Introduction
Cytochrome c is a highly conserved,
water-soluble protein of 12.3 kD with a net positive charge at
neutral pH, residing loosely attached in the mitochondrial
intermembrane space. It has a dual function; it is involved in
energy production in mitochondria by interaction with redox
partners and it also has a critical function in the induction of
intrinsic (cytochrome c/mitochondria-mediated) apoptosis.
Intrinsic apoptosis is activated by cellular stress originating
from inside the cell (e.g. DNA damage or the presence of reactive
oxygen species) and is strictly dependent on the release of
cytochrome c from the mitochondria into the cytoplasm upon
an intrinsic (i.e. of intracellular origin) stimulus. Cytochrome
c is then, together with other cytosolic factors, including
apoptotic protease activating factor 1 (Apaf-1) and pro-caspase-9,
assembled into the apoptosome. Following apoptosome assembly and
activation of pro-caspase-9 (initiator caspase), the downstream
caspases-3 and −7 (effector caspases) are cleaved and thereby
activated. This leads to the execution of the apoptotic program,
culminating in the dismantling of the cell (1–4).
Apoptosis also occurs through the extrinsic (cytochrome
c-independent) Fas/FasL-mediated pathway, which merges with
the intrinsic pathway at the level of the effector caspases-3 and
−7 (5).
The findings that exogenous cytochrome c,
either microinjected directly into the cytoplasm or delivered into
the cytoplasm by electroporation, activates apoptosis without the
requirement for additional apoptotic stimuli supports the critical
role of cytochrome c in apoptosis (6–8).
Related studies have demonstrated that apoptosis in tumor cells is
activated by cytochrome c delivered by nanoparticles,
including nanotubes or polylactic-co-glycolic acid (PLGA)
microspheres (9,10). This suggests that the cytoplasmic
delivery of exogenous cytochrome c through suitable carriers
with subsequent apoptosis activation is a potential therapeutical
approach against cancer. Contrary to necrosis, apoptosis does not
induce an immune response of the surrounding tissue, which may be
of clinical significance.
Cell-penetrating peptides (CPPs) are a group of
peptides that are often ~20 amino acids long and contain a cluster
of basic residues. Based on their property of translocating across
the hydrophobic cell membrane, they are also capable of delivering
protein- and DNA-based macromolecules and drug molecules to cells
without the loss of biological activity of the conveyed materials.
CPPs are intensively studied and considered as important carriers
in drug delivery (11–15).
Antennapedia (Antp) is one member of the family of
CPPs. Antp was originally derived from the 60 amino acid long
homeodomain of the Drosophila transcription factor Antennapedia
(16). Later on, its translocation
ability was narrowed down to a 16-mer, termed as penetratin (Antp
PTD, 43–58 residues, RQIKIWFQNRRMKWKK) present in the homeodomain
(17). In the present study we
describe the effects of Antp-SMCC-cytochrome c, a conjugate
molecule synthesized from cytochrome c and Antp on apoptosis
activation and proliferation inhibition in HeLa cervical tumor
cells.
Materials and methods
Cell culture and compounds
HeLa cervical cancer cells (obtained from Dr G.
Marra, Institute of Molecular Cancer Research, University of
Zurich) were routinely cultured in Iscove's modified Dulbecco's
medium (IMDM)-21980 (Invitrogen, Basel, Switzerland) containing 10%
fetal calf serum (Oxoid, Basel, Switzerland) at 37°C and in an
atmosphere of 5% carbon dioxide and 95% humidity. Horse heart
cytochrome c was purchased from Sigma-Aldrich Chemie GmbH
(Buchs, Switzerland) and a stock solution (20 mg/ml, 1.63 mM) was
prepared in sterile water and stored at −20°C. The 19-mer
synthetically synthesized Antp peptide was purchased from Bachem
(Bubendorf, Switzerland) and solutions were prepared in
phosphate-buffered saline (PBS) containing 2 mM tributylphosphine
prior to use. This Antp peptide (amino acid sequence,
Ser-Gly-Arg-Gln-Ile-Lys-Ile-Trp-Phe-Gln-Asn-Arg-Arg-Met-Lys-Trp-Lys-Lys-Cys)
was biotinylated at the 5′-carboxy terminus and functionalized at
the 3′-amino terminus with a trifluoroacetate group.
Sulfo-succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate
(SMCC) was purchased from Pierce Biotechnology Inc. (Lausanne,
Switzerland) and solutions were freshly prepared in PBS. The
pan-caspase inhibitor peptide z-VAD-fmk was purchased from Enzo
Life Sciences (Laufen, Switzerland) and a stock solution in
dimethyl sulfoxide (DMSO) was stored at −20°C.
Conjugate synthesis
The Antp-SMCC-cytochrome c conjugate
synthesis was a two-step reaction, where sulfo-SMCC was used as a
cross-linker molecule (also referred to as a bifunctional coupling
reagent). The conjugate synthesis was performed as follows: In the
first step, cytochrome c was incubated with crystalline
sulfo-SMCC in PBS at a molar ratio of protein molecules to
succinimidyl groups of 1:4 for 60 min under continuous stirring at
room temperature. This coupled the sulfo-SMCC covalently to
cytochrome c. Excess sulfo-SMCC was removed by overnight
dialysis at 4°C against PBS. In the second step, the
sulfo-SMCC-coupled cytochrome c was incubated with freshly
prepared Antp solution containing 2 mM tributylphosphine (to
prevent dimerization of the Antp peptides) at a molar ratio of
cytochrome c-SMCC:Antp of 1:5 for 48 h under continuous
stirring at 4°C. The reddish conjugate solution was then filtered
[Millex-HV polyvinylidene fluoride (PVDF) 0.45-μm pore-size sterile
filter]. The concentration of cytochrome c in the conjugate
was determined by a cytochrome c (human) enzyme-linked
immunosorbent assay (ELISA) kit (Enzo Life Sciences) according to
the manufacturer's instructions.
Cell lysates and immunoblot analysis
Immunoblot analysis was performed in cell lysates to
assess apoptosis on the basis of the treatment-induced proteolytic
cleavage of the 116 kDa PARP-1 precursor into its 89 kDa fragment.
Proteolytic PARP-1 cleavage is an acknowledged measure of ongoing
apoptosis. Cell lysates were produced from untreated HeLa control
cultures or HeLa cultures treated with either the
Antp-SMCC-cytochrome c conjugate or the non-conjugated
compounds (cytochrome c, Antp) for 24 h, washed in PBS and
lysed according to standard laboratory protocols. In certain
cultures the pan-caspase inhibitor peptide z-VAD-fmk was added (10
or 20 μM) 2 h before the addition of Antp-SMCC-cytochrome c.
The protein concentration of cell lysates was determined using the
BCA Protein Assay kit (Pierce Biotechnology Inc.). For immunoblot
analysis (performed following standard laboratory protocols), 20 μg
cell lysate protein was separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by
blotting onto a PVDF membrane (Amersham Biosciences, Otelfingen,
Switzerland). Proteins were detected by the specific primary
antibodies and the respective secondary antibodies: horseradish
peroxidase (HRP)-conjugated anti-mouse (M15345; BD Transduction
Laboratories, Lexington, KY, USA) or HRP-conjugated anti-rabbit
(7074, Cell Signaling Technology Inc./BioConcept, Allschwil,
Switzerland). The primary antibodies used were PARP-1 (9542, Cell
Signaling; recognizing the 116 kDa full-length PAPR-1 and the
cleaved 89 kDa fragment) and anti-mouse β-actin (A5441, Sigma) or
anti-rabbit α/β-tubulin (2148, Cell Signaling) as sample loading
controls. Complexes were visualized by enhanced chemiluminescence
(Amersham Biosciences) and autoradiography. A HeLa cell culture
treated with 0.8 mM H2O2 for 6 h served as
the positive control sample for apoptosis.
Clonogenic assay
The sensitivity of HeLa cells to the treatments was
determined by the clonogenic assay. HeLa cells (500 cells in 2 ml
culture medium) were plated in 35 mm cell culture plates. Then, 24
h after plating, the cells were treated with various concentrations
of either the conjugate or the non-conjugated compounds for 24 h.
Then, the drug-containing medium was replaced with drug-free
medium. Seven days after treatment, cells were fixed with 25%
acetic acid in ethanol and stained with Giemsa. Colonies of ≥50
cells were scored visually. Each experiment was performed three
times. Clonogenic survival was presented as the percentage of the
untreated control as a function of the compound concentration.
Results
Antp-SMCC-cytochrome c conjugate
activates caspase-dependent apoptosis
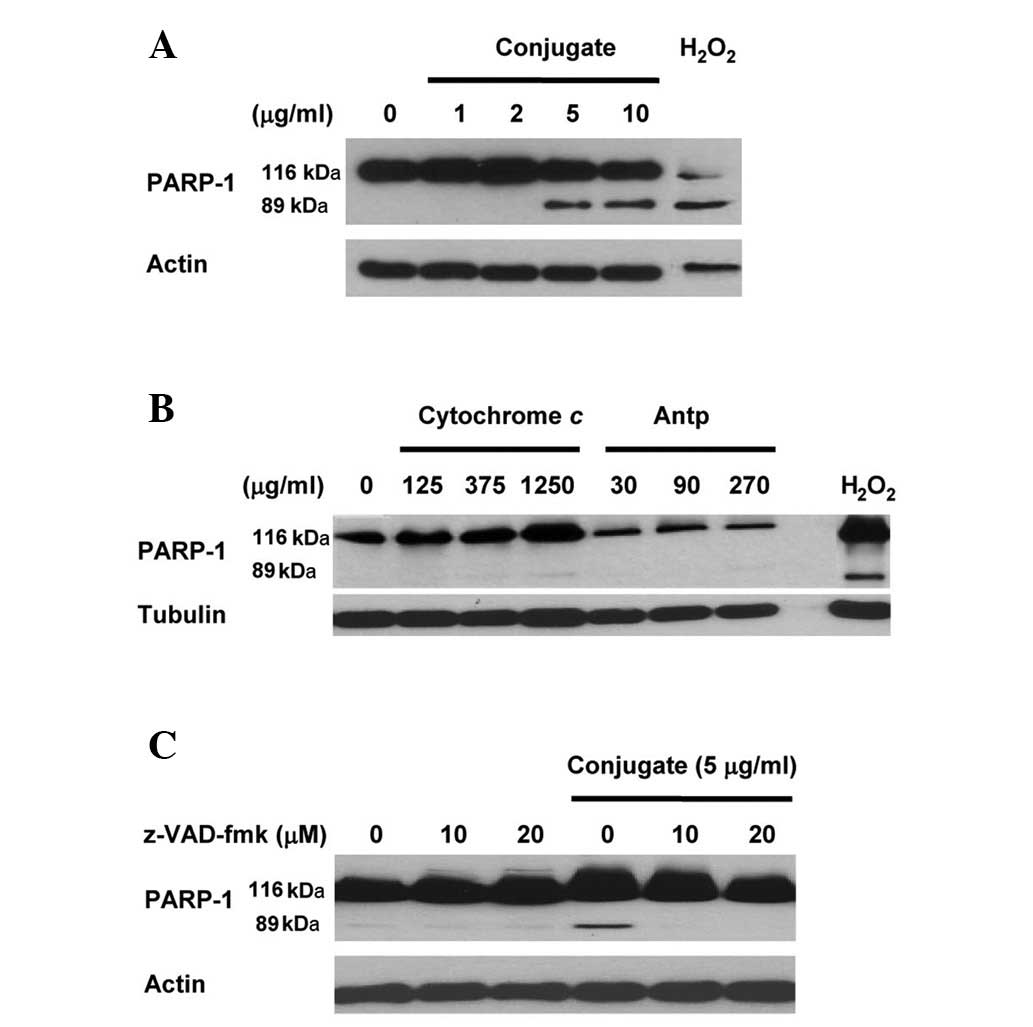
Immunoblot data (Fig.
1A) revealed that, in comparison with the untreated control
sample, the treatment of HeLa cells with Antp-SMCC-cytochrome
c resulted in the cleavage of the 116-kDa PARP-1 precursor
into an 89-kDa cleaved fragment (a measure for ongoing apoptosis).
A concentration of cytochrome c (contained in the conjugate
and measured by cytochrome c-specific ELISA) as low as 5
μg/ml was sufficient to result in PARP-1 cleavage, i.e. to activate
apoptosis. By contrast, PARP-1 cleavage was not observed when HeLa
cells were treated with either cytochrome c or Antp alone at
concentrations of up to 1,250 μg/ml or 270 μg/ml, respectively
(Fig. 1B). This indicates that
apoptosis is activated by treatment with the Antp-SMCC-cytochrome
c conjugate but not with Antp or cytochrome c
alone.
The 2-h pretreatment of HeLa cultures with 10 or 20
μM z-VAD-fmk and the subsequent treatment with Antp-SMCC-cytochrome
c (5 μg/ml) eliminated the Antp-SMCC-cytochrome
c-induced apoptosis. This was manifested by the failure to
detect PARP-1 precursor cleavage (Fig.
1C). As a broad spectrum caspase inhibitor peptide, z-VAD-fmk
irreversibly inhibits the activity of the majority of the members
of the caspase-family, indicating that the Antp-SMCC-cytochrome
c-induced apoptosis was caspase-dependent.
Antp-SMCC-cytochrome c conjugate inhibits
clonogenic survival
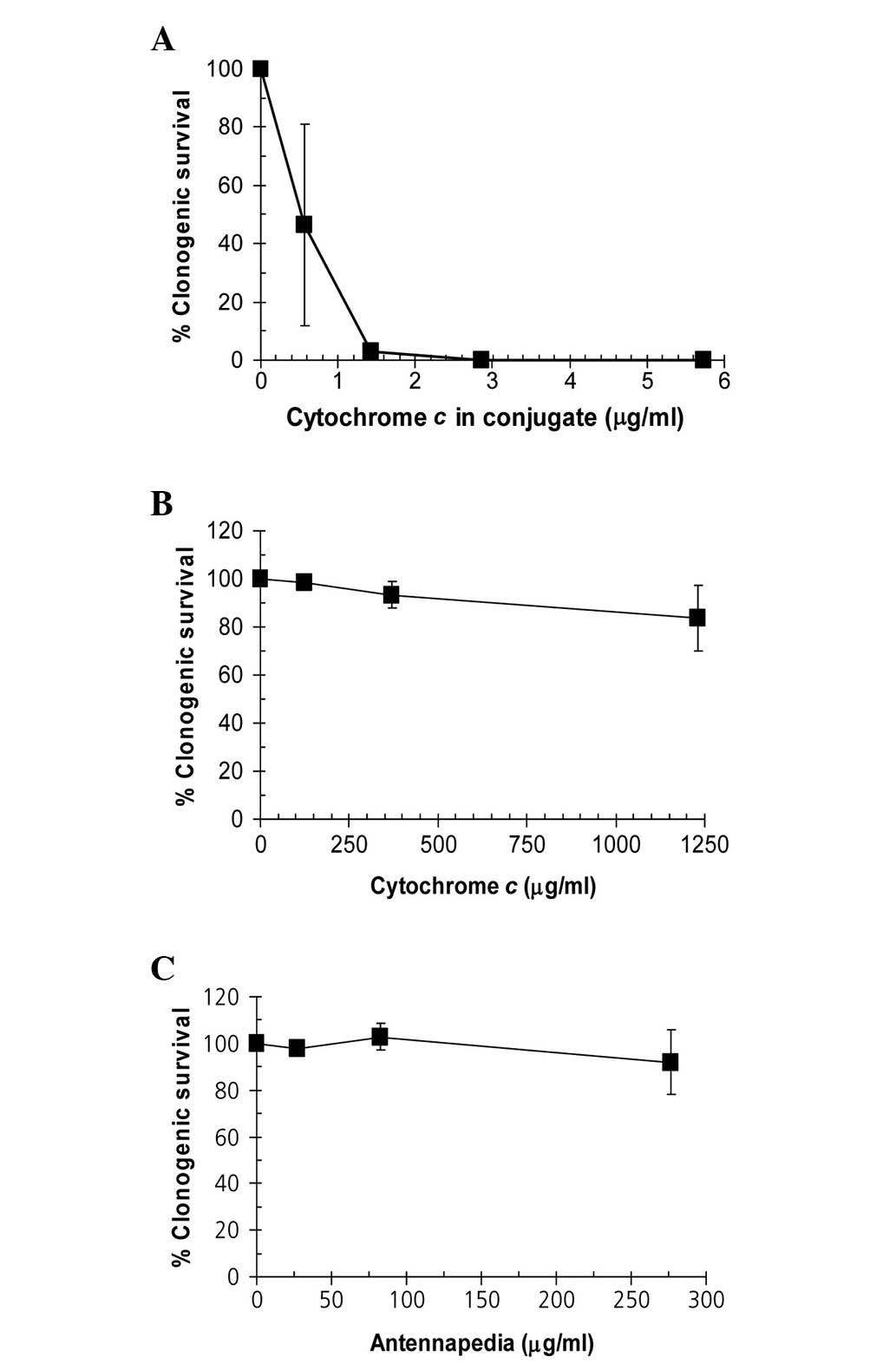
The Antp-SMCC-cytochrome c conjugate reduced
the clonogenic survival of Hela cells (Fig. 2A). A concentration as low as 1.3
μg/ml cytochrome c (contained in the conjugate) was
sufficient to completely block the clonogenic potential of HeLa
cells. By contrast, cytochrome c alone (≤1,250 μg/ml) or
Antp alone (≤275 μg/ml) did not produce a substantial negative
effect on clonogenic survival (Fig. 2B
and C).
Discussion
Cytochrome c has been shown to activate
apoptosis when directly microinjected or delivered into tumor cells
via electroporation or nanoparticles. CPPs, including Antp,
facilitate the penetration of various biomolecules and particles
into cells. On this basis, we synthesized the conjugate molecule
Antp-SMCC-cytochrome c from the respective compounds
(cytochrome c and Antp) using the sulfo-SMCC crosslinker and
determined the effects of this Antp-SMCC-cytochrome c
conjugate on survival, i.e. apoptosis activation and proliferation
in HeLa cervical cancer cells.
The aim of the present study was to determine
whether apoptosis in HeLa tumor cells is activated by exogenous
cytochrome c delivered into the cytoplasm through the CPP
Antp in the form of a conjugate molecule consisting of Antp
covalently linked to cytochrome c.
In the current study, we demonstrated that
cytochrome c covalently conjugated to Antp applied to HeLa
cervical cancer cell cultures activates caspase-dependent apoptosis
and inhibits proliferation, whereas neither cytochrome c nor
Antp alone affected survival and proliferation. Therefore, we
conclude that the inhibitory effects on survival and proliferation
are attributed to cytochrome c delivered to HeLa cells via
Antp. This suggests that the Antp-aided delivery of cytochrome
c into tumor cells may be a candidate strategy for
activating apoptosis and consequently inhibiting the survival and
proliferation of tumor cells.
In a pilot set of experiments, we demonstrated that
the presence of non-conjugated cytochrome c alone in the
culture medium did not activate apoptosis nor substantially reduce
the clonogenic potential at concentrations of up to 1,250 μg/ml,
suggesting that cytochrome c is not accumulated in the
cytoplasm. This suggestion is supported by findings that cytochrome
c is unable to translocate across membranes on its own and
therefore requires the so-called translocases in the outer membrane
(TOM) complex for the translocation across the mitochondrial outer
membrane (18). The presence of
(non-conjugated) Antp (concentrations ≤270 μg/ml) alone in the
culture medium had no effect on apoptosis and clonogenic potential.
This suggests that Antp is not harmful in this experimental
setting. It is known that CPPs are toxic to cells due to membrane
perturbation at higher levels of the peptides (19).
The key finding in the present study was that,
unlike non-conjugated cytochrome c and Antp, the incubation
of HeLa cultures with the Antp-SMCC-cytochrome c conjugate
resulted in the activation of apoptosis and reduction of the
clonogenic potential of HeLa cells. Antp-SMCC-cytochrome
c-induced apoptosis is caspase-dependent, since it was
inhibited by the pan-caspase inhibitor z-VAD-fmk.
The following series of events that eventually lead
to apoptosis may be proposed on the basis of the results of the
current study. The Antp-SMCC-cytochrome c conjugate
translocates across the cellular membrane and accumulates in the
cytoplasm, where the conjugate is hydrolyzed into its components
(the SMCC-crosslinker is pH-sensitive). Cytochrome c is then
assembled into the apoptosome that, in turn, finally results in the
activation and the execution of apoptosis. This implies that the
structural integrity and the biological function of cytochrome
c are not compromised by the chemical modifications made
during Antp-SMCC-cytochrome c conjugate synthesis and its
subsequent hydrolysis. Studies have shown that injection of ~10 fg
cytochrome c is sufficient to activate apoptosis (6), corresponding to an estimated
intracellular cytochrome c concentration of ~20 μM (7). Whether and to what extent cytochrome
c molecules with covalently bound SMCC retain functional
integrity in terms of proper apoptosome formation remains unclear.
Likewise, the possible effects of the other products of the
hydrolysis with respect to apoptosis activation and clonogenic
survival are unknown, but may be marginal.
It is important to acknowledge that the results of
the present study should be considered as proof-of-concept only,
and that more detailed studies should be performed. However,
hypotheses towards important features related to antitumor studies
may be proposed.
Conventional chemotherapy is an indispensable
therapeutic option for the treatment of a number of malignancies.
It kills tumor cells through the activation of the apoptotic
machinery by the use of foreign-to-body chemicals or biological
compounds. These compounds are by definition toxic and are
frequently of limited bio-tolerability and bio-degradability.
Clinicians and patients are therefore often confronted with
limitations, including adverse side-effect profiles. Cytochrome
c as the therapeutically active compound against tumor cells
appears appealing and may be a candidate alternative to
conventional chemotherapy. It is intrinsic to cells and not toxic;
however, it is able to activate apoptosis when delivered to cells
from outside in femtogram quantities.
Exogenous cytochrome c as the
‘therapeutically’ active compound may help overcome certain types
of chemotherapy resistance. Resistance to chemotherapeutic
compounds emerges through the expression of multidrug resistance
drug efflux transporters or drug detoxifiers, or through the
enhanced repair of damaged DNA (20). This leads to ineffective
mitochondrial cytochrome c release due to the absence of
apoptotic stimuli, to ineffective apoptosome assembly and caspase
activation, and eventually to ineffective apoptosis execution.
Absent release of intrinsic cytochrome c may be compensated
by the exogenously delivered cytochrome c, thereby
overcoming chemoresistance. It may also be hypothesized that
exogenous cytochrome c does not cause the acquisition of
drug resistance in tumor cells, a major problem of conventional
chemotherapies.
Despite its intriguing characteristics, there are
critical issues with the concept of CPP-aided cytochrome c
delivery. One is that CPPs have limited target specificity; CPPs
are likely to deliver their cargo not only to tumor cells, but also
to normal cells. Further studies are required to render CPP-aided
delivery target cell-specific. An alternative to CPP-aided
cytochrome c delivery may be cytochrome c delivery
via tumor cell-targeted immunoliposomes; however, this approach may
suffer from limitations associated with the intrinsic disadvantages
of endocytotic-based mechanisms. Another issue is what the
potential clinical application of the CPP-aided cytochrome
c-therapy may be. We performed this study with HeLa cervical
cancer cells; therefore, it may be applied as a therapy of
inoperable, local cervical cancers or advanced primary inoperable
vulvar and vaginal cancers that are easily accessible to, for
instance, an Antp-SMCC-cytochome c-containing ointment. A similar
application may also be suitable for superficial cancers, including
skin cancer.
Acknowledgements
The authors thank Professor Reto Schwendener,
Institute of Molecular Cancer Research, University of Zurich, for
assistance in the generation of the conjugates. This study was
supported by the Lydia Hochstrasser Foundation.
References
|
1
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schafer ZT and Kornbluth S: The
apoptosome: physiological, developmental, and pathological modes of
regulation. Dev Cell. 10:549–561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riedl SJ and Salvesen GS: The apoptosome:
signalling platform of cell death. Nat Rev Mol Cell Biol.
8:405–413. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ow YL, Green DR, Hao Z and Mak TW:
Cytochrome c: functions beyond respiration. Nat Rev Mol Cell
Biol. 9:532–542. 2008.
|
|
5
|
Peter ME and Krammer PH: The
CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26–35. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li F, Srinivasan A, Wang Y, Armstrong RC,
Tomaselli KJ and Fritz LC: Cell-specific induction of apoptosis by
microinjection of cytochrome c. Bcl-xL has activity
independent of cytochrome c release. J Biol Chem. 272:30299–30305.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhivotovsky B, Orrenius S, Brustugun OT
and Døskeland SO: Injected cytochrome c induces apoptosis.
Nature. 391:449–450. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gabriel B, Sureau F, Casselyn M, Teissié J
and Petit PX: Retroactive pathway involving mitochondria in
electroloaded cytochrome c-induced apoptosis. Protective
properties of Bcl-2 and Bcl-XL. Exp Cell Res. 289:195–210. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kam NW and Dai H: Carbon nanotubes as
intracellular protein transporters: generality and biological
functionality. J Am Chem Soc. 127:6021–6026. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frauke Pistel K, Breitenbach A,
Zange-Volland R and Kissel T: Brush-like branched biodegradable
polyesters, part III. Protein release from microspheres of
poly(vinyl alcohol)-graft-poly (D,L-lactic-co-glycolic acid). J
Control Release. 73:7–20. 2001.PubMed/NCBI
|
|
11
|
Temsamani J and Vidal P: The use of
cell-penetrating peptides for drug delivery. Drug Discov Today.
9:1012–1019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zorko M and Langel U: Cell-penetrating
peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv
Rev. 57:529–545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mäe M and Langel U: Cell-penetrating
peptides as vectors for peptide, protein and oligonucleotide
delivery. Curr Opin Pharmacol. 6:509–514. 2006.PubMed/NCBI
|
|
14
|
Howl J, Nicholl ID and Jones S: The many
futures for cell-penetrating peptides: how soon is now? Biochem Soc
Trans. 35:767–769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aroui S, Ram N, Appaix F, Ronjat M, Kenani
A, Pirollet F and De Waard M: Maurocalcine as a non toxic drug
carrier overcomes doxorubicin resistance in the cancer cell line
MDA-MB 231. Pharm Res. 26:836–845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perez F, Joliot A, Bloch-Gallego E,
Zahraoui A, Triller A and Prochiantz A: Antennapedia homeobox as a
signal for the cellular internalization and nuclear addressing of a
small exogenous peptide. J Cell Sci. 102:717–722. 1992.PubMed/NCBI
|
|
17
|
Derossi D, Joliot AH, Chassaing G and
Prochiantz A: The third helix of the Antennapedia homeodomain
translocates through biological membranes. J Biol Chem.
269:10444–10450. 1994.PubMed/NCBI
|
|
18
|
Diekert K, de Kroon AI, Ahting U,
Niggemeyer B, Neupert W, de Kruijff B and Lill R: Apocytochrome
c requires the TOM complex for translocation across the
mitochondrial outer membrane. EMBO J. 20:5626–5635. 2001.PubMed/NCBI
|
|
19
|
Saar K, Lindgren M, Hansen M, Eiríksdóttir
E, Jiang Y, Rosenthal-Aizman K, et al: Cell-penetrating peptides: a
comparative membrane toxicity study. Anal Biochem. 345:55–65. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Redmond KM, Wilson TR, Johnston PG and
Longley DB: Resistance mechanisms to cancer chemotherapy. Front
Biosci. 13:5138–5154. 2008. View
Article : Google Scholar : PubMed/NCBI
|