the
surrounding normal tissue. After one week, new blood vessels are
formed as a permanent dermal replacement. In one study (3), matrix enzyme treatment was used to
obtain ADM via cyclical compression technology. Ma et al
(4) reported that porcine ADM and
an autologous split-thickness skin graft film composite were able
to effectively treat full-thickness skin defects in animals and
improve the quality of wound healing. Since 1997, the Foshan First
People’s Hospital Burns and Plastic Surgery Unit have used
xenogeneic ADM as a temporary wound-covering material to treat burn
wounds and have obtained satisfactory clinical results (5). Since the satisfactory outcome of the
first successful use of an acellular dermal allograft and an
autologous mesh cograft by Wainwright et al (6,7),
composite sheets comprising a combination of different dermal
matrices and autologous films, including skin films and skin
particles, have been widely used in clinical applications (8–10).
Acellular dermal grafts have been used to repair perineal hernia
(11), complex scalp defects
(12) and eyelid defects (13). To identify improved methods of
repairing deep-burn wounds or scar removal wounds, 30 patients with
deep burns who underwent crust cutting were treated using a
combination of meshed acellular dermal xenograft (ADX) and
split-thickness skin autograft from January 2002 to December
2003.
Subjects and methods
General data
A total of 30 cases were enrolled in the present
study (20 males and 10 females, aged 18–60 years) between January
2002 and December 2003. The burn area was 25–60% of the total body
surface area (TBSA), with the third-degree burn area ≤40%. The
smallest region of cografting was ∼0.5% and the largest was ∼3%.
All patients presented thermal burns, with no exposed bones,
joints, nerves or tendons, no serious heart, liver, kidney and
blood system complications and no systemic infection. This study
was conducted in accordance with the Declaration of Helsinki and
with approval from the Ethics Committee of The First People’s
Hospital of Foshan. Written informed consent was obtained from all
participants. Acellular (porcine) dermis was obtained from the
Institute of Qidong Medical Supplies, China.
ADX preparation
ADX was prepared according to a previously described
method (2). The crust of the
deep-burn wound was cut up to the plane of the normal tissue. The
scar wound was excised to the faulty adipose tissue or deep fascial
plane. Following complete hemostasis, cografting was conducted on
the base of the wound. A control wound area next to the cograft
plot was selected for pure grafting of split-thickness skin.
Application of ADX in deep full-thickness
burn wounds
A total of 30 deep-burn patients were selected for
wound crust cutting 1 week after injury. The wounds of 20 patients
were cut to the deep fascia and the wounds of the remaining 10
patients to the superficial fascia. Following complete hemostasis,
the patients were treated with a combination of ADX and
split-thickness skin autograft transplanted in ∼1–2% of the area,
while a pure graft of split-autologous epidermal skin ∼0.15–0.25 mm
thick was placed on the control wound area beside the cograft plot.
The cografted and controlled areas were opened after 5–14 days.
Wound healing observation
The observation was performed by two clinical
physicians to maintain consistency. Two weeks after transplantation
was considered the skin graft survival observation endpoint. The
area of skin graft survival was observed using the grid number
method and graft survival rate was calculated using the following
formula: Skin graft survival area/original graft area ×100.
Vancouver scar hyperplasia
evaluation
Scars were described using the Vancouver Scar Scale
(VSS). Melanin, hardness and scar hyperplasia height were observed
at 1, 3, 6 and 12 months after transplantation and were scored by
the VSS (2). The scars were
evaluated descriptively using the following four indices: melanin
(M), height (H), vascularity (V) and pliability (P).
The score criteria were as follows: i) M: 0, scar
color similar to that of normal body parts; 1, lighter color; 2,
mixed color; and 3, darker color. ii) H: 0, normal; 1, <1 mm; 2,
1–2 mm; 3, 2–4 mm; and 4, >4 mm. iii) V: 0, scar skin color
similar to that of normal body parts; 1, partial pink color; 2,
reddish color; and 3, purple color. iv) P: 0, normal; 1, soft
(deformation under the skin in the least resistance); 2, flexible
(deformation under pressure); 3, hard (inflexible, moving in
blocks, resistant to pressure); 4, banding (rope-like organization
and scar blanches when stretched); and 5, contracture (shortening
leads to permanent deformity and scar distortion). The maximum
possible score was 15 points and higher scores represented more
severe scarring.
Morphological observations
With patient consent, specimens were excised from
the composite graft and control areas at 2 weeks, 8 weeks, 12
weeks, and 1 year and 10 months after surgery and were
pathologically assessed by a single-blind method. The specimens
were divided into two groups under sterile operating conditions.
One group contained the specimens for observation under a light
microscope. The specimens (0.3×1.0 cm in size) were fixed in 10%
formalin, dehydrated for 5 min, embedded in paraffin, sectioned in
series (5 μm thick) and finally stained with
hematoxylin-eosin. The specimens were then observed under an
optical microscope. The second group contained specimens for
observation under the electron microscope. The specimens (0.2×1.0
cm) were fixed in 10 g/l osmium tetroxide, dehydrated, impregnated
in an epoxy resin, embedded and cut into thinner sections (60
μm thick). The specimens then underwent uranium and lead
double-staining and were observed under a CM10 transmission
electron microscope (Philips, Amsterdam, The Netherlands).
Postoperative follow-up
All the wounds of patients underwent postoperative
follow-up for 6 months to 2 years.
Statistical analysis
All data were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). Results were compared using a
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Wound-healing process and changes in
appearance and function
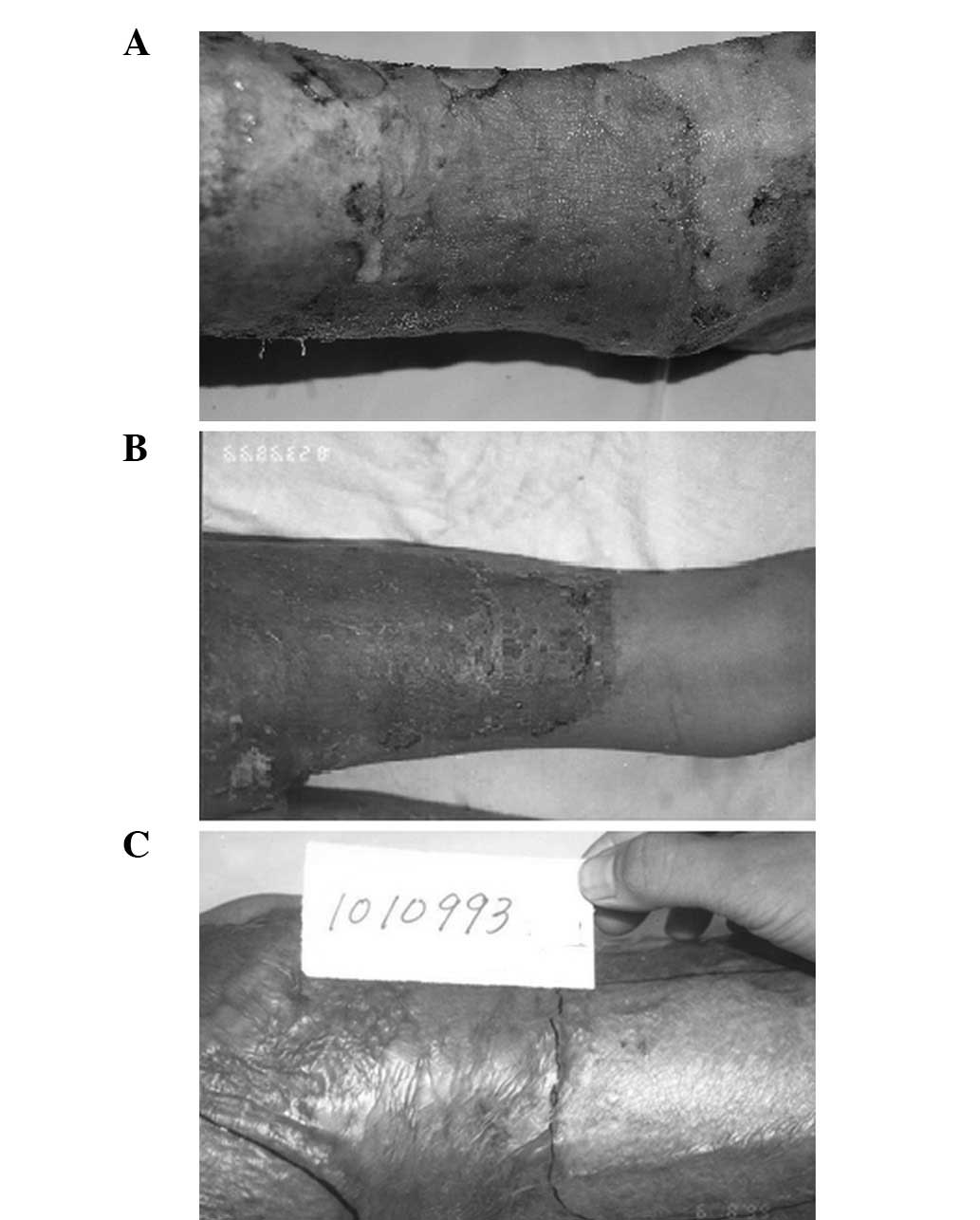
At 5 days after cograft surgery, which used a
combination of meshed ADX and split-thickness skin autograft, the
wounds, which were slightly dark red in color, were opened using an
autologous skin graft survival method. The meshed dermal xenograft
was indistinct throughout the split-autologous skin grafts. The
unstable combination of the skin grafts and the base was felt when
the wound was touched. At 10 days after surgery, light-colored
thick-layer scalings were observed on the cografted wound. The
dermal xenograft was barely visible under the autologous epidermal
skin graft and the wound achieved a stabilized composite graft
survival based on touch (Fig. 1A).
Two weeks after transplantation, the survival rates of the
composite transplant wounds and simple autologous split-thickness
transplant wounds were 100%. The autologous skin graft in the
control area exhibited a small amount of skin scaling, with a color
similar to that of the cograft area observed 10 days after surgery.
At 30 days after cografting, the composite skin was soft, smooth
and thick and exhibited no shrinkage or wear, and an even interface
with the flat edge of the normal skin (Fig. 1B). The autologous epidermal skin
graft on the control area started to exhibit varying degrees of
thick, wrinkled and hard-textured mild scar uplift. After 45 days,
the cografted skin was shiny, remained soft and smooth and was
slightly redder than normal; however, it was lighter in color
compared with that of the control area. After 90 days of general
observation, the skin on the cografted area was even and smooth.
The thick and hard-wearing epidermis demonstrated no scar
contracture, was pale yellow-white, exhibited good function and had
traits similar to those of the full-thickness skin graft at the
time of excision (Fig. 1C). The
control wound exhibited surface shrinkage and elevation, and
hardened with the formation of dark red contractures and a
conspicuous hyperplasia scar.
Vancouver scar hyperplasia
evaluation
VSS at 1 month after burn wound healing demonstrated
no significant differences between the experimental area and
control area (P>0.05), while the difference was statistically
significantly after 3, 6 and 12 months (P<0.05; Table I).
 | Table I.Comparison of the Vancouver Scar Scale
score between the experimental and control areas. |
Table I.
Comparison of the Vancouver Scar Scale
score between the experimental and control areas.
| Time point
|
|---|
| Group | 1 month | 3 months | 6 months | 12 months |
|---|
| Experimental | 8.35±1.16 | 8.98±1.48 | 7.48±1.61 | 6.35±1.03 |
| Control | 8.98±1.22 | 10.35±1.68 | 12.23±1.24 | 10.54±1.75 |
| P-value | P>0.05 | P<0.05 | P<0.05 | P<0.05 |
Morphological observation
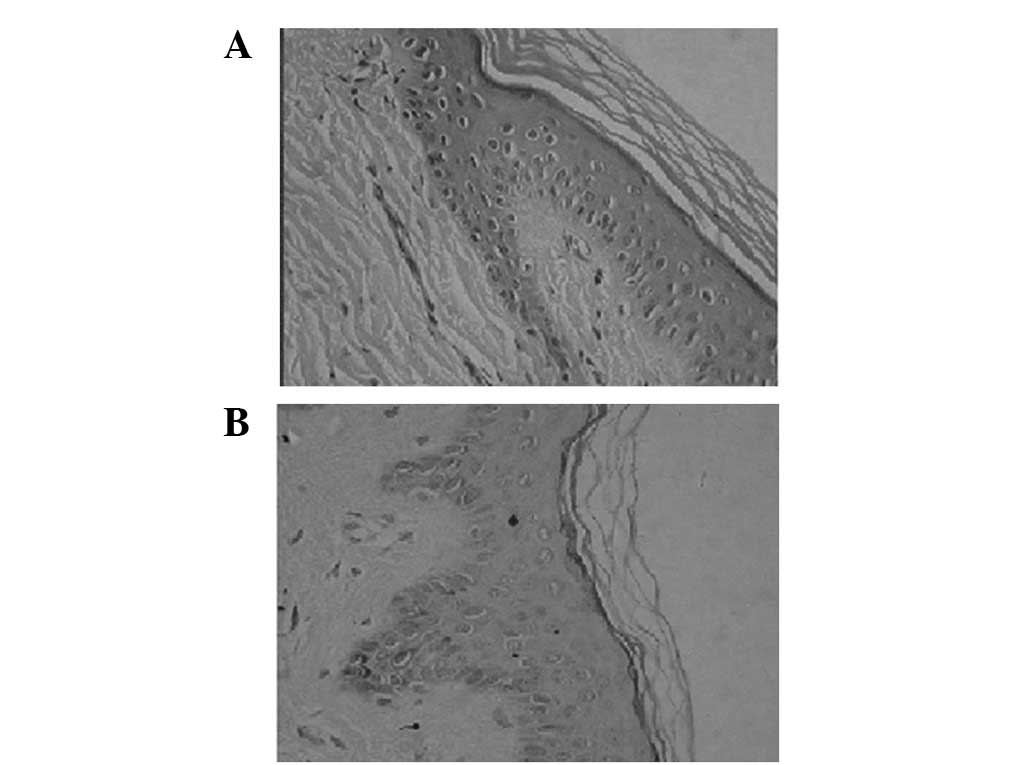
Two weeks after transplantation and with patient
consent, three biopsy specimens were randomly selected and observed
under a light microscope using a scaled layer of the thick
cografted prickle cell hyperplasic area. Numerous inflammatory
cells and fibroblasts in the junction of the dermal xenograft and
the base of the wound were observed. The autologous epidermis,
dermal xenograft and adipose layers closely interweaved with one
another and were hardly separated (Fig. 2A). Under the scale-like epithelium,
rich capillaries and fiber mother cells, lymphocytes, neutral white
blood cells and multinucleated cells were clearly observed.
However, no skin appendages were observed. Twelve weeks after
cograft surgery, the skin structures had combined. The corneous
layer was continuous, mature and presented downward-stretching rete
pegs and dermal collagen fibers with similar structural thickness
and a regular arrangement. The number of capillaries was lower than
before and no skin appendages were observed (Fig. 2B). Masson’s staining revealed a
more regular arrangement of the dermal collagen in the cograft,
which was uniformly stained (Fig.
3A), while there was a large number of dermal fibroblasts and
collagen deposition in the control area, accumulated in a
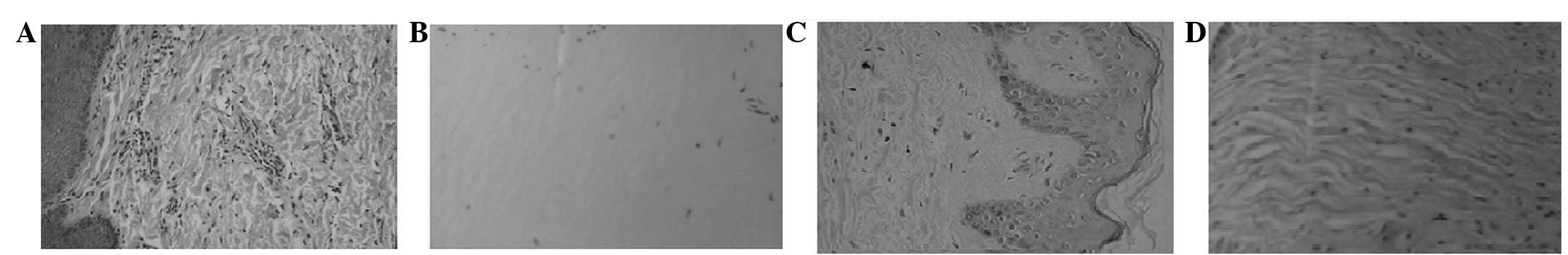
disorderly manner (Fig. 3B). Six
months after cograft surgery, the dermal composition had integrity,
inflammatory cells were reduced and collagen was arranged regularly
(Fig. 4A). Approximately 1 year
and 10 months after cografting, the skin structure appeared similar
to normal skin under a light microscope (Fig. 4B and C), while in the control area,
collagen was disordered and the density was variable (Fig. 4D). Eight weeks after
transplantation, electron microscopy examinations revealed a clear
spike of desmosomes between cells, the basal membrane and
hemidesmosomes. However, the basal membrane of the control area
appeared fuzzy and discontinuous.
Postoperative follow-up
Patients were followed up for 6 months to 2 years
after surgery. The appearance and functional recovery of the
composite transplant wound were better than those of the simple
autologous split-thickness skin transplant. The survival, color,
elasticity, thickness and mobility of the transplanted skin area
were good. Patients treated with the composite acellular (porcine)
and autologous split-thickness skin graft presented good expected
results.
Adverse reactions
No significant adverse reactions were observed
during the treatment with the two types of graft, including skin
ulcers or scar hyperplasia at the donor sites.
Discussion
In patients with extensive deep burns, the use of
the patient’s own skin to treat the wounds is recommended, using
the principles and techniques of plastic surgery, depending on the
skin source and the skin conditions. This treatment has great
significance in the reduction of late surgeries, as well as in the
recovery of the function and appearance of burns or scars (14). However, when deep-burn wounds and
scar-excision wounds, particularly extensive wounds, are treated,
the use of the patient’s own thick-skinned or autologous
full-thickness skin for repair is challenging. For a timely and
effective wound closure, several methods, including split-thickness
skin autografting, autologous meshed skin grafting and autologous
particle grafting, are currently available (9). Although these methods are effective
to a certain degree, they have also demonstrated varying degrees of
postoperative scar formation and dysfunction due to the lack of
adequate dermal components. Balasubramani et al (15) considered such a lack of dermal
structure or dermal components as the cause of overexpression of
fibroblast cells in the wound-healing process, eventually resulting
in conspicuous scar hyperplasia. Such a phenomenon may be
significantly changed with dermal component (dermal structure) or
dermal substitute replenishment, which greatly improves the quality
of wound-healing. Hence, ADM was developed and applied to dermal
wounds (2). The authors reported
that dermal substitution guided the tissue regeneration. A dermal
substitute may be used as a dermal regeneration template to support
the infiltration of host fibroblasts, neovascularization and
epithelialization during the wound-healing process, thereby
reducing the scar and improving the quality of wound-healing
(16). Certain scholars (17) identified that adding hyaluronic
acid to porcine ADM promotes the expression of collagen types I and
III and reduces the ratio of collagen type I to type III. Porcine
ADM was also shown to be conducive to wound-healing, skin graft
reconstruction of the basement membrane and reduction of shrinkage.
Xu et al (18) grafted
porcine ADM onto a simian rotator cuff and observed no
hypersensitivity. Moreover, the skin was safely repaired and the
rotator cuff of the human structure was strengthened. The effects
of using human ADM and non-crosslinked porcine ADM were compared in
a study concerning the repair of porcine abdominal hernia (19). The results demonstrated that
vascular tissues and cells exhibited infiltration in four weeks and
the muscle fascia and bioprosthesis interface had similar
strengths; however, the human ADM presented a greater amount of
cell and blood vessel infiltration. An animal study (20) revealed that, compared with pure
autologous skin, vascular cell adhesion molecule 1 on the cograft
exhibited high expression levels of latency, suggesting that the
expression of vascular cell adhesion molecule 1 is significantly
related to angiogenesis and the remnants of the composite skin.
This result also suggested that the expression levels of vascular
cell adhesion molecule 1 in the homologous and heterologous ADM
cografts are different.
The structure of porcine skin is similar to that of
human skin and its composition and collagen content are also
similar to those of human skin grafts. Porcine collagen adhesion,
hemostasis function and pain reduction traits are the same as those
of humans. Hence, porcine skin as a temporary covering for wounds
is widely used in clinical studies (21–23).
Since 1997, we have used porcine cells in derma-clinical
applications and have identified that the cograft survival time is
not significantly different from that of the pure split-thickness
skin autograft. At 5 days after surgery and after opening the
cograft area wound, the split-thickness skin autograft was living
tissue; however, the combination with the base was not stable. At
this time, the fibrin exudate of the base infiltrated the bottom of
the epidermis through the ADX mesh. The fibrin exudate nourished
the epidermis and established a blood supply with the epidermis. At
10 days after surgery, the split-thickness skin autograft gradually
blended with the stent (xenograft), which initially completed the
healing process of the composite graft. The tissue sectioning
confirms that two weeks after cografting, the autologous epidermis,
dermis and adipose layers via cografting generally had more closely
connected skin bonding that was difficult to separate.
Approximately 12 weeks after cografting, the composite skin graft
was soft, smooth and easy to lift. Under light microscopic
observation, the skin structure integrated into one continuous and
mature stratum corneum with stretched spikes. The thickness of the
collagen fibers in the dermis was essentially the same and had a
relatively regular arrangement. Masson’s staining revealed a
generally regular arrangement of the dermal collagen, which was
uniformly stained. These results suggest that the three-dimensional
structure of the dermal tissue plays a guiding role in cell repair.
The structure not only induces cell repair ingrowth, but also
adjusts the function of repair cells to improve the mechanical
state of the wound. The integrity and continuity of the dermal
tissue is a necessary prerequisite in fully executing the guiding
role in cell repair. The loss of dermal tissue integrity and
continuity following injury, which induces the lack of guiding
function, may be one of the important mechanisms affecting cell
repair function and may result in cicatrix formation. The clinical
application results of the 30 cases in the present study show no
conspicuous rejections of the cograft wound surface after
cografting with ADX and the split-thickness skin autograft, which
had a high survival rate and produced a satisfactory grafting form
and function. This type of cografting saves the patient’s
autologous skin source and skin donor area without leaving any
scars. The price of acellular dermal xenograft in China is 127
USD/10×10 cm, while the price of Integra™ in China is 3,500
USD/10×10 cm. There are no significant differences of clinical
efficacy between the two methods. In conclusion, the cografting of
ADX and split-thickness skin autograft is an ideal treatment method
for the repair of deep full-thickness burns and scar wounds and has
extensive clinical applications.
Acknowledgements
This study was supported by the Foshan
Key Scientific and Technological Research Projects (0308022) and
Foshan Medical Scientific and Technological Research Projects
(201008084).
References
|
1.
|
Yannas IV: Studies on the biological
activity of the dermal regeneration template. Wound Repair Regen.
6:518–523. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Livesey SA, Herndon DN, Hollyoak MA,
Atkinson YH and Nag A: Transplanted acellular allograft dermal
matrix. Potential as a template for the renconstruction of viable
dermis. Transplantation. 60:1–9. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Prasertsung I, Kanokpanont S, Bunaprasert
T, Thanakit V and Damrongsakkul S: Development of acellular dermis
from porcine skin using periodic pressurized technique. J Biomed
Mater Res B Appl Biomater. 85:210–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ma ZF, Chai JK, Yang HM, Liu Q, Xu MH and
Yin HN: Acellular porcine dermal matrix produced with different
methods and an experimental study on its transplantation to skin
wound. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 17:92–94. 2005.(In
Chinese).
|
|
5.
|
Feng XS, Pan YG, Tan JJ, et al: Treatment
of deep partial thickness burns by a single dressing of porcine
acellular dermal matrix. Zhonghua Wai Ke Za Zhi. 44:467–470.
2006.(In Chinese).
|
|
6.
|
Wainwright D, Madden M, Luterman A, et al:
Clinical evaluation of an acellular allograft dermal matrix in
full-thickness burns. J Burn Care Rehabil. 17:124–136. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wainwright DJ: Use of an acellular
allograft dermal matrix (AlloDerm) in the management of
full-thickness burns. Burns. 21:243–248. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
MacLeod TM, Sarathchandra P, Williams G,
Sanders R and Green CJ: Evaluation of a porcine origin acellular
dermal matrix and small intestinal submucosa as dermal replacements
in preventing secondary skin graft contraction. Burns. 30:431–437.
2004. View Article : Google Scholar
|
|
9.
|
Liu Q, Chai JK, Yang HM and Yin HN:
Experimental study on composite transplantation of acellular
porcine dermal matrix and macro-autograft to repair deep burn
wound. Zhongguo Wei Zhong Ji Jiu Yi Xue. 16:77–80. 2004.(In
Chinese).
|
|
10.
|
Xiao S, Zhu S, Li H, Yang J, Lv K and Xia
Z: Feasibility study of composite skin reconstructed by mixing
keratinocytes and acellular dermal matrix for wound repair. Swiss
Med Wkly. 139:16–21. 2009.PubMed/NCBI
|
|
11.
|
Kathju S, Lasko LA and Medich DS: Perineal
hernia repair with acellular dermal graft and suture anchor
fixation. Hernia. 15:57–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Brunetti B, Tenna S, Segreto F and
Persichetti P: The use of acellular dermal matrix in reconstruction
of complex scalp defects. Demrmatol Surg. 37:527–529. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
McCord C, Nahai FR, Codner MA, Nahai F and
Hester TR: Use of porcine acellular dermal matrix (Enduragen)
grafts in eyelids: a review of 69 patients and 129 eyelids. Plast
Reconstr Surg. 122:1206–1213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Song HF, Chai JK, Jing SH, et al: Early
repair and reconstruction for the wounds of face and joints of mass
burn casualties. Medical Journal of Chinese People’s Liberation
Army. 23:1222–1223. 2007.(In Chinese).
|
|
15.
|
Balasubramani M, Kumar TR and Babu M: Skin
substitutes: a review. Burns. 27:534–544. 2001. View Article : Google Scholar
|
|
16.
|
Xiang J, Hu QS, Qing CH, et al: The
dynamic histological observations of grafting dermal regeneration
template with thin autogenic skin in burn patients. Acta
Universitatis Medicinalis Secondae Shanghai. 23:492–494. 2007.(In
Chinese).
|
|
17.
|
Zhao JY, Chai JK, Song HF, et al: Effects
on collagen I and III after transplantation of porcine acellular
dermal matrix with hyaluronic acid. Zhonghua Yi Xue Za Zhi.
91:1276–1280. 2011.(In Chinese).
|
|
18.
|
Xu H, Sandor M, Qi S, et al: Implanted of
a porcine acellular dermal graft in a primate model of rotator cuff
repair. J Shoulder Elbow Surg. 21:580–588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Campbell KT, Burns NK, Rios CN, Mathur AB
and Butler CE: Human versus non-cross-linked porcine acellular
dermal matrix used for ventral hernia repair: comparison of in vivo
fibrovascular remodeling and mechanical repair strength. Plast
Reconstr Surg. 127:2321–2332. 2011. View Article : Google Scholar
|
|
20.
|
Pan Y, Xu J and Chen S: Expression of
vascular cell adhesion molecule 1 in acellular dermal matrix
grafting in pigs. Zhonguo Xiu Fu Chong Jian Wai Ke Za Zhi.
21:382–385. 2007.(In Chinese).
|
|
21.
|
Chiu T and Burd A: “Xenograft” dressing in
the treatment of burns. Clin Dermatol. 23:419–423. 2005.
|
|
22.
|
Feng X, Shen R, Tan J, et al: The study of
inhibiting systematic inflammatory response syndrome by applying
xenogenic (porcine) acellular dermal matrix on second-degree burns.
Burns. 33:477–479. 2007. View Article : Google Scholar
|
|
23.
|
Chiu T, Pang P, Ying SY and Burd A:
Porcine skin: friend or foe? Burns. 30:739–741. 2004. View Article : Google Scholar : PubMed/NCBI
|