Introduction
A great deal of attention has been paid to the
theory of inflammation and certain researchers consider that
diabetes, obesity and atherosclerosis represent a low-grade,
chronic inflammatory condition. Pickup (1) hypothesized that stimulation from
dietary surplus and other environmental factors results in the
activation of a specific cell population of the innate immune
system. Certain sentinel cells, including macrophages and fat
cells, secrete tumor necrosis factor (TNF)-α, IL-6 and other
inflammatory agents that cause a low-grade inflammatory condition
and accordingly trigger insulin resistance, diabetes and related
diseases. Toll-like receptor 4 (TLR4) is one of the receptors that
is able to identify pathogenic microorganisms in a natural immune
system, bind the specific ligand and produce corresponding
inflammation following identification. One study identified that
TLR4 is related to whole-body, low-grade chronic inflammatory
diseases, including those mentioned above (2).
Previous studies have demonstrated that TLR4 may be
a central link between insulin resistance, inflammation and
obesity, and that a point mutation in TLR4, which inactivates the
receptor, prevents the diet-induced obesity (DIO) activation of IκB
kinase (IKKβ) and c-Jun NH2-terminal kinase (JNK), and inhibits
insulin resistance, suggesting that TLR4 is a key modulator in the
cross-talk between inflammatory and metabolic pathways (3–8).
TLR4 exogenous ligands include lipopolysaccharides
(LPS), endogenous ligands, including free fatty acids (FFA)
(9), high-molecular-weight sugar
(10) and the surface of fungi
polysaccharides (10). Myenteric
neurons mediate LPS recognition via TLR4, resulting in neuronal
cell death (11). Continuous FFA
stimulation activates TLR4 signaling pathways; however, when TLR4
is blocked or knocked out, FFA stimulation is also blocked
(12). In addition, triglycerides
(TG) also activate TLR4/NF-κB and increase the expression of
inflammatory cytokines. One study demonstrated that acute and
chronic, physical exercise in DIO rats induces significant
suppression in the TLR4 signaling pathway in the liver, muscle and
adipose tissue, reduces LPS serum levels and improves insulin
signaling and sensitivity (13).
Therefore, low-grade inflammation triggered by a high-fat diet is
closely associated with TLR4 expression. The main focal point of
current research is TLR4 receptors in insulin-sensitive organs and
tissues, including fat, liver, pancreas and skeletal muscle;
however, studies concerning intestinal TLR4 have not yet been
reported.
The intestines are not only primary immune organs,
but they also have the most extensive surface area compared with
other immune organs. The TLR4 receptors, which are distributed on
the intestinal surface, recognize enteric pathogen-associated
molecular patterns (PAMPs) and activate NF-κB. Then, signals
incorporated into the corresponding promoter and enhancer DNA
binding sites in the cytokine gene regulation area initiate gene
transcription and expression (14). Induced cytokines further activate
immunocytes or cytokine receptors, expand the immune reaction,
cause excessive release of inflammatory mediators and produce a
cascade reaction of inflammatory factors. As a sensor of endogenous
lipid and fatty acids, TLR4 regulates metabolism and the immune
system (15).
On the basis of data from previous studies, we
hypothesize that intestinal TLR4, activated by components of the
high-fat diet, may become a key trigger for intestinal low-grade
inflammation. Thus, the present study aimed to analyze the effect
of mouse intestinal TLR4/NF-κB signaling pathway activation caused
by a short-term, high-fat diet, as well as the function of the
signaling pathway in the local enteric inflammatory response.
Materials and methods
Animals
Adult male C57BL/6 mice (n=60) aged 6 weeks,
weighing 18±2 g, were purchased from Xinjiang Medical University
Animal Experimental Center (Urumqi, China). All animals were housed
and used in accordance with the Chinese Regulations for Animal
Care. This study was performed in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of the First Affiliated Hospital of
Xinjiang Medical University. All experiments were conducted with
the approval of the animal ethics committees of Xinjiang Medical
University. The mice were randomly divided into six groups and each
group of ten mice was fed with a high-fat diet for 0, 1, 3, 5, 7
and 9 days, respectively. The formula of the high-fat diet was
added to common fodder as follows: 10% sucrose, 28% maltodextrin,
0.12% choline chloride, 10% lard, 27.5% casein, 0.24% methionine
and 0.1% sodium chloride.
Hematoxylin and eosin (H&E)
staining
The mice were sacrificed by decapitation after 6 h
of fasting in accordance with experimental requirements. The
intestines were removed, absterged with physiological saline
solution and then stored at −80°C until analysis.
A 1 cm length of intestinal tissue from each sample
was obtained. A microscope slide with rehydrated tissue sections
was fixed in alcohol. The slide was immersed for 30 sec with
agitation by hand in H2O. The slide was dipped into a
Coplin jar containing Mayer’s hematoxylin and agitated for 30 sec.
The slide was rinsed with H2O for 1 min. The slide was
stained with 1% eosin Y solution for 10–30 sec with agitation. The
sections were dehydrated with two changes of 95% alcohol and two
changes of 100% alcohol for 30 sec each. The alcohol was removed
with two changes of xylene. One or two drops of mounting medium was
added and the section was covered with a coverslip. The intestinal
mucosa was observed by light microscopy using the single-blind
method.
Immunohistochemistry
Intestinal tissue was fixed with paraformalin (40
g/l), embedded in paraffin, sectioned using a microtome (4 μm),
deparaffinized by xylene, dehydrated with a graded alcohol series,
blocked with 10% goat serum for 30 min at room temperature in order
to block nonspecific binding and repaired. TNF-α and IL-6 were
detected using rabbit anti-TNF-α and anti-IL-6 polyclonal
antibodies (Abs), followed by application of horseradish peroxidase
(HRP)-labeled goat secondary Abs. The presence of brown staining in
the cytoplasm and/or nuclei indicated that cells were positive for
TNF-α or IL-6. Each sample was randomly assessed with five dyes at
high magnification (x400). Positive cells were graded and scored
according to a coloring scale: no coloring (−, 0 points), coloring
area <25% (+, 1 point), coloring area 25–50% (++, 2 points) and
coloring area >50% (+++, 3 points).
Western blot analysis
Briefly, holoprotein extracts (70 mg) of the mouse
intestinal tissue in each sample were electrophoresed on a 10%
sodium dodecyl sulfate (SDS)-polyacrylamide gel and transferred to
polyvinylidene fluoride (PVDF) membranes. Activated TLR4, NF-κB and
phosphorylated IκB (PIκB) were detected using rabbit Abs. Membranes
were blocked and incubated with appropriate Abs at 4°C overnight,
then imaged by conjugation with a HRP-linked secondary antibody and
enhanced chemiluminescence (ECL) detection reagent. All experiments
were performed in triplicate with similar results. All protein
expression was divided by the amount of β-actin of individual
samples as analyzed by image software. The blots were analyzed by
densitometry.
Real-time polymerase chain reaction
(PCR)
The mRNA expression was analyzed using reverse
transcription (RT)-PCR. Total RNA from mouse intestines was
extracted using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Each RNA sample (5 μg) was diluted and
reverse-transcribed into complementary DNA (cDNA), to provide
transcripts (3 μg) for amplification. The primer sequences for
amplification of the cDNA were as follows: TLR4, forward:
5′-CACTGTTCTTCTCCTGCCTGAC-3′ and reverse, 5′-TGG
TTGAAGAAGGAATGTCATC-3′); NF-κB, forward: 5′-CCT
CTGGCGAATGGCTTTAC-3′ and reverse: 5′-GCTATGGAT ACTGCGGTCTGG-3′;
β-actin, forward: 5′-CACGATGGA GGGGCCGGACTCATC-3′ and reverse:
5′-TAAAGACCTCTA TGCCAACACAGT-3′). The PCR conditions were as
follows: i) 95°C for 2 min for one cycle; ii) 95°C for 45 sec; iii)
54°C for 45 sec; iv) 72°C for 1 min (modified for each primer set);
v) steps ii, iii and iv were repeated for 30 cycles for TLR4 mRNA,
35 cycles for NF-κB mRNA and 27 cycles for β-actin mRNA; vi) 72°C 5
min for one cycle. The identification of the PCR fragments was
confirmed by size following electrophoretic migration on ethidium
bromide (0.5 mg/l)-stained agarose gels and imaging. The amount of
the PCR products was imaged and expressed as optical density. The
target cDNA present in each sample was corrected for the respective
β-actin values.
Statistical analysis
Data are presented as mean ± standard deviation
(SD). Student t-tests were performed to determine the statistical
significance of protein and mRNA expression levels among the
different groups. Enumeration data was analyzed by a rank sum test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
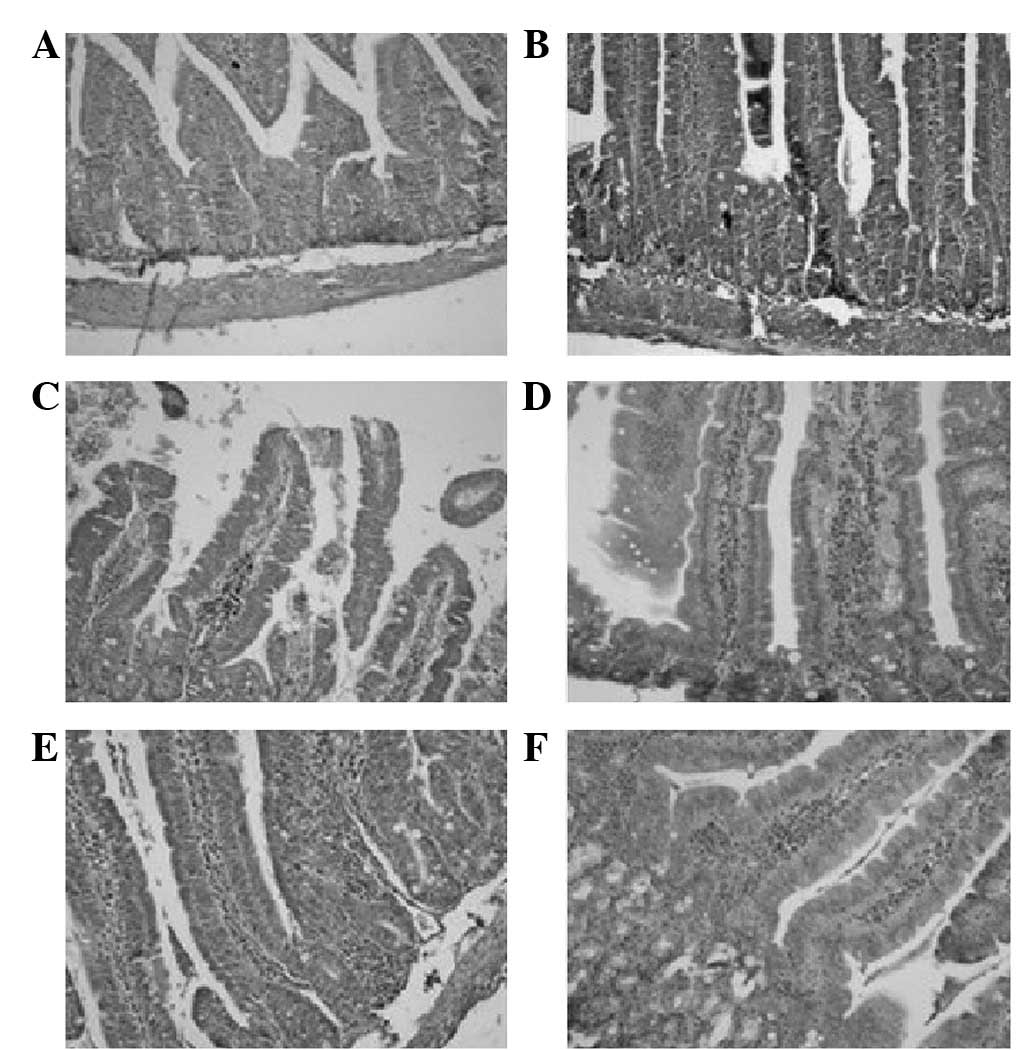
H&E staining
By macroscopic observation, there were no clear
changes and no hyperemia or hydrops in the enteric cavity. By light
microscopic observation, there was no ulceration or interruption of
the intestinal mucosa and no mass neutrophilic granulocyte
infiltration. Diffused macrophage distribution was observed on days
7 and 9 (Fig. 1).
Immunohistochemistry
All the groups expressed TNF-α and IL-6, with the
exception of the day 0 group. The presence of brown staining in the
cytoplasm and/or nuclei indicated that cells were positive for
TNF-α and IL-6, which were mainly expressed in the nuclei. We
observed that the quantity and distribution of TNF-α-positive cells
presented dynamic changes with sustained stimulation by a high-fat
diet. The expression gradually strengthened and demonstrated
cluster distribution in the epithelial mucosa, lamina propria
mucosa and submucosa (Table I and
Fig. 2). The expression of
IL-6-positive cells increased gradually in the epithelial mucosa
and lamina propria mucosa, and diffused distribution demonstrated a
clustering trend (Table II and
Fig. 3).
 | Figure 2Mouse intestinal tissue with
immunohistochemical staining of tumor necrosis factor (TNF)-α. On
day 0 of high-fat diet stimulation, there were no TNF-α-positive
cells. However, there were TNF-α-positive cells during the
following days; a changing trend was observed with time. On day 1,
there were a few cells expressing TNF-α that were continuously
distributed in the intestinal epithelium. On day 3, the
TNF-α-positive cells in the intestinal epithelium markedly deepened
in color, while a few cells began to distribute in the lamina
propria mucosa. On day 5, the TNF-α-positive cells in the lamina
propria mucosa began to darken in color, increase in number and
there was a clustering trend in the distribution. On day 7, the
TNF-α-positive cells in the lamina propria mucosa were distributed
in clusters; however, few were distributed and scattered in the
submucosa. On day 9, the TNF-α-positive cells were distributed in
clusters and dispersed in the lamina propria mucosa and submucosa.
(A) Day 0; (B) day 1; (C) day 3; (D) day 5; (E) day 7; (F) day 9.
Magnification, ×40. |
 | Figure 3Mouse intestinal IL-6
immunohistochemical staining. On day 0 of high-fat diet
stimulation, there were no IL-6-positive cells in the intestines.
However, there were IL-6-positive cells during the following days,
which gradually changed over time on days 1–9. On day 1, there were
a few cells weakly expressing IL-6, which were sporadically
distributed in the intestinal epithelium. On day 3, the
IL-6-positive cells in the intestinal epithelium were continuously
distributed and the color markedly deepened. There were a few pale
colored IL-6-positive cells in the lamina propria mucosa. On day 5,
the IL-6-positive cells in the lamina propria mucosa darkened in
color; however, no changes in quantity or range occurred. On day 7,
the amount of IL-6-positive cells in the lamina propria mucosa
increased markedly and demonstrated a clustering trend in
distribution. On day 9, there were IL-6-positive cells distributed
in clusters, dispersed in the lamina propria mucosa but not in the
submucosa. (A) Day 0; (B) day 1; (C) day 3; (D) day 5; (E) day 7;
(F) day 9. Magnification, ×40. |
 | Table IDistribution of TNF-α in mouse
intestines. |
Table I
Distribution of TNF-α in mouse
intestines.
| Time (days) |
|---|
|
|
|---|
| Score | 0 | 1 | 3 | 5 | 7 | 9 |
|---|
| − | 6 | 5 | 4 | 2 | 0 | 0 |
| + | 0 | 1 | 2 | 4 | 4 | 2 |
| ++ | 0 | 0 | 0 | 0 | 2 | 2 |
| +++ | 0 | 0 | 0 | 0 | 0 | 2 |
 | Table IIDistribution of IL-6 in mouse
intestines. |
Table II
Distribution of IL-6 in mouse
intestines.
| Times (days) |
|---|
|
|
|---|
| Score | 0 | 1 | 3 | 5 | 7 | 9 |
|---|
| − | 6 | 5 | 4 | 2 | 1 | 1 |
| + | 0 | 1 | 2 | 3 | 3 | 2 |
| ++ | 0 | 0 | 0 | 1 | 2 | 2 |
| +++ | 0 | 0 | 0 | 0 | 0 | 1 |
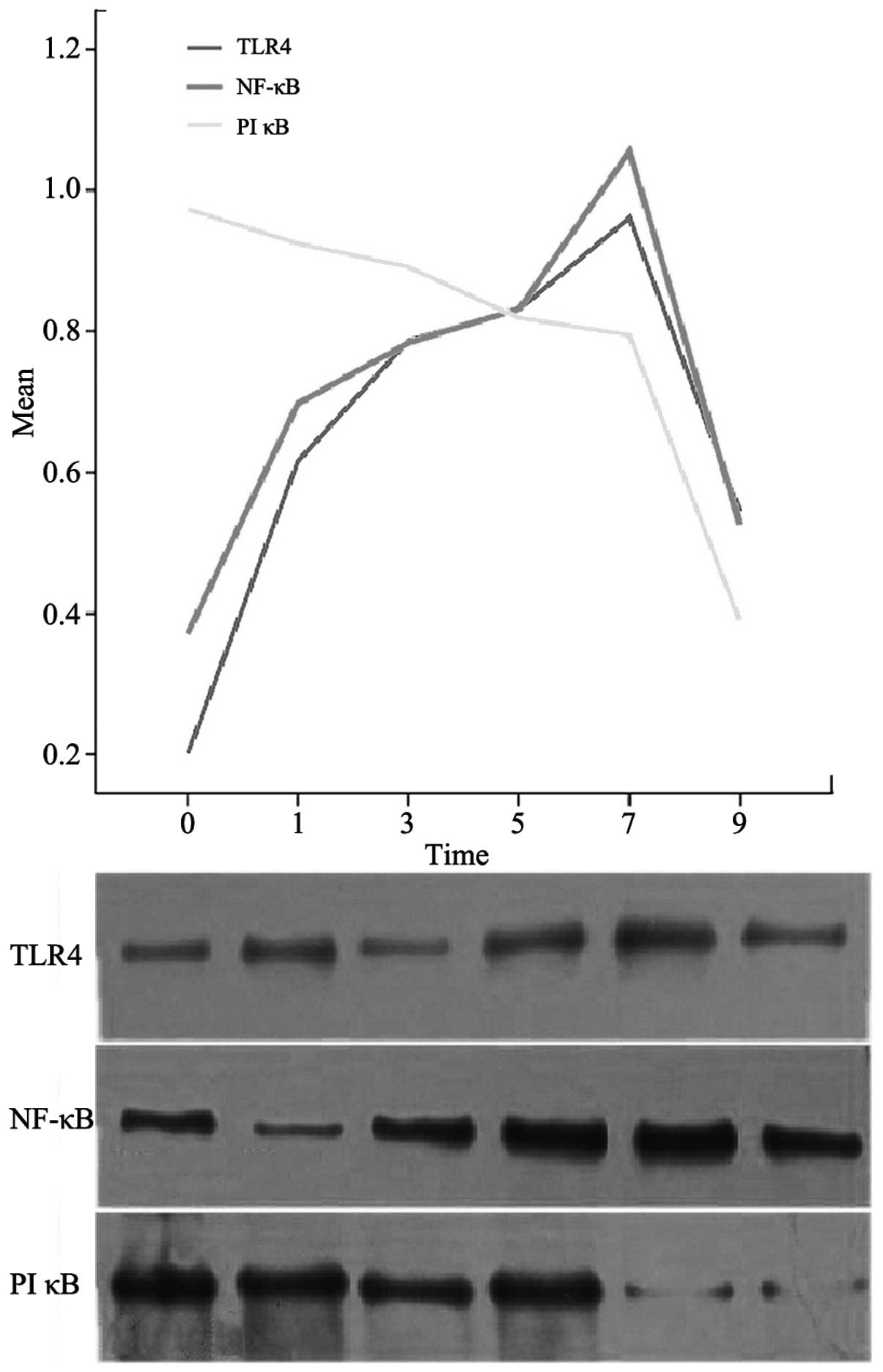
Western blot analysis
On day 0, no TLR4 or NF-κB protein expression was
observed in the mouse intestines; however, PIκB protein expression
was present and was at its peak level (P<0.05). At 1, 3, 5, 7
and 9 days, the TLR4 and NF-κB protein expression levels increased
progressively until day 7, when peaks were reached (P<0.05). The
expression levels then decreased. The expression levels of PIκB
protein decreased gradually and reached a minimum on day 9
(P<0.05; Fig. 4).
RT-PCR
On day 0, there was no expression of TLR4 or NF-κB
mRNA in the mouse intestines. On days 1, 3, 5, 7 and 9, the mRNA
expression levels of TLR4 and NF-κB increased gradually until day
7, when maximum levels were reached (P<0.05). Then, a gradual
reduction was observed. As a reference, β-actin expression remained
relatively stable (Fig. 5).
Discussion
Our results show that a short-term, high-fat diet
induces local inflammation in mouse intestine, accompanied by
activation of TLR4/NF-κB signaling. This indicates that TLR4/NF-κB
signaling is related to the induction of local inflammation in the
intestines caused by a short-term, high-fat diet. The results
revealed no clear changes in the physiological structure of
intestinal tissue following stimulation with a short-term high-fat
diet. From day 1 to 9, the intestinal mucosa was complete and a
large accumulation of macrophages was not detected. On days 7 and
9, we observed a distribution of macrophages, indicating that the
inflammatory reaction shown in this study is different from
pathological damage caused by diseases such as ulcerative colitis,
since it is low-grade inflammation.
Cani et al(16) hypothesized that bacterial LPS acts
as a triggering factor, linking inflammation to high-fat
diet-induced diabetes and obesity. The authors identified that
consumption of a high-fat diet resulted in significant modulation
of the dominant bacterial populations within the gut microflora.
Additionally, the authors observed a reduction in the number of
bifidobacteria, Eubacterium rectale-Clostridium
coccoides species and bacteroides, which favored an increase in
the gram-negative to gram-positive ratio. However, in the present
study, the observation cycle was shorter than the cycle for
bacterial enhancement and LPS release, thus avoiding the
interference of LPS generated by intestinal gram-negative bacteria.
The results of the present study indicated that a high-fat diet, as
an endogenous TLR4 ligand, causes increasing intestinal TLR4
expression. After 1 day of high-fat diet, there was mild activation
of intestinal TLR4/NF-κB, which increased gradually, peaking on day
7. According to the analysis of the results, the expression of TLR4
and NF-κB was coincident with the production of TNF-α and IL-6.
This is in agreement with the results of the study by Tsujimoto
et al(17), which
demonstrated that the amount of TLR4 expressed was related to the
quantity of the inflammatory factor released. PIκB, formed by the
phosphorylation of IκB, is the key step for activating the
TLR4/NF-κB signaling pathway. With the continual consumption of
intracellular PIκB, the activation level of TLR4/NF-κB increased.
After 7 days, the expression of TLR4/NF-κB began to decrease. This
may signify that PIκB was almost exhausted or a protection
mechanism was activated.
The local intestinal inflammatory response and
inflammatory factors complement each other. Ou et
al(18) investigated the
expression of TLR4 on human mononuclear/macrophage (THP 1) cell
surfaces with flow cytometry. The results demonstrated that the
expression of TLR4 on the cell surface may be significantly
activated within 24 h after stimulation by IL-6. Abreu et
al(19) identified that
activated NF-κB induces the transcription of TNF-α; TNF-α further
promotes the expression of NF-κB. Studies have demonstrated that
TNF-α induces the apoptosis of intestinal epithelium cells
(20–22), as well as a change in the structure
and function of tight junction proteins between cells (23), which leads to increased intestinal
epithelium permeability (24) and
eventually causes diffusion of the local inflammatory reaction.
Inflammatory agents in the local intestinal reaction, including
TNF-α and IL-6, may accelerate the activation of the TLR4/NF-κB
pathway. This may explain why in the current study the activation
of the TLR4/NF-κB pathway demonstrated an increasing trend with
time.
Reactive oxygen species (ROS) produced as a result
of the activation of TLRs, induce a change in cells. Ko et
al identified that activation of TLRs in the innate immune
response causes retinal photoreceptor oxidative stress and
mitochondrial DNA (mtDNA) damage (25). Ye et al(26) expanded our knowledge of TLR4 in a
well-characterized mouse model of fatty liver disease induced by a
westernized diet. The authors identified that a genetic deletion
model of TLR4 (Apoe−/−/TLR4−/−) led to a
reduction in high-fat, high-cholesterol diet-induced liver
inflammation and injury compared with that observed in wild-type
mice (Apoe−/−/TLR4+/+), which is associated
with the reduced expression of ROS and pro-inflammatory
cytokines.
The intestine is the body’s largest ‘bank of
bacteria and endotoxins’ with a mucous membrane barrier that is
highly selective and maintains normal intestinal physiological
activities. The intestinal mucous membrane barrier is chiefly
composed of mechanical, immunological and biological barriers. The
mechanical barrier is primarily constructed of epithelial cells
with a tight junction between them; it is the first line of defense
against antigens and toxins from outside. TLR4, which is well
distributed on the surface, is able to identify pathogens quickly
and rapidly induces an immune inflammatory response. The role of
macrophages in local intestinal inflammation should not be ignored.
The intestinal immune barrier is mainly constructed of secretory
immunoglobulin (SIgA) and SIgA is made up of poly-immunoglobulin
(pIgA) and pIgA receptors (pIgR). Since there are a number of
different types of glycosyls that are significant bacterial ligands
on pIgR, the enteric cavity avoids attack by LPS and enjoys immune
protection (27). Multiple factors
impact the expression of pIgR, including the nutritional state, as
well as cytokines, such as TNF-α (28). An increased level of TNF-α induces
enterocytes to secrete more pIgR at the protein and molecular
level, and to increase intestinal immunoprotection. Furthermore,
TNF-α also combines with cell surface receptors, including
lymphatic toxin receptors and B-cell activators, to participate in
the activation of lymphoid organ genes and enable the transcription
of the pIgR gene. This is extremely important in limiting the
intestinal inflammation caused by viruses and bacteria and in
promoting tissue repair (29). It
may explain why the protein and mRNA levels of TLR4 and NF-κB were
markedly reduced on day 9 compared with their levels on day 7.
The hypothesis that TLR4 signaling is involved in
autoimmune diseases has prompted research into TLR4 inhibition. One
study demonstrated that the binding of mAbs to distinct regions on
TLR4 inhibits LPS-dependent activation, providing a novel method
for manipulating TLR4 activation and also a rationale for designing
drugs targeted to TLR4 (30).
As mentioned previously, we successfully established
a model of local intestinal inflammation using a high-fat diet. The
digested products of the high-fat diet acted as ligands to rapidly
activate the intestinal TLR4/NF-κB pathway and cause local
inflammation. Whether the local intestinal low-grade inflammation
induced by high-fat diet is a prologue to a systemic inflammation
response or a partial expression of systemic inflammation remains
unknown and requires further study.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (30801019) and was also
supported by the Department of Metabolic Diseases, VIP Laboratory,
First Affiliated Hospital of Xinjiang Medical University.
References
|
1
|
Pickup JC: Inflammation and activated
innate immunity in the pathogenesis of type 2 diabetes. Diabetes
Care. 27:813–823. 2004. View Article : Google Scholar
|
|
2
|
Michelsen KS, Doherty TM, Shah PK and
Arditi M: TLR signaling: an emerging bridge from innate immunity to
atherogenesis. J Immunol. 173:5901–5907. 2004. View Article : Google Scholar
|
|
3
|
Kim F, Pham M, Luttrell I, et al:
Toll-like receptor-4 mediates vascular inflammation and insulin
resistance in diet-induced obesity. Circ Res. 100:1589–1596. 2007.
View Article : Google Scholar
|
|
4
|
Kopp A, Buechler C, Neumeier M, et al:
Innate immunity and adipocyte function: ligand-specific activation
of multiple Toll-like receptors modulates cytokine, adipokine, and
chemokine secretion in adipocytes. Obesity (Silver Spring).
17:648–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nguyen MT, Favelyukis S, Nguyen AK, et al:
A subpopulation of macrophages infiltrates hypertrophic adipose
tissue and is activated by free fatty acids via Toll-like receptors
2 and 4 and JNK-dependent pathways. J Biol Chem. 282:35279–35292.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song MJ, Kim KH, Yoon JM and Kim JB:
Activation of Toll-like receptor 4 is associated with insulin
resistance in adipocytes. Biochem Biophys Res Commun. 346:739–745.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsukumo DM, Carvalho-Filho MA, Carvalheira
JB, et al: Loss-of-function mutation in Toll-like receptor 4
prevents diet-induced obesity and insulin resistance. Diabetes.
56:1986–1998. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Barnes GT, Yang Q, et al: Chronic
inflammation in fat plays a crucial role in the development of
obesity-related insulin resistance. J Clin Invest. 112:1821–1830.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dasu MR, Devaraj S, Zhao L, Hwang DH and
Jialal I: High glucose induces Toll-like receptor expression in
human monocytes. Diabetes. 57:3090–3098. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smiley ST, King JA and Hancock WW:
Fibrinogen stimulates macrophage chemokine secretion through
toll-like receptor 4. J Immunol. 167:2887–2894. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arciszewski MB, Sand E and Ekblad E:
Vasoactive intestinal peptide rescues cultured rat myenteric
neurons from lipopolysaccharide induced cell death. Regul Pept.
146:218–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi H, Kokoeva MV, Inouye K, Tzameli I,
Yin H and Flier JS: TLR4 links innate immunity and fatty acid
induced insulin resistance. J Clin Invest. 116:3015–3025. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oliveira AG, Carvalho BM, Tobar N, et al:
Physical exercise reduces circulating lipopolysaccharide and TLR4
activation and improves insulin signaling in tissues of DIO rats.
Diabetes. 60:784–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grigoryev DN, Finigan JH, Hassoun P and
Garcia JG: Science review: searching for gene candidate in acute
lung injury. Crit Care. 8:440–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tschöp M and Thomas G: Fat fuels insulin
resistance through Toll-like receptors. Nat Med. 12:1359–1361.
2006.PubMed/NCBI
|
|
16
|
Cani PD, Bibiloni R, Knauf C, et al:
Changes in gut microbiota control metabolic endotoxemia-induced
inflammation in high-fat diet-induced obesity and diabetes in mice.
Diabetes. 57:1470–1481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsujimoto H, Ono S, Efron PA, Scumpia PO,
Moldawer LL and Mochizuki H: Role of Toll-like receptors in the
development of sepsis. Shock. 29:315–321. 2008.PubMed/NCBI
|
|
18
|
Ou HQ, Ma XH, Shen J and Shen HJ: Effects
of human interleukin-6 (IL-6) on the expression of Toll like
receptor-4 (TLR4) of THP-1. Acta Universitatis Medicinalis Nanjing
(Natural Science). 30:319–323. 2010.
|
|
19
|
Abreu MT, Vora P, Fature E, Thomas LS,
Arnold ET and Arditi M: Decreased expression of Toll-like
receptor-4 and MD-2 correlates with intestinal epithelial cell
protection against dysregulated proinflammatory gene expression in
response to bacterial lipopolysaccharide. J Immunol. 167:1609–1616.
2001. View Article : Google Scholar
|
|
20
|
Bojarski C, Bendfeldt K, Gitter AH, et al:
Apoptosis and intestinal barrier function. Ann NY Acad Sci.
915:270–274. 2000.PubMed/NCBI
|
|
21
|
Bruewer M, Luegering A, Kucharzik T, et
al: Proinflammatory cytokines disrupt epithelial barrier function
by apoptosis-independent mechanisms. J Immunol. 171:6164–6172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pinkoski MJ, Droin NM and Green DR: Tumor
necrosis factor alpha up-regulates non-lymphoid Fas-ligand
following superantigen-induced peripheral lymphocyte activation. J
Biol Chem. 277:42380–42385. 2002. View Article : Google Scholar
|
|
23
|
Poritz LS, Garver KI, Tilberg AF and
Koltun WA: Tumor necrosis factor alpha disrupts tight junction
assembly. J Surg Res. 116:14–18. 2004. View Article : Google Scholar
|
|
24
|
Ma TY, Iwamoto GK, Hoa NT, et al:
TNF-alpha induced increase in intestinal epithelial tight junction
permeability requires NF-kappa B activation. Am J Physiol
Gastrointest Liver Physiol. 286:G367–G376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ko MK, Saraswathy S, Parikh JG and Rao NA:
The role of TLR4 activation in photoreceptor mitochondrial
oxidative stress. Invest Ophthalmol Vis Sci. 52:5824–5835. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye D, Li FY, Lam KS, et al: Toll-like
receptor-4 mediates obesity-induced non-alcoholic steatohepatitis
through activation of X-box binding protein-1 in mice. Gut.
61:1058–1067. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bruno ME and Kaetzel CS: Long-term
exposure of the HT-29 human intestinal epithelial cell line to TNF
causes sustained up-regulation of the polymeric Ig receptor and
pro-inflammatory genes through transcriptional and
posttranscriptional mechanisms. J Immunol. 174:7278–7284. 2005.
View Article : Google Scholar
|
|
28
|
Schjerven H, Tran TN, Brandtzaeg P and
Johansen FE: De novo synthesized RelB mediates TNF-induced
up-regulation of the human polymeric Ig receptor. J Immunol.
173:1849–1857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murthy AK, Dubose CN, Banas JA, Coalson JJ
and Arulanandam BP: Contribution of polymeric immunoglobulin
receptor to regulation of intestinal inflammation in dextran
sulfate sodium induced colitis. J Gastroenterol Hepatol.
21:1372–1380. 2006.PubMed/NCBI
|
|
30
|
Tsukamoto H, Fukudome K, Takao S, et al:
Multiple potential regulatory sites of TLR4 activation induced by
LPS as revealed by novel inhibitory human TLR4 mAbs. Int Immunol.
24:495–506. 2012. View Article : Google Scholar : PubMed/NCBI
|