Introduction
Autoimmune hepatitis (AIH) was first described in
1950 as a progressive liver disease of unknown cause (1). The disease is characterized by
interface hepatitis on histological examination, hyper
immunoglobulin G (IgG) and autoantibodies (2–4). The
absence of specific clinical presentation and serological markers
in AIH patients may make a correct and timely diagnosis difficult
(5). The International Autoimmune
Hepatitis Group (IAIHG) proposed the diagnostic criteria for AIH
and a diagnostic scoring system in 1993 (6), which were subsequently revised in
1999 (7). The merit of the revised
scoring system is that it is capable of diagnosing individuals who
lack certain classical features (hypergammaglobulinemia or
autoantibodies) or who exhibit atypical manifestations
(antimitochondrial antibodies, cholestasis or atypical histological
features). However, the diagnostic criteria have been criticized
since they are complex (13 components and 29 possible grades) and
are not widely available as an easily applicable clinical tool
(7–9). As a result, a simplified scoring
system based on four components [level of autoantibody expression,
serum IgG concentration, liver histology and the absence of viral
markers] was developed in 2008 by the IAIHG (10). In a retrospective study, the
simplified scoring system performed with a sensitivity of 95% and a
specificity of 90% (11). Czaja
(11) analyzed 153 individuals
with AIH by codified clinical criteria and concluded that the
performance parameters of each scoring system were the same. The
selected patients from previous studies were including not only AIH
patients but also primary biliary cirrhosis (PBC) or primary
sclerosing cholangitis (PSC) patients, and, to the best of our
knowledge, no recent study has evaluated the independent factors
that affect the diagnostic discrepancy between the revised and
simplified scoring systems, particularly in Asia. The aim of this
study was to evaluate the independent parameters associated with
the diagnostic discrepancy between the two scoring systems by
analyzing the clinical and laboratory characteristics of 77
patients with AIH.
Patients and methods
A retrospective analysis was performed of the
patients diagnosed with definite or probable AIH, according to the
revised criteria of the IAIHG in 1999, in the Second Xiangya
Hospital (Changsha, China) over a nine-year period (2002–2011). For
each patient, age, gender, clinical presentation, the prevalence of
concurrent autoimmune diseases, laboratory and immunological data,
and serological markers of viral hepatitis were obtained. The
patients were enrolled in the present study prior to undergoing
specific therapy. Liver biopsy results were also obtained. Patients
with viral liver diseases, hereditary hemochromatosis, Wilson’s
disease, nonalcoholic fatty liver disease (NFLD), PBC and PSC were
excluded. Thia study was approved by the Ethics Committee of
Central South University (Changsha, China). The procedures were in
accordance with the ethical standards of the Committee on Human
Experimentation of Central South University and/or were performed
in accordance with the Helsinki Declaration of 1975. Informed
consent was obtained from all patients.
Serum IgG concentration was evaluated using
immunonephelometry. In addition, hepatitis B serum markers (HBsAg,
HBsAb, HBcAb, HBeAb and HBeAg), Hepatitis A Virus IgM antibody
(HAVAb-IgM) and antibodies to hepatitis C and E were assessed in
the patients using second-generation enzyme-linked immunosorbent
assays (ELISAs). Smooth muscle antibody (SMA), antineutrophil
cytoplasmic antibody (ANCA) and antinuclear antibody (ANA) levels
were examined using indirect immunofluorescence (IIF). Antibodies
to liver kidney microsome type 1 (LKM-1), antimitochondrial
antibodies (AMA) and soluble liver antigen/liver-pancreas antigen
(SLA/LP) were assessed using ELISA.
Liver tissue examinations were performed in 33
patients, and the liver specimens were evaluated by two
pathologists who specifically identified the characteristic
histological features defined by the IAIHG, including interface
hepatitis, lymphoplasmacytic infiltrate, liver cell rosettes and
biliary changes. Professor H.P. Dienes (Institute of Pathology,
University of Cologne, Cologne, Germany) and Professor A.W. Lohse
(Department of Medicine, Johannes Gutenberg-University, Mainz,
Germany) defined three categories for grading histology: Atypical
histology, histology compatible with AIH and typical histology.
Interface hepatitis, lymphocytic/lymphoplasmacytic infiltrates in
portal tracts and extending into the lobule, emperipolesis (active
penetration by one cell into and through a larger cell) and hepatic
rosette formation were regarded as typical for the diagnosis of
AIH. To be considered typical, each of the three features of
typical AIH histology had to be present. Compatible features
consisted of chronic hepatitis with lymphocytic infiltration
without all the features considered typical. Histology was
considered atypical when showing signs of another diagnosis, such
as steatohepatitis.
The revised scoring system (as the ‘gold standard’)
and the simplified scoring system were used to provide diagnoses of
definite, probable or negative for AIH in the patients. According
to the revised criteria, a pretreatment score of 10–15 points
indicated probable AIH and >15 points indicated definite AIH. In
the simplified scoring system, a score ≥6 and <7 points
supported probable AIH and a score ≥7 points supported a diagnosis
of definite AIH.
Statistical analysis
Continuous data are expressed as a percentage and
mean ± standard deviation (SD). The χ2 test or Fisher’s
exact test were used for the comparison of single parameters.
Logistic regression models were utilized to evaluate factors
associated with discrepancies in the AIH diagnosis between the
revised and the simplified criteria. A backward elimination
strategy was used to identify factors independently associated with
disease; the probability for removal was set at 0.15. Variables
included in the analysis were gender, age, concurrent autoimmune
disease, serum IgG concentration, alkaline phosphatase
(ALP)/aspartate aminotransferase (AST) levels, AMA, the presence
and level of autoantibody expression [ANA or SMA and SLA/LP or
perinuclear antineutrophil cytoplasmic antibody (pANCA)], liver
histology (compatible or typical), viral markers and drug history.
Data were analyzed using the SPSS statistical software package
version 17.0 (SPSS, Chicago, IL, USA). During statistical analysis,
serum ALP:AST ratios were categorized into <1.5, 1.5–3 and
>3, and serum IgG levels were transformed into Normal (N) group,
1–1.1 × N group and >1.1 × N group, and the titers of ANA or SMA
were grouped into <1:40, 1:80>x≥1:40 and ≥1:80. The results
are presented as odds ratios (ORs), 95% confidence intervals (CIs),
Wald values and P-values. P≤0.05 was considered to indicate a
statistically significant difference.
Results
Clinical profile and laboratory findings
in patients with AIH
A total of 77 patients, including 67 females (87%)
and 10 males (13%), who were classified as having probable or
definite AIH via the IAIHG revised scoring system were identified
as those who also had complete clinical, laboratory and
histological data. Patient age ranged between 10 and 78 years (mean
± SD, 50.4±14.7). Other associated autoimmune diseases were
observed in 44 patients (57.1%). Among the 44 patients, systemic
lupus erythematosus was the most common disorder [18 patients
(23.4%)]. Autoimmune thyroiditis was present in five patients
(6.5%), Sjögren’s syndrome in nine (11.7%), diabetes in seven
(9.1%), ulcerative colitis in one (1.3%) and rheumatoid arthritis
in three (3.9%).
With regard to biochemical data, conventional liver
function tests, serum IgG concentration and coherent antibodies
were assessed prior to the treatment of the patients. Sixty-two of
the 77 patients (80.5%) showed high serum IgG levels of >2,500
mg/dl. Fifty-nine patients (76.6%) had an ALP:AST ratio of <1.5.
Fifty patients (70.1%) were positive for ANA, including 29 patients
(37.7%) with titers of ≥1:80. Anti-SLA/LP tests were positive in 13
patients (16.9%) and pANCA was positive in nine patients
(11.7%).
Histological biopsies were conducted prior to
treatment, and histological assessments were performed for 33
patients (42.9%). The results indicated that 33 patients had
interface hepatitis. Plasma cell infiltration in the portal area
was observed in 18 patients and three had liver cell rosettes.
According to the simplified scoring system, compatible histological
manifestation was observed in 30 patients and typical in three
patients.
Comparison of the two scoring systems for
the diagnosis of AIH
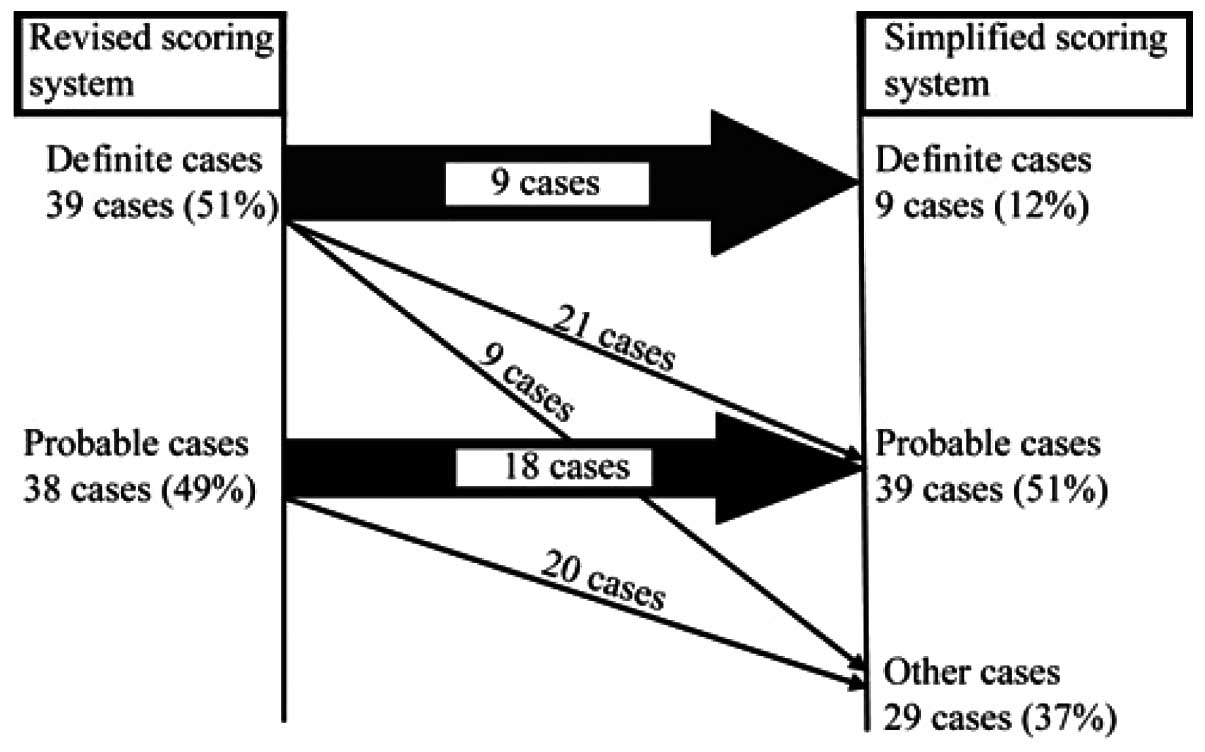
The revised scoring system was applied to 77
patients prior to treatment. The revised system graded 39 subjects
(50.6%) with the diagnosis of definite AIH and 38 subjects (49.4%)
with probable AIH. By contrast, the simplified scoring system
diagnosed 48 patients (62.3%) with AIH. Of the 39 patients with
definite AIH diagnosed by the revised criteria, the simplified
criteria classified nine as definite AIH, 21 as probable AIH and
nine without AIH. Of the 38 patients with probable AIH diagnosed by
the revised scoring system, 20 patients were classified as being
without AIH by the simplified scoring system (Fig. 1). The concordance rate between the
two scoring systems was 62.3% (48/77). Among the 77 patients, the
results indicated that 50 patients (64.9%) had a discrepant AIH
diagnosis and 27 patients had an accordant diagnosis by the two
scoring systems. A χ2 test was applied to evaluate the
clinical characteristics of the 77 patients and compare the single
parameter that affected the diagnosis when using the revised
scoring system and the simplified scoring system. With regard to
the revised scoring system, patients with definite AIH had higher
titers of ANA or SMA (χ2=10.08, P=0.01) than those
diagnosed with probable AIH. In addition, whether the patients
underwent a liver biopsy (χ2=5.93, P=0.021) was also a
statistically significant factor affecting the diagnosis. Using the
simplified scoring system, patients with a definite or probable
diagnosis had higher levels of IgG (χ2=17.35, P=0.002)
and higher titers of ANA or SMA (χ2=34.63, P<0.001)
than those diagnosed without AIH. The clinical and laboratory
features of the 77 patients diagnosed using the revised scoring
system and the simplified scoring system are listed in Tables I and II.
 | Table IComparison of the clinical features of
77 patients with definite and probable AIH diagnoses according to
the revised scoring system. |
Table I
Comparison of the clinical features of
77 patients with definite and probable AIH diagnoses according to
the revised scoring system.
| Parameters | Definite AIH (score
>15) (n=39) | Probable AIH (score
10–15) (n=38) | χ2 | P-value |
|---|
| Female | 34 | 33 | 0.002 | 0.965 |
| Concurrent autoimmune
diseases | 23 | 21 | 0.108 | 0.820 |
| ALP:AST ratio | | | 0.004 | 0.998 |
| 1.5–3 | 5 | 5 | | |
| <1.5 | 30 | 29 | | |
| IgG | | | 4.293 | 0.117 |
| Normal | 3 | 8 | | |
| Abnormal | 36 | 30 | | |
| ANA or SMA | | | 10.085 | 0.010 |
| ≥1:80>x≥1:40 | 13 | 6 | | |
| ≥1:80 | 18 | 11 | | |
| Anti-SLA/LP or
pANCA | 7 | 7 | 0.003 | 1.000 |
| Liver histology | 22 | 11 | 5.927 | 0.021 |
 | Table IIComparison of the clinical features of
77 patients with diagnoses of definite, probable and no AIH
according to the simplified scoring system. |
Table II
Comparison of the clinical features of
77 patients with diagnoses of definite, probable and no AIH
according to the simplified scoring system.
| Parameters | Definite (score ≥7)
(n=9) | Probable (score
6≤x<7) (n=39) | Other (score <6)
(n=29) | χ2 | P-value |
|---|
| Female | 8 | 33 | 26 | 0.406 | 0.816 |
| Concurrent autoimmune
diseases | 5 | 16 | 12 | 0.672 | 0.715 |
| ALP:AST ratio | | | | 3.881 | 0.422 |
| 1.5–3 | 0 | 7 | 3 | | |
| <1.5 | 7 | 28 | 24 | | |
| IgG | | | | 17.348 | 0.002 |
| Normal | 1 | 0 | 10 | | |
| Abnormal | 8 | 39 | 19 | | |
| ANA or SMA | | | | 34.627 | <0.001 |
| 1:80>x≥1:40 | 2 | 2 | 2 | | |
| ≥1:80 | 7 | 37 | 17 | | |
| Anti-SLA/LP or
pANCA | 2 | 9 | 3 | 1.924 | 0.382 |
Factors influencing the diagnostic
discrepancy in the scoring systems
The characteristics of the 50 patients with
discrepant diagnoses were as follows: Six (12.0%) were male, 31
(62.0%) had other concurrent autoimmune diseases, 39 (78.0%) had
ALP/AST ratios of <1.5, 24 (48.0%) had low ANA titers
(<1:40), 28 (56.0%) did not undergo liver biopsy and 10 (20%)
had normal levels of IgG.
The patients were divided into two groups (accordant
and discrepant diagnoses using the revised and simplified scoring
systems) to compare those parameters that contributed to the
discordant diagnoses of the two scoring systems.
According to the statistical analysis, single factor
analysis (χ2 test or Fisher’s exact test) showed that
patients with a discrepant diagnosis had lower titers of ANA or SMA
(<1:40) than those with accordant diagnosis (48 vs. 18.5%,
χ2=15.0, P=0.001). In addition, patients with a
discordant diagnosis presented more frequently with compatible
histology than those with an accordant diagnosis (44 vs. 29.6%,
χ2=6.5, P=0.038). The data are listed in Table III.
 | Table IIIComparison of important parameters
between 77 patients with discrepant and accordant diagnoses by
χ2 test. |
Table III
Comparison of important parameters
between 77 patients with discrepant and accordant diagnoses by
χ2 test.
| Parameters | Discrepant diagnoses
(n=50) | Accordant diagnoses
(n=27) | χ2 | P-value |
|---|
| Female | 44 | 23 | 0.123 | 0.734 |
| Concurrent autoimmune
diseases | 31 | 13 | 1.374 | 0.335 |
| ALP:AST ratio | | | 0.932 | 0.628 |
| 1.5–3 | 7 | 3 | | |
| <1.5 | 39 | 20 | | |
| IgG | | | 4.013 | 0.134 |
| Normal | 10 | 1 | | |
| Abnormal | 40 | 26 | | |
| ANA or SMA | | | 15.000 | 0.001 |
| 1:80>x≥1:40 | 15 | 4 | | |
| ≥1:80 | 11 | 18 | | |
| Anti-SLA/LP or
pANCA | 8 | 6 | 0.456 | 0.499 |
| Liver
histology | | | 6.517 | 0.038 |
| Not tested | 28 | 16 | | |
| Compatible | 22 | 8 | | |
| Typical | 0 | 3 | | |
The parameters were subsequently entered into the
logistic regression model to determine which independent factor was
associated with diagnostic discordance. The results are presented
in Table IV. The analysis showed
that the presence of concurrent autoimmune diseases (OR=7.25;
P=0.018; 95% CI, 1.41–37.29) was the only important independent
risk factor associated with the discrepant diagnosis by the scoring
systems. However, the presence of anti-SLA/LP or pANCA (OR=0.12;
P=0.022; 95% CI, 0.02–0.74), the level of IgG with 1–1.1 × N
(OR=0.02; P=0.044; 95% CI, 0.00–0.89) and titers of ANA or SMA of
≥1:80 (OR=0.04; P<0.001; 95% CI, 0.01–0.23) were three
independent protective factors. However, the presence of normal or
>1.1 × N levels of IgG, titers of ANA or SMA of ≥1:40, liver
histology and whether the patient underwent a liver biopsy were not
statistically significant factors contributing to discrepant
diagnoses when using the revised and simplified scoring systems
(P>0.05).
 | Table IVLogistic regression analysis of
independent factors associating with a discrepant diagnosis by the
two systems. |
Table IV
Logistic regression analysis of
independent factors associating with a discrepant diagnosis by the
two systems.
| Parameters | B | Odds ratios
(OR) | Wald value | P-value | 95% CI |
|---|
| Drug history | 24.68 | 5.24E10 | 0.00 | 1.000 | 0.00 |
| Concurrent
autoimmune diseases | 1.98 | 7.25 | 5.62 | 0.018 | 1.41–37.29 |
| IgG | | | | | |
| Normal | 4.12 | 0.128 | | | |
| 1–1.1 ×
Normal | −3.96 | 0.02 | 4.07 | 0.044 | 0.00–0.89 |
| >1.1 ×
Normal | −2.05 | 0.13 | 2.38 | 0.123 | 0.01–1.73 |
| ANA or SMA | | | | | |
| <1:40 | | 14.40 | 0.001 | | |
|
1:80>x≥1:40 | 0.41 | 1.51 | 0.18 | 0.676 | 0.22–10.26 |
| ≥1:80 | −3.32 | 0.04 | 12.27 | <0.001 | 0.01–0.23 |
| Anti-SLA/LP or
pANCA | −2.15 | 0.12 | 5.24 | 0.022 | 0.02–0.74 |
| Liver
histology | | | | | |
| Not tested | 0.59 | 0.743 | | | |
| Compatible | −0.68 | 0.51 | 0.59 | 0.441 | 0.09–2.84 |
| Typical | −21.95 | 0.00 | 0.00 | 0.999 | 0.00 |
Discussion
Previous studies have demonstrated that compared
with the revised scoring system, the simplified scoring system
exhibits high sensitivity and specificity for the diagnosis of
definite or probable AIH, and even superior specificity and
predictability for the disease (11–14).
The study by Qiu et al(15), supported the simplified criteria
for the diagnosis of patients, noting that the simplified criteria
had high sensitivity and specificity for the diagnosis of AIH. The
study also showed that lower IgG levels and less frequent
positivity for autoantibodies or lower titers may be two causes
contributing to downgraded or excluded diagnosis by the simplified
criteria. Although the sensitivity and specificity was predicted,
the parameters affecting the discordant diagnosis by the simplified
criteria have not, until now, been independently evaluated in
patients.
Czaja (11)
suggested that the simplified scoring system may underestimate the
diagnosis of AIH in patients who have few or atypical features of
the disease. In the present patient cohort, the application of the
simplified criteria was less likely to ascribe a definite diagnosis
of AIH than the revised scoring system. The concordance rate
between the two scoring systems was 62.3%. Fifty of the 77 patients
(64.9%) identified as having AIH by the revised scoring system were
downgraded by the simplified scoring system. Twenty-four of the 50
patients with a discrepant diagnosis who were downgraded by the
simplified scoring system had low ANA titers (<1:40). Patients
with accordant diagnoses had higher titers of ANA than those with
discrepant diagnosis by the two scoring systems. From the logistic
regression analysis, titers of ANA of ≥1:80 and the presence of
anti-SLA/LP or pANCA were likely to decrease the ratio of
downgraded risk via the simplified scoring system. In addition, IgG
levels of 1–1.1 × N was an independent protective factors; however,
these levels were not considered to be statistically significant
with regard to discordant diagnosis. Therefore, it was suggested
that lower or higher IgG levels were not the main determinants that
affected the discrepancies in diagnosis. This differed from the
study by Qiu et al(15), in
which low IgG levels were one of the main discriminants that
downgraded or even excluded from the diagnosis of AIH by the
simplified scoring system.
The present study attempted to identify certain
notable clinical features in the patients with AIH. Notably,
another discrepancy between the scoring systems was the presence of
other concurrent autoimmune diseases (excluding liver diseases). Of
the AIH patients diagnosed by the revised scoring system, 57.1% had
other associated autoimmune diseases. This presence ratio was
higher than that observed in other regions. García-Torres et
al(16) revealed that
associated autoimmune diseases were observed in 24.7%of patients
with AIH in Spain. According to a nationwide survey in Japan
(17), the prevalence of
complicating Sjögren syndrome in patients with AIH was ~10%.
Furthermore, 62% patients with a discordant diagnosis by the two
scoring systems had other concurrent autoimmune diseases. The
present study further showed that the presence of other concurrent
autoimmune diseases increases the risk of discrepant diagnosis by
the two scoring systems. The revised scoring system may facilitate
the diagnosis of AIH, rather than that of other autoimmune
diseases, in cases that are unrecognized by the simplified scoring
system. In China, the detection of other associated autoimmune
diseases is a pivotal factor in the diagnosis of AIH. A previous
study (14) showed that the
simplified scoring system excluded the diagnosis of other diseases
with concurrent immune features more frequently than the revised
scoring system.
Certain studies (13,15)
demonstrated that liver histological scores were critical for
making the differential diagnosis between AIH and other chronic
liver diseases. However, in the present study, it was revealed that
liver biopsy was not a factor contributing to the discrepant
diagnosis by the two scoring systems. In this study, liver
histology with compatible or typical manifestation may enhance the
diagnosis with AIH via the simplified scoring system.
In the present study, the revised scoring system
performed better in the patients than the simplified criteria. The
revised scoring system was developed to support the diagnosis of
AIH in patients who lacked the conventional serological markers of
the disease and particularly in the patients with other associated
autoimmune diseases. Its golden standard in this regard remains
unchallenged (18). Therefore, the
revised scoring system was capable of diagnosing AIH in those
patients who were downgraded or unrecognized using the simplified
scoring system. Despite this, the simplified scoring system was
utilized as a convenient tool for the four parameters, and was
capable of easily classifying the diagnosis. Notably in the present
study, which was performed in China, the patients with AIH had
various characteristics that differed from patients from other
regions, particularly Europe (19,20).
Those features indicate that the simplified scoring system may be
less sensitive than the revised scoring system in ascribing an
overall (probable or definite) diagnosis of AIH (62.3 vs. 100%).
However, the two scoring systems were created to support the
clinical diagnosis, not to supersede each other. The combination of
systems may ensure a correct diagnosis of patients with AIH.
In conclusion, in this study it was concluded that
other concurrent autoimmune diseases present frequently in Chinese
patients with AIH. The presence of other concurrent autoimmune
diseases, the high titer of ANA or SMA and the presence of other
autoantibodies are important in the discrepant diagnosis of AIH by
the two scoring systems. The golden standard of the revised scoring
system remains unchallenged. Due to the convenience of the
simplified scoring system, it was suggested that this system may be
utilized as a subordinate tool, and that the revised scoring system
should be applied to enhance the diagnosis of AIH.
References
|
1
|
Waldenstrom J: The diagnostic importance
of ACTH. Acta Endocrinol (Copenh). 5:235–242. 1950.
|
|
2
|
Czaja AJ: Autoimmune hepatitis. Evolving
concepts and treatment strategies. Dig Dis Sci. 40:435–456.
1995.PubMed/NCBI
|
|
3
|
Czaja AJ and Freese DK; American
Association for the Study of Liver Disease. Diagnosis and treatment
of autoimmune hepatitis. Hepatology. 36:479–497. 2002. View Article : Google Scholar
|
|
4
|
Czaja AJ: Behavior and significance of
autoantibodies in type 1 autoimmune hepatitis. J Hepatol.
30:394–401. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feld JJ, Dinh H, Arenovich T, et al:
Autoimmune hepatitis: effect of symptoms and cirrhosis on natural
history and outcome. Hepatology. 42:53–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson PJ and McFarlane IG: Meeting
report: International Autoimmune Hepatitis Group. Hepatology.
18:998–1005. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alvarez F, Berg PA, Bianchi FB, et al:
International Autoimmune Hepatitis Group Report: review of criteria
for diagnosis of autoimmune hepatitis. J Hepatol. 31:929–938. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Czaja AJ, Bianchi FB, Carpenter HA, et al:
Treatment challenges and investigational opportunities in
autoimmune hepatitis. Hepatology. 41:207–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czaja AJ and Carpenter HA: Validation of a
scoring system for diagnosis of autoimmune hepatitis. Dig Dis Sci.
41:305–314. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hennes EM, Zeniya M, Czaja AJ, et al:
Simplified criteria for the diagnosis of autoimmune hepatitis.
Hepatology. 48:169–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Czaja AJ: Performance parameters of the
diagnostic scoring systems for autoimmune hepatitis. Hepatology.
48:1540–1548. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chandok N, Silveira MG and Lindor KD:
Comparing the simplified and international autoimmune hepatitis
group criteria in primary sclerosing cholangitis. Gastroenterol
Hepatol (NY). 6:108–112. 2010.PubMed/NCBI
|
|
13
|
Gatselis NK, Zachou K, Papamichalis P, et
al: Comparison of simplified score with the revised original score
for the diagnosis of autoimmune hepatitis: a new or a complementary
diagnostic score? Dig Liver Dis. 42:807–812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeoman AD, Westbrook RH, Al-Chalabi T, et
al: Diagnostic value and utility of the simplified International
Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic
liver disease. Hepatology. 50:538–545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiu D, Wang Q, Wang H, et al: Validation
of the simplified criteria for diagnosis of autoimmune hepatitis in
Chinese patients. J Hepatol. 54:340–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
García-Torres ML, Primo J, Ortuño JA, et
al: A clinical study of adult autoimmune hepatitis in Valencia,
Spain. Rev Esp Enferm Dig. 100:400–404. 2008.(In Spanish).
|
|
17
|
Monna T, Kuroki T and Yamamoto S:
Autoimmune hepatitis: the present status in Japan. Gastroenterol
Jpn. 20:260–272. 1985.
|
|
18
|
Krawitt EL: Clinical features and
management of autoimmune hepatitis. World J Gastroenterol.
14:3301–3305. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Omagari K, Masuda J, Kato Y, et al:
Re-analysis of clinical features of 89 patients with autoimmune
hepatitis using the revised scoring system proposed by the
International Autoimmune Hepatitis Group. Intern Med. 39:1008–1012.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oo YH, Hubscher SG and Adams DH:
Autoimmune hepatitis: new paradigms in the pathogenesis, diagnosis,
and management. Hepatol Int. 4:475–493. 2010. View Article : Google Scholar : PubMed/NCBI
|