Introduction
Primary testicular lymphoma is a collection of
neoplasms that constitutes only 1–9% of testicular tumors (1). Although uncommon in the general
population, it is the most common type of malignant testicular
tumor in men ≥50 years of age (2).
There are various subtypes, including diffuse large B-cell lymphoma
(DLBCL), Burkitt’s lymphoma and follicular lymphoma. In the adult
testis, primary DLBCL represents the most frequent subtype of
lymphoma (80–90%), whereas the majority of testicular lymphomas in
children consist of secondary involvement by Burkitt’s lymphoma,
DLBCL or lymphoblastic lymphoma (3). The typical clinical sign is a
painless testicular mass of variable size that is usually
unilateral. Primary testicular lymphoma may be identified during
the initial presentation of primary or systemic malignant
lymphomas, or during a clinical follow-up of patients with lymphoma
(4). Historically, primary
testicular lymphoma has been reported to exhibit a poor prognosis
with an overall 5-year survival rate of 17–48%, particularly
primary testicular DLBCL, whose clinical behavior has been reported
to be aggressive and to demonstrate a high propensity to
disseminate to the central nervous system (CNS) and skin at
presentation and relapse (5,6). The
underlying mechanisms responsible for this aggressive behaviour
have yet to be elucidated.
In the present study, a patient with primary
testicular DLBCL was examined and touch imprint specimens from the
patient’s tumor were obtained. While the histomorphology of
testicular DLBCL is well described, no information with regard to
the cytological diagnosis of this tumor is currently available in
the literature. Furthermore, the imprint cytological features of
primary testicular DLBCL have yet to be reported; thus, our results
are considered to be of interest. The imprint cytological findings
were compared with those obtained from histological examination and
immunohistochemical staining in order to evaluate the significance
of touch imprint cytology in the diagnosis of testicular DLBCL.
Case report
A 64-year-old male presented with a slowly growing,
painless enlargement in the left scrotum that was discovered by the
patient ∼2 months beforehand. The patient had a history of mild
alcohol ingestion, inguinal hernia, benign prostatic hyperplasia
and lobectomy due to non-small cell lung carcinoma that had been
fully removed 2 years previously. There was no history suggestive
of cryptorchidism or any endocrine symptoms. The patient had a
heavy feeling in the left scrotum and physical examination revealed
a left testicular mass measuring approximately the size of an
adult’s fist. The right testicle was normal. The patient had no
lymphadenopathy or hepatosplenomegaly. Examination of the
oronasopharynx revealed no abnormal results. Laboratory test
results, including hematological, urinary and biochemical values,
were within normal range. No abnormal results were observed
following an abdominal computed tomography scan. Results of a
thoracic computed tomography scan were also normal. Positron
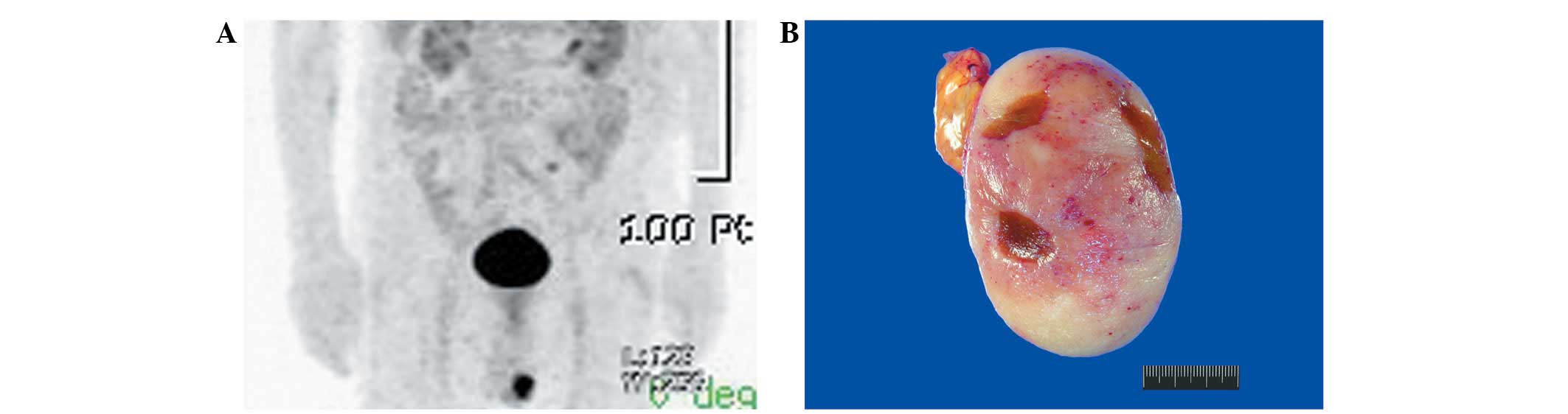
emission tomography scanning demonstrated a conspicuous
hypermetabolic lesion in the left scrotum (Fig. 1A). As a testicular neoplasm or
orchitis was clinically suspected, a left orchiectomy was
performed.
The resected specimen demonstrated the formation of
a well-circumscribed tumor measuring 7.5×5.5×4.8 cm. Grossly, the
cut surface of the tumor was solid, fleshy, lobulated and pale
yellow-to-pink with hemorrhagic punctuations (Fig. 1B). The tumor exhibited a
homogeneous texture and diffusely replaced the testicular
parenchyma. The epididymis, spermatic cord and adjacent soft
tissues appeared normal. Smears obtained from a touch imprint of
the lesion were highly cellular, consisting of discretely arranged
monomorphic large cells (Fig. 2A).
The individual tumor cells exhibited a high nucleo-cytoplasmic
ratio and clear cytoplasm forming a narrow rim around the nucleus
and a distinct outer cell border (Fig.
2B). The nuclei were enlarged, clumped and hyperchromatic, with
irregular nuclear membranes and conspicuous single to multiple
nucleoli (Fig. 2C). Intermingled
amongst the large tumor cells were small, round lymphocytes. In
some areas, tumor cells arranged in cohesive groups were also
detected (Fig. 2D).
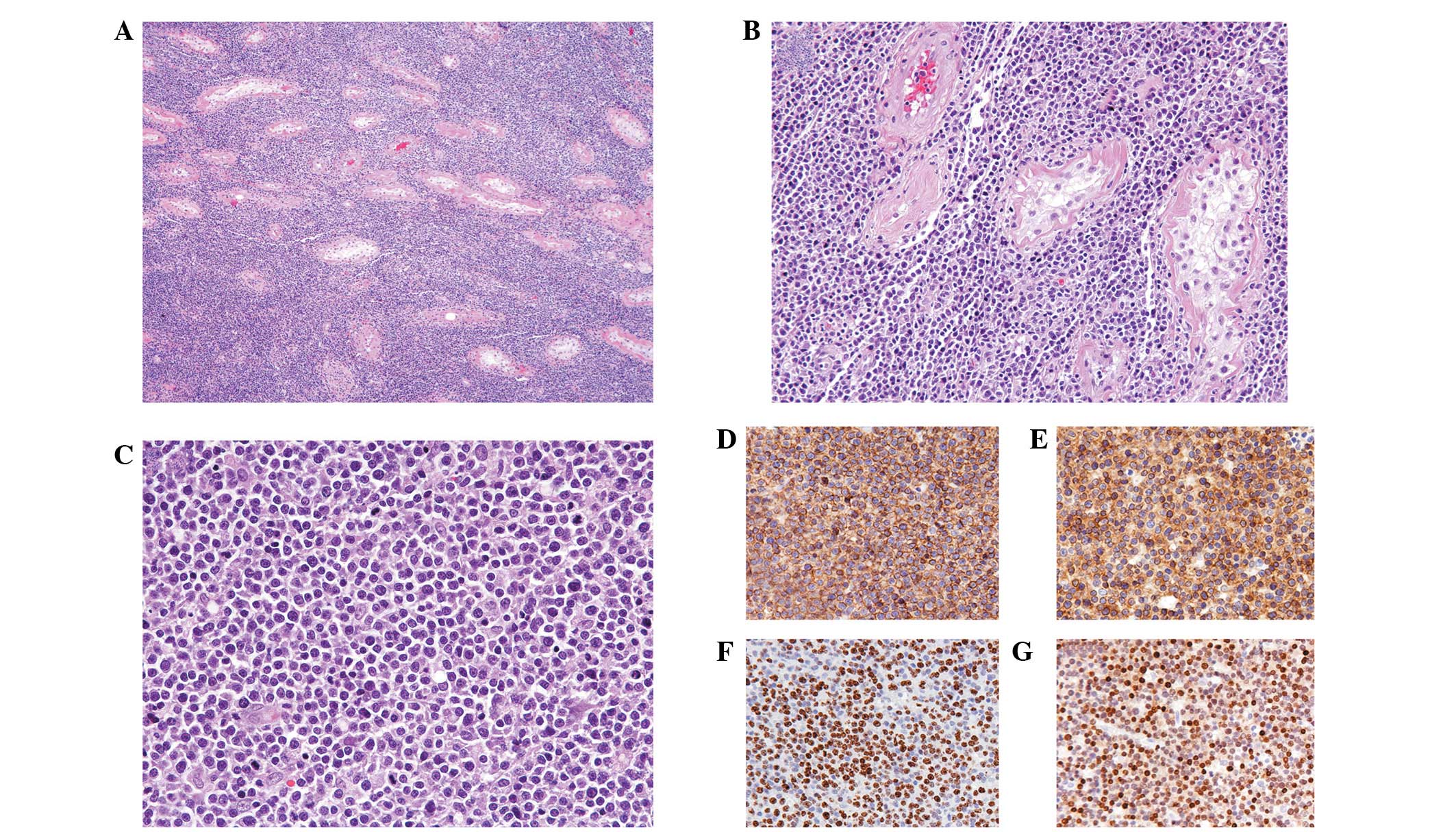
Histologically, the testicular tumor demonstrated
complete replacement by a monomorphic population of neoplastic
lymphocytes with a diffuse growth pattern (Fig. 3A). The tumor cells penetrated
diffusely into tissue spaces, producing a wide separation of intact
seminiferous tubules (Fig. 3B).
Spermatogenic arrest, interstitial fibrosis and tubular
hyalinization were also observed. Similar to the results observed
in the touch imprint specimens, the tumor cells demonstrated
enlarged nuclei with irregular nuclear membranes and conspicuous
nucleoli (Fig. 3C). In a few
areas, there was a destruction of the tubular wall and blood vessel
wall with invasion of the lumen. The tumor did not infiltrate the
epididymis, spermatic cord, tunica albuginea or tunica vaginalis.
Immunohistochemical assays revealed that the tumor cells were
markedly positive for CD45 (Fig.
3D) and CD20 (Fig. 3E);
however, they were negative for CD3, epithelial membrane antigen
and pancytokeratin, indicative of a B-cell lymphoid malignancy. The
proliferation index as detected by Ki-67 staining was high (∼90%;
Fig. 3F). In addition, since the
tumor demonstrated a CD10-negative, MUM1-positive (Fig. 3G) and BCL6-negative immunoprofile,
this case was classified as non-germinal center B-cell-like DLBCL
(non-GCB-DLBCL) (7). This study
was approved by and conducted in accordance with the policies of
the Institutional Review Board of Republic of Korea Air Force.
Informed consent was obtained from the patient.
Discussion
Although the greatest accuracy of cytological
examination of the testis is observed in azoospermic males whose
smears demonstrate normal spermatogenesis, the diagnostic accuracy
of cytological examination in testicular neoplasms has also been
reported to be extremely high (8,9).
Previous studies have described the cytomorphology of numerous
types of testicular malignancy, including classic and spermatocytic
seminoma, embryonal carcinoma and metastatic lesions (8,10,11);
however, no information with regard to the cytological diagnosis of
primary testicular DLBCL is available in the literature. To the
best of our knowledge, the present study is the first to describe
the cytological features of testicular DLBCL. Smears obtained from
the touch imprints exhibited a high cellular yield predominantly
consisting of discretely arranged monomorphic lymphocytes with
irregular nuclear membranes, scant cytoplasm and conspicuous
nucleoli. These findings were identical to those of primary nodal
DLBCL.
The observation of cohesive cellular aggregates in
specific areas of the imprint cytological smear slides was notable.
A pattern of cohesive groups of tumor cells is not common in DLBCL,
although it may occasionally occur. This atypical feature mimics
metastatic carcinoma and may confound diagnosis. In fact,
distinguishing DLBCL from metastatic carcinoma on cytological
examination is usually a straightforward procedure for an
experienced pathologist. Generally, benign or malignant lymphoid
cells are characterized by a predominant single-cell pattern on
cytology specimens, whereas carcinoma cells typically exhibit
cohesive clusters (12). Although
lymphoma cells may occasionally artificially demonstrate focal
cohesion, particularly in highly cellular specimens, the
predominant single-cell pattern in the background usually aids in
establishing the correct diagnosis.
Previously, it has been shown that DLBCLs may be
divided into three prognostically distinct subtypes by gene
expression profiles using a cDNA microarray (7): GCB-DLBCLs, activated B-cell-like
DLBCLs and type 3. The immunohistochemical expression of CD10, BCL6
and MUM1 may be used to categorize DLBCLs into GCB and non-GCB
types, the latter including activated B-cell-like types and type 3
(13). GCB-DLBCLs are assigned to
those that express CD10 and/or are positive for BCL6 but negative
for MUM1, and non-GCB-DLBCLs are assigned to those negative for
CD10 and positive for MUM1. These subtypes differ in clinical
behavior, i.e., GCB-DLBCLs have an improved clinical outcome
compared with non-GCB types. Al-Abbadi et al (14) demonstrated that primary testicular
DLBCL exhibited non-GCB type gene expression almost exclusively. Li
et al (6) also demonstrated
that the majority of primary testicular DLBCLs exhibited non-GCB
type characteristics and the overall survival rate of patients with
non-GCB-DLBCL was significantly lower compared with patients with
GCB-DLBCL. Based on these data, it is reasonable to hypothesize
that the main explanation for the poor prognosis of primary
testicular DLBCL may be its correlation with the non-GCB
phenotype.
Differential diagnosis of primary testicular DLBCL
may involve a number of germ cell tumors, including classic
seminoma, spermatocytic seminoma and embryonal carcinoma (1). Granulomatous and viral orchitis may
also mimic lymphoma histologically. Seminoma cells, unlike the
majority of lymphoma cells, have distinct cell membranes, abundant
glycogen-rich cytoplasms and rounded but focally flattened central
nuclei. The cells of spermatocytic seminoma are polymorphous and
belong to three distinct types. Embryonal carcinoma has a
characteristic epithelioid appearance that frequently forms
glandular, papillary or tubular structures. Lymphomas often possess
smaller cells with less cytoplasm and a higher nucleo-cytoplasmic
ratio. In addition, they demonstrate diffuse intertubular
infiltration with recognizable tubular remnants. This
characteristic intertubular growth pattern of lymphoma is initially
suggestive of the diagnosis in numerous cases. Furthermore, in
contrast to seminoma and embryonal carcinoma, lymphomas lack
precursor intratubular germ cell neoplasia. Although lymphoma cells
may invade the seminiferous tubules, they do not demonstrate the
regular basal alignment within the tubules that is observed in
intratubular germ cell neoplasia. Viral and granulomatous orchitis
have heterogeneous and benign-appearing inflammatory cellular
infiltrates, in contrast to the more homogeneous and
malignant-appearing infiltrate of lymphoma.
The treatment for patients with primary testicular
DLBCL may be divided into limited disease (stage I/II) and advanced
disease (stage III/IV) treatments. For limited disease, a standard
treatment has yet to be established (2). Orchiectomy provides histological
tissue for diagnosis and also removes a potential sanctuary site,
as the blood-testis barrier renders testicular tumors inaccessible
to systemic chemotherapy (15).
The cyclophosphamide, doxorubicin, vincristine and prednisone
(CHOP) regimen has been the mainstay of therapy for several
decades. More recently, the addition of the anti-CD20 monoclonal
antibody rituximab to the CHOP regimen (R-CHOP) has led to a marked
improvement in progression-free and overall survival (16). Routine CNS prophylaxis is
recommended in patients with primary testicular lymphoma of any
stage due to the high rate of CNS recurrence. Radiation therapy may
be used as a prophylactic therapy to prevent relapse in the
regional lymph nodes or in the controlateral testis, or to treat
lymphomatous lesions, including retroperitoneal lymphadenopathies.
For advanced disease, patients should be treated according to the
guidelines for the treatment of advanced stage nodal DLBCL. The
standard therapeutic option for patients with stage III/IV disease
is conventional-dose anthracycline-containing chemotherapy plus
rituximab with the addition of prophylactic scrotal radiotherapy
and intrathecal chemotherapy. The standard therapeutic option for
patients with relapsed disease has yet to be defined in prospective
trials. However, the therapeutic strategy should be identical to
the strategies used for other relapsed aggressive forms of
non-Hodgkin’s lymphoma.
In conclusion, careful observation of the touch
imprint specimen of testicular DLBCL reveals a high cellularity
with a predominant single-cell pattern of monomorphic cells
demonstrating irregular nuclear membranes and conspicuous nucleoli.
In addition, taking into consideration that DLBCL is capable of
developing in the testis and forming a predominantly discohesive
cell population that suggests a lymphoid malignancy, it may be
possible to detect morphological features characteristic of DLBCL
using imprint cytology. To the best of our knowledge, this is the
first study describing the touch imprint cytological diagnosis of
testicular DLBCL. It is important to identify primary testicular
DLBCL correctly and to distinguish it from other entities due to
differences in therapy, management and prognosis.
Acknowledgements
The author would like to thank Ja Ok
Kim (Aerospace Medical Library, Aerospace Medical Center, Republic
of Korea Air Force) for valuable expertise in assisting with the
literature search.
References
|
1.
|
Horne MJ and Adeniran AJ: Primary diffuse
large B-cell lymphoma of the testis. Arch Pathol Lab Med.
135:1363–1367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shahab N and Doll DC: Testicular lymphoma.
Semin Oncol. 26:259–269. 1999.
|
|
3.
|
Miedler JD and MacLennan GT: Primary
testicular lymphoma. J Urol. 178:26452007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Doll DC and Weiss RB: Malignant lymphoma
of the testis. Am J Med. 81:515–524. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zucca E, Conconi A, Mughal TI, et al
International Extranodal Lymphoma Study Group: Patterns of outcome
and prognostic factors in primary large-cell lymphoma of the testis
in a survey by the International Extranodal Lymphoma Study Group. J
Clin Oncol. 21:20–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Li D, Xie P and Mi C: Primary testicular
diffuse large B-cell lymphoma shows an activated B-cell-like
phenotype. Pathol Res Pract. 206:611–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Alizadeh AA, Eisen MB, Davis RE, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rammou-Kinia R, Anagnostopoulou I,
Tassiopoulos F and Lykourinas M: Fine needle aspiration of the
testis. Correlation between cytology and histology. Acta Cytol.
43:991–998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Verma K, Ram TR and Kapila K: Value of
fine needle aspiration cytology in the diagnosis of testicular
neoplasms. Acta Cytol. 33:631–634. 1989.PubMed/NCBI
|
|
10.
|
Pandey A, Nandini N, Jha A and Manjunath
G: Fine needle aspiration cytology and cell block in the diagnosis
of seminoma testis. J Cytol. 28:39–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Saran RK, Banerjee AK, Gupta SK and
Rajwanshi A: Spermatocytic seminoma: a cytology and histology case
report with review of the literature. Diagn Cytopathol. 20:233–236.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Das DK: Value and limitations of
fine-needle aspiration cytology in diagnosis and classification of
lymphomas: A review. Diagn Cytopathol. 21:240–249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hans CP, Weisenburger DD, Greiner TC, et
al: Confirmation of the molecular classification of diffuse large
B-cell lymphoma by immunohistochemistry using a tissue microarray.
Blood. 103:275–282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Al-Abbadi MA, Hattab EM, Tarawneh MS, Amr
SS, Orazi A and Ulbright TM: Primary testicular diffuse large
B-cell lymphoma belongs to the nongerminal center B-cell-like
subgroup: a study of 18 cases. Mod Pathol. 19:1521–1527. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Vitolo U, Ferreri AJ and Zucca E: Primary
testicular lymphoma. Crit Rev Oncol Hematol. 65:183–189. 2008.
View Article : Google Scholar
|
|
16.
|
Coiffier B: Rituximab therapy in malignant
lymphoma. Oncogene. 26:3603–3613. 2007. View Article : Google Scholar : PubMed/NCBI
|