Introduction
Multiple myeloma (MM) is a malignant disorder of
plasma cells characterized by the proliferation of neoplastic
plasma cells in the bone marrow (BM). These cells impair
hematopoiesis, activate osteoclastic bone resorption and secrete a
monoclonal paraprotein (M-protein) in the serum and/or urine. MM
accounts for ∼1% of all human neoplasms, ∼2% of cancer mortalities
and 12–15% of all cases of hematological malignancy (1).
Primary MMs are normally located in the BM, but
neoplastic cells may invade other tissues and organs, such as the
liver, lung, spleen, pancreas, kidney and lymph nodes (2). In the current study, we present the
case of a 60-year-old female patient with MM and extramedullary
plasmacytoma (EMP) that had invaded the skin and eyeballs following
autologous stem cell transplantation (auto-SCT). Concomitant
presentation of EMP is a poor prognostic factor, it is observed in
approximately 13% of patients with multiple myeloma, and the
incidence is rising (2). The study
was approved by the ethics committee of Beijing Chaoyang Hospital,
Capital Medical University (Beijing, China) and the informed
consent was obtained from the patient
Case report
A 60-year-old female patient presented with a
painless subcutaneous mass (∼3.0×1.0 cm) in the lower right limb.
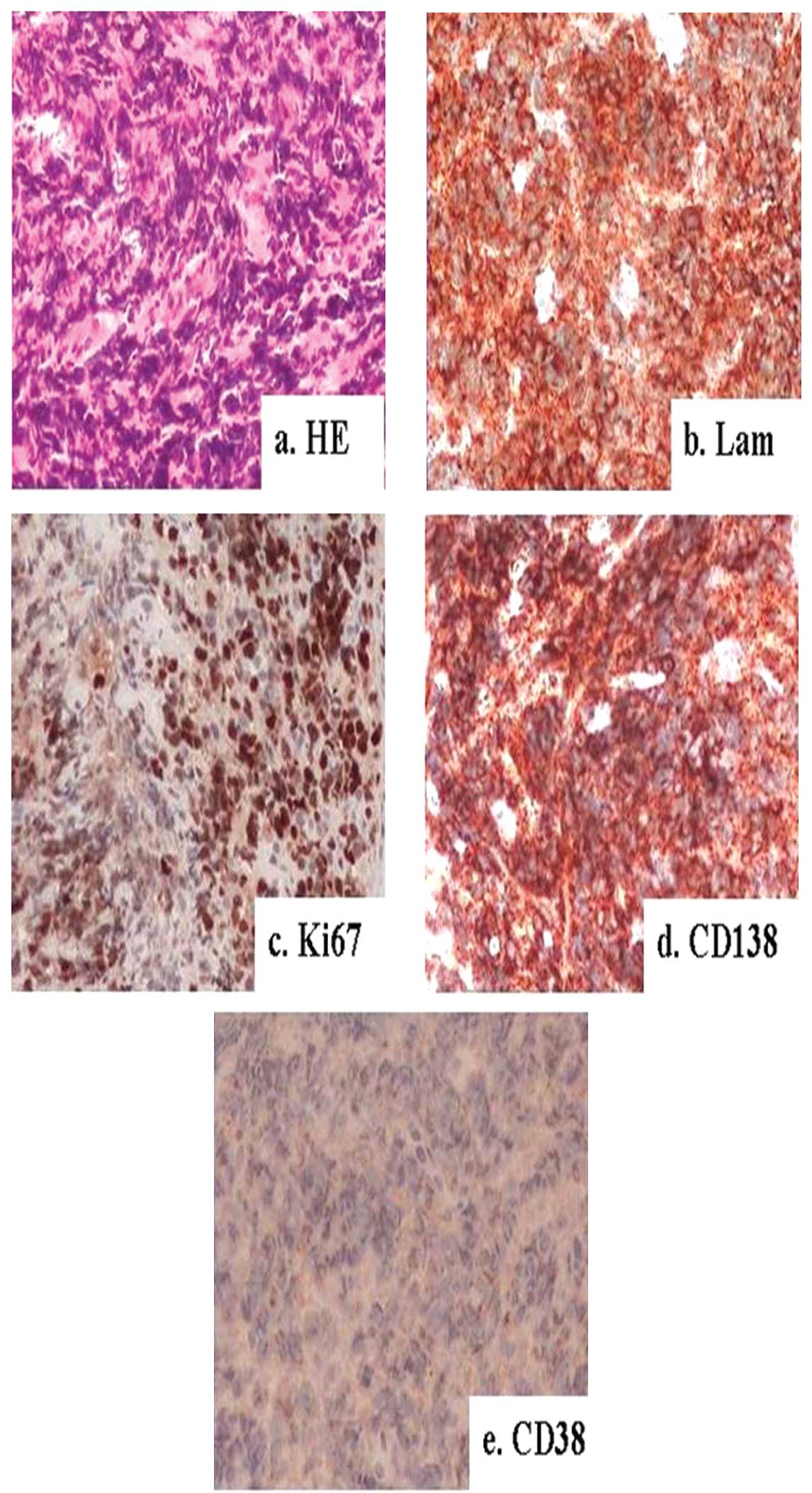
The mass was confirmed as a plasmacytoma that was CD38 (+), CD138
(+), λ (+) and κ (−). The patient was diagnosed with MM λ type
stage III international stage system (ISS) (3) by BM aspiration (the plasmacyte level
was 21.5% in the BM) and protein electrophoresis with
immunofixation electrophoresis (light chain λ in urine, 13.6 g/24
h).
The patient received three cycles of a therapeutic
PDT regimen (bortezomib, dexamethasone and thalidomide) at the
Beijing Chaoyang Hospital (Beijing, China) and achieved complete
remission. The patient received a further two cycles of the PDT
regimen and subsequently proceeded to receive a high dose
cyclophosphamide (3.5 g/m2) regimen combined with
granulocyte-colony stimulating factor (G-CSF) for stem cell
mobilization and harvesting. Fourteen months later, the patient
received a high-dose therapy (melphalan 200 mg/m2 and
bortezomib) supported by auto-SCT. Following the auto-SCT, the
patient remained in complete remission.
Six months later, a painless subcutaneous mass
(∼1.5×1.0 cm) was identified in the left side of the patient’s neck
and the mass gradually increased in size. One month later, the
patient exhibited exophthalmos and loss of sight. We located a mass
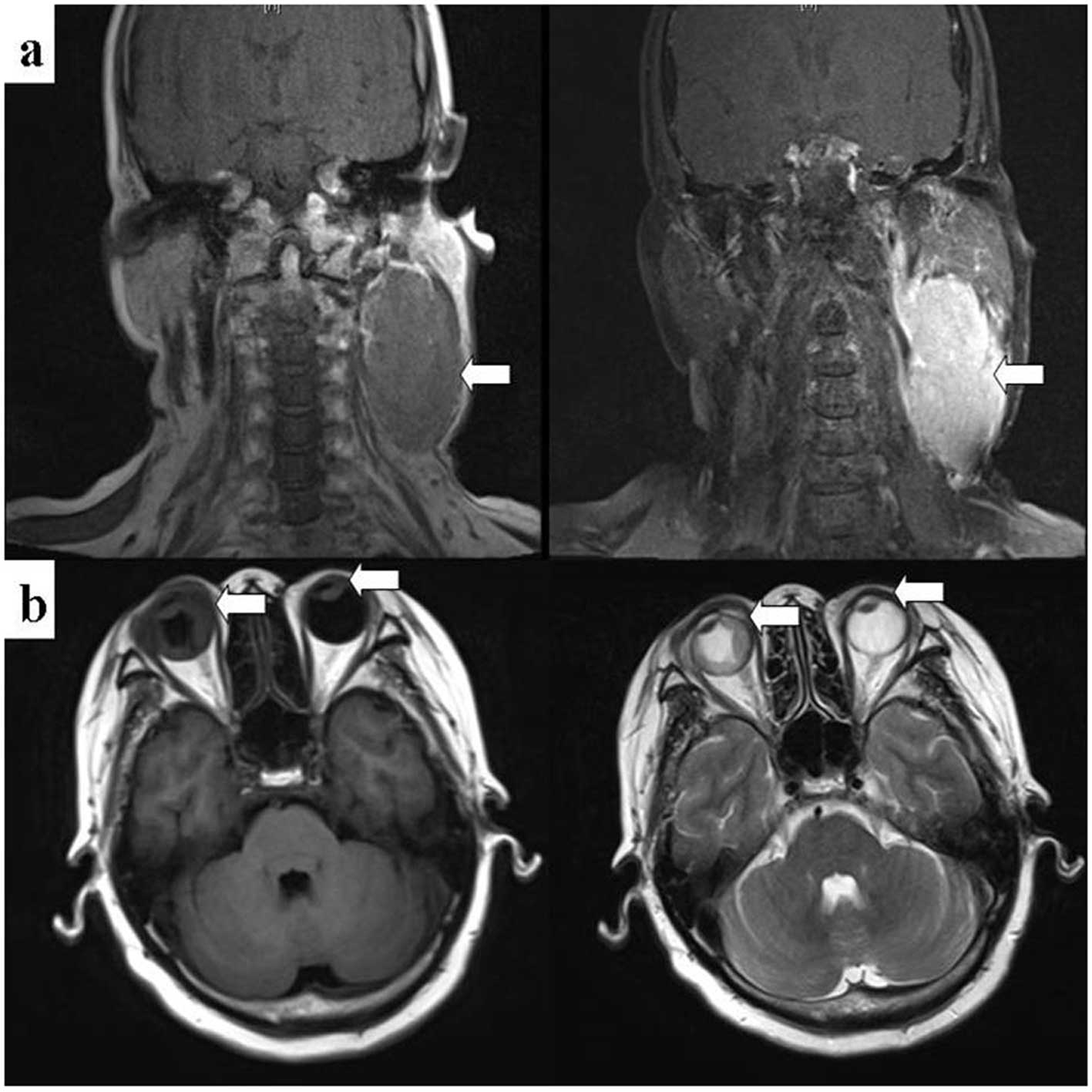
∼7.8×4.2 cm in the left side of the patient’s neck and observed
that the patient’s eyeballs were abnormal by using magnetic
resonance imaging (MRI). A 6.9×2.8 mm soft mass was observed in the
left anterior chamber and was enhanced markedly following the
infusion of a contrast-enhancing agent (Fig. 1). Pathology results from the masses
in the neck and right eyelid revealed that the masses were
plasmacytomas (Figs. 2 and
3). The pathology results and MRI
combined with the results of BM aspiration (plasmacyte levels of
11.0% in the BM) and protein electrophoresis with immunofixation
electrophoresis (light chain λ in urine, 1.2 g/24 h) revealed that
the disease had relapsed. The patient received two cycles of the
therapeutic CPADT regimen (cyclophosphamide, bortezomib,
pharmorubicin, dexamethasone and thalidomide). The response to the
treatment was assessed by BM aspiration and protein electrophoresis
with immunofixation electrophoresis. The patient achieved complete
remission again with negative immunofixation electrophoresis and
plasmacyte levels of 1% in the BM. Furthermore, the patient’s
exophthalmos improved, the mass in the patient’s neck was markedly
reduced in size and the mass in the eyeball ring was also smaller
(Fig. 4). However, the patient’s
visual acuity did not improve. The patient refused to continue
receiving bortezomib and pharmorubicin for therapy due to severe BM
inhibition and infection. The patient continued to receive four
cycles of the therapeutic CTD regimen (cyclophosphamide,
dexamethasone and thalidomide). Subsequently, the patient received
local radiotherapy for the neck and eyes. The patient remained
stable since the treatment that followed the initial relapse with a
progression-free survival (PFS) time of eight months.
Discussion
EMP may occur either at diagnosis or during the
course of MM. In a longitudinal study, 13% of MM patients had EMP
(7% at diagnosis and 6% during follow-up) (2). A high incidence of EM relapses has
been reported following autologous and allogeneic SCT (4,5,6). The
patient in the current study exhibited EM and medullory progression
following auto-SCT. However, the incidence of EMP relapse is not
higher in patients receiving high-dose therapy (HDT) compared with
other treatments (2). The
widespread use of more sensitive imaging techniques such as
computed tomography (CT) and MRI may partially explain the
phenomena.
The patient in the current study was diagnosed with
two plasmacytomas, in the eyeball and the neck. The majority of MM
patients with EMP have a single plasmacytoma. In a previous study,
in 85% of cases the sites affected were the soft tissues
surrounding the axial skeleton; plasmacytomas of the lymph nodes,
liver, kidney, airways, skin and breast, accounted for 15%
(2).
The patient in the current study was identified as
having low M-protein levels during disease progression, but the
positive rate of Ki-67 in EMP was very high. However, the lactate
dehydrogenase (LDH) level of the patient was normal. Notably,
Dawson et al (7) reported
three MM patients who underwent EM relapse associated with a shift
in the secretion of intact immunoglobulins to free light chains,
known as the ‘light chain escape from plateau phase’ (LEPP). The
syndrome was characterized by multiple EM sites of relapse,
plasmablastic features, renal failure, high LDH and
β2-microglobulin levels and an aggressive course of clinical
treatment. The authors hypothesized that LEPP results from clonal
selection and the expansion of precursors that have lost the
ability to secrete intact immunoglobulins while acquiring stromal
independence and the ability to spread outside the BM (7). Furthermore, they indicated that LEPP
may be derived from the effect of novel agents, including
bortezomib and lenalidomide, on the BM microenvironment since LEPP
occurred following novel therapies such as thalidomide or
lenalidomide. Other authors have not identified a relationship
between the EM spread of disease and prior exposure to novel agents
(2).
In the current study, a combination therapy,
including thalidomide and bortezomib, was administered. Following
two cycles of therapy, the patient experienced a marked remission.
The introduction of thalidomide, bortezomib, and lenalidomide has
expanded the therapeutic armamentarium for MM (8–10).
However, to date no studies have focused on the treatment of MM
patients with EMP. Certain studies have indicated that bortezomib
is more promising in this environment (11,12).
Radiotherapy is normally associated as a systemic treatment with
chemotherapy or other novel agents.
In a study of 19 patients with EMP and extraosseous
MM, the disease was observed to follow an aggressive course, with a
median overall survival (OS) of 15 months (13). Terpos et al (5) noted that isolated EMP relapses
following HDT were almost invariably followed by systemic
progression with short OS. However, in another study of 78 patients
who relapsed following autologous or allogeneic SCT, the outcome of
patients with EMP or medullary relapse was not significantly
different (4). To the best of our
knowledge, data concerning the prognosis of EMP in MM are limited
and controversial since certain studies show that the patients with
EMP and extraosseous MM had a poor prognosis, but others show that
the outcome of patients with EMP or medullary relapse was not
significantly different (4,5,13).
In conclusion, the patient with extramedullary
plasma-cytoma invading skin and eyeballs following autologous stem
cell transplantation in the present study had a favorable response
after combination therapy with bortezomib. Such patients require
clinical studies with novel treatment strategies for a better
prognosis.
References
|
1.
|
Lorsbach RB, Hsi ED, Dogan A and Fend F:
Plasma cell myeloma and related neoplasms. Am J Clin Pathol.
136:168–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Varettoni M, Corso A, Pica G,
Mangiacavalli S, Pascutto C and Lazzarino M: Incidence, presenting
features and outcome of extramedullary disease in multiple myeloma:
a longitudinal study on 1003 consecutive patients. Ann Oncol.
21:325–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Greipp PR, San Miguel J, Durie BG, et al:
International staging system for multiple myeloma. J Clin Oncol.
23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Zeiser R, Deschler B, Bertz H, et al:
Extramedullary vs medullary relapse after autologous or allogeneic
hematopoietic stem cell transplantation (HSCT) in multiple myeloma
(MM) and its correlation to clinical outcome. Bone Marrow
Transplant. 34:1057–1065. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Terpos E, Rezvani K, Basu S, et al:
Plasmacytoma relapses in the absence of systemic progression
post-high-dose therapy for multiple myeloma. Eur J Haematol.
75:376–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Perez-Simon JA, Sureda A, Fernandez-Aviles
F, et al: Reduced-intensity conditioning allogeneic transplantation
is associated with a high-incidence of extramedullary relapses in
multiple myeloma patients. Leukemia. 20:542–545. 2006.
|
|
7.
|
Dawson MA, Patil S and Spencer A:
Extramedullary relapse of multiple myeloma associated with a shift
in secretion from intact immunoglobulin to light chains.
Haematologica. 92:143–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Rajkumar SV, Blood E, Vesole D, et al
Eastern Cooperative Oncology Group: Phase III clinical trial of
thalidomide plus dexamethasone compared with dexamethasone alone in
newly diagnosed multiple myeloma: a clinical trial coordinated by
the Eastern Cooperative Oncology Group. J Clin Oncol. 24:431–436.
2006. View Article : Google Scholar
|
|
9.
|
Richardson PG, Sonneveld P, Schuster MW,
et al: Bortezomib or high-dose dexamethasone for relapsed multiple
myeloma. N Engl J Med. 352:2487–2498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Weber DM, Chen C, Niesvizky R, et al:
Lenalidomide plus dexamethasone for relapsed multiple myeloma in
North America. N Engl J Med. 357:2133–2142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Laura R, Cibeira MT, Uriburu C, et al:
Bortezomib: an effective agent in extramedullary disease in
multiple myeloma. Eur J Haematol. 76:405–408. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Patriarca F, Prosdocimo S, Tomadini V, et
al: Efficacy of bortezomib therapy for extramedullary relapse of
myeloma after autologous and non-myeloablative allogeneic
transplantation. Haematologica. 90:278–279. 2005.PubMed/NCBI
|
|
13.
|
Damaj G, Mohty M, Vey N, et al: Features
of extramedullary and extraosseous multiple myeloma: a report of 19
patients from a single center. Eur J Haematol. 73:402–406. 2004.
View Article : Google Scholar : PubMed/NCBI
|