Introduction
Schwannomas are benign peripheral nerve sheath
tumors composed exclusively of Schwann cells. The peak incidence
occurs in the fourth to the sixth decades of life, with no gender
predilection. Patients usually present with a slowly growing mass
in the head, neck and the flexor surfaces of the upper and lower
extremities (1). Pain and
neurological symptoms are uncommon unless the tumor reaches a
certain size. The etiology of sporadic schwannoma is not well
understood.
The histological hallmark of schwannoma is the
alternating pattern of Antoni A and B areas. The relative levels of
these two components vary, and they may blend imperceptibly or
change abruptly. Antoni A areas are highly cellular and demonstrate
nuclear palisading and Verocay bodies. Antoni B areas are less
cellular and far less orderly. A number of schwannomas have
thick-walled vessels with fibrinoid and hyaline changes in the
vessel walls (1).
Sural nerve schwannoma is exceptionally rare. This
study presents an unusual case of solitary schwannoma originating
from the sural nerve in a middle-aged man. Written informed consent
for publication was obtained from the patient.
Case report
A 42-year-old male patient was referred to Fukuoka
University Hospital (Fukuoka, Japan) with a >30-year history of
a slowly growing, painless mass in the posterolateral aspect of the
left distal lower leg. Physical examination revealed a 3-cm,
elastic-hard, mobile, non-tender mass, while neurovascular
examinations, including Tinel’s sign, were normal. The patient’s
past medical history included nothing of note. Magnetic resonance
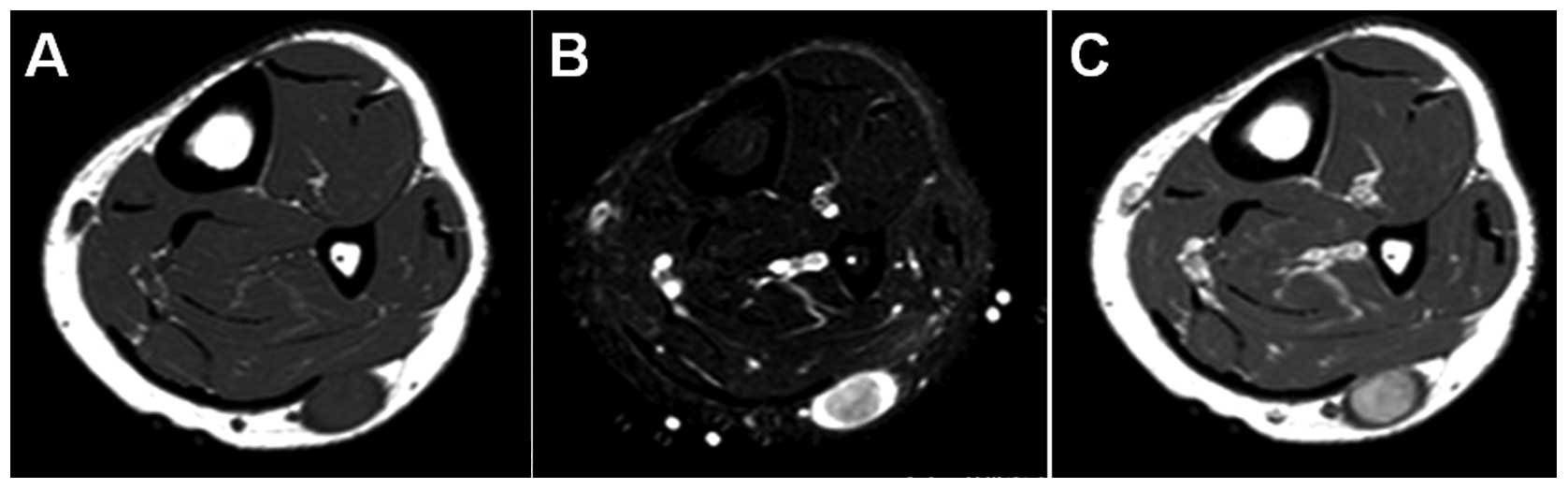
imaging (MRI) demonstrated an oval-shaped subcutaneous mass. The
mass showed iso-signal intensity relative to adjacent muscle on
T1-weighted sequences (Fig. 1A),
and higher signal intensity peripherally and lower signal intensity
centrally, representing a target sign, on T2-weighted spectral
presaturation with inversion recovery sequences (Fig. 1B). Contrast-enhanced T1-weighted
sequences demonstrated a marked central enhancement of the mass
(Fig. 1C). Based on these results,
a benign neurogenic tumor, such as schwannoma, was strongly
suspected.
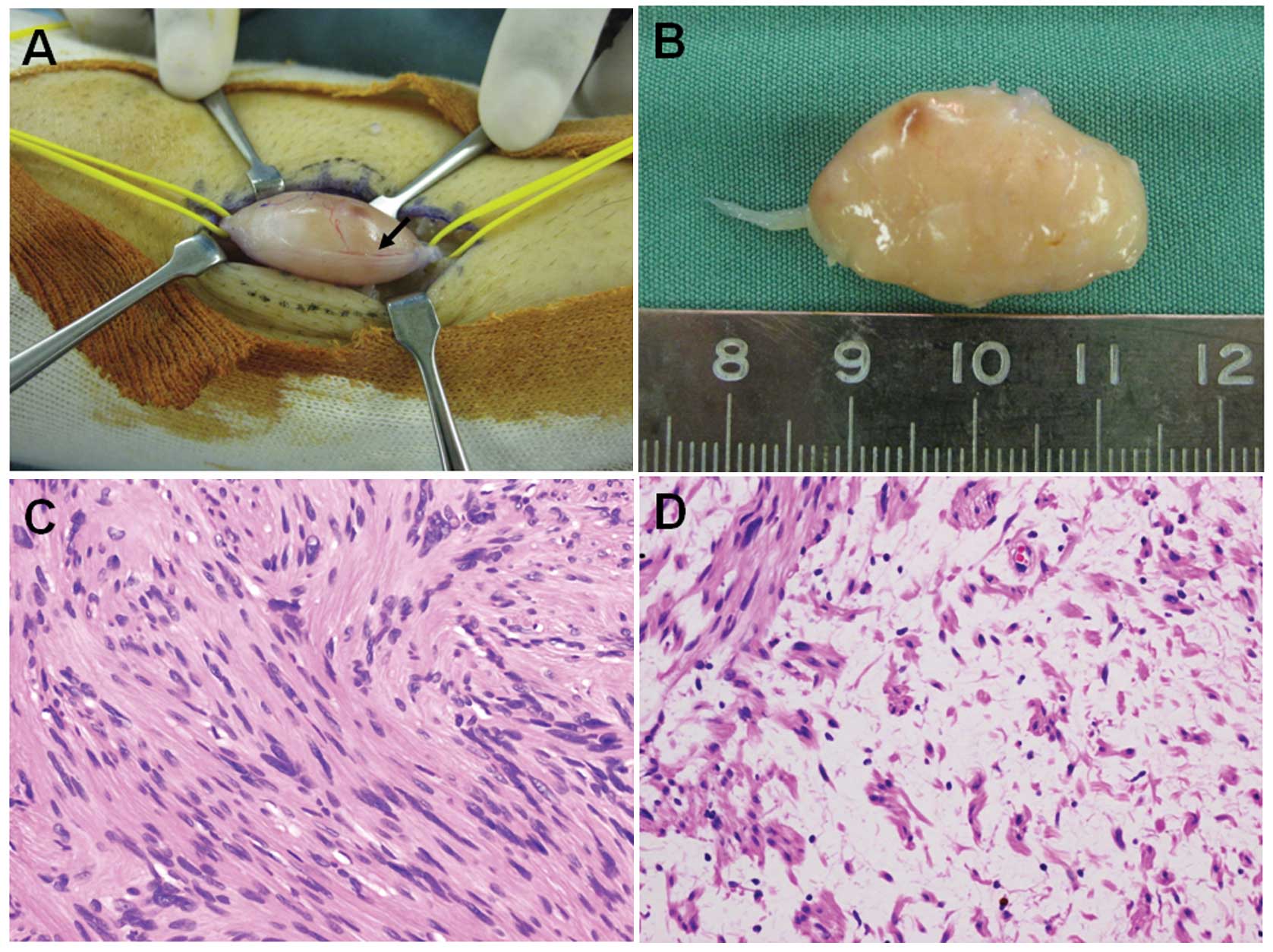
The surgery was performed under general anesthesia
with tourniquet control and loupe magnification. The tumor and the
proximal and distal portions of the affected nerve were exposed
(Fig. 2A), and then a longitudinal
incision was carefully made in the epineurium, distal to the
fascicles. The epineurial layers were gently peeled out until the
shiny surface of the tumor was exposed. The entire tumor mass was
subsequently shelled out in one piece without damage to the
fascicles. The intraoperative observations were consistent with the
diagnosis of schwannoma. Grossly, the smooth-surfaced tumor was
tan-white (Fig. 2B).
Microscopically, the tumor showed a proliferation of spindle-shaped
cells arranged in interlacing fascicles in Antoni A areas (Fig. 2C). Edematous, hypocellular areas,
known as Antoni B, were also observed (Fig. 2D). Neither nuclear atypia nor
mitotic figures were observed. These features confirmed the
diagnosis of schwannoma.
The postoperative course was uneventful. At two
months of follow-up, the patient had no evidence of recurrence and
no neurological deficit.
Discussion
The sural nerve is a sensory nerve that lies close
to the small saphenous vein and provides sensory innervation to the
lateral surface of the foot and ankle. It is typically composed of
two merging components: A medial component from the tibial nerve
and a lateral component from the lateral sural cutaneous nerve or
common peroneal nerve (2).
Solitary schwannoma originating from this nerve is extremely rare;
only few cases have been described in the English literature
(3–5). Moreover, Ogose et al(6) described a case of multiple
schwannomas in the sural nerve. Despite its rare occurrence, it is
important to be aware of the possible existence of sural nerve
schwannoma in the lower leg.
The radiographic features of schwannomas are
non-specific. On MRI, the lesions usually demonstrate iso- or low
signal intensity relative to skeletal muscle on T1-weighted images
and high signal intensity on T2-weighted images. A fascicular
appearance and a thin hyperintense rim may be observed on
T2-weighted images (7).
Furthermore, the target sign is one of the characteristic imaging
features in schwannomas (8), as
observed in the present case. Contrast-enhanced T1-weighted images
show strong central enhancement. Previous studies have described a
substantial variability in the 18F-fluorodeoxyglucose
(FDG) uptake by schwannomas (9–11).
These studies suggested that schwannomas may not be reliably
discriminated from malignant peripheral nerve sheath tumors by FDG
positron emission tomography imaging.
In the present case, the possible differential
diagnosis included neurofibroma and benign perivascular tumors,
such as angioleiomyoma. Neurofibromas may assume one of three
growth patterns: Solitary, diffuse or plexiform. Solitary
neurofibromas usually arise in a cutaneous nerve of the dermis or
subcutis. In the majority of patients, solitary neurofibromas
present as a slowly growing, painless mass. Histologically,
solitary neurofibromas consist of a mixture of Schwann cells,
perineurial cells and fibroblasts in a matrix of wavy collagenous
fibers. In contrast to schwannomas, the cells are loosely arranged
and diffusely infiltrate the involved nerve (12). On MRI, the lesions usually exhibit
iso- or low signal intensity relative to skeletal muscle on
T1-weighted images and heterogeneous high signal intensity on
T2-weighted images. Contrast-enhanced T1-weighted images show
central enhancement. The target sign has been described as being
nearly pathognomonic of neurofibroma on T2-weighted images
(7,13). In the opinion and experience of the
authors, it is often difficult or impossible to distinguish
schwannomas from neurofibromas solely on the basis of MRI.
Angioleiomyomas are relatively common soft-tissue
tumors that occur in the subcutaneous tissues of the extremities,
particularly the lower leg (14).
Pain is the major complaint in approximately half of patients.
Angioleiomyoma typically presents as a small, slowly growing, firm
mass. Histologically, angioleiomyomas are composed of
well-differentiated smooth muscle cells with intervening vascular
channels. On MRI, the lesions usually exhibit iso- or slightly high
signal intensity relative to skeletal muscle on T1-weighted images
and heterogeneous high signal intensity on T2-weighted images.
Contrast-enhanced T1-weighted images show marked enhancement
(15). Unlike neurogenic tumors,
angioleiomyomas usually do not exhibit the target sign on
T2-weighted images.
In conclusion, a rare example of solitary schwannoma
originating from the sural nerve in a middle-aged male patient has
been described. Clinicians should consider schwannoma as a possible
diagnosis for a well-defined, oval, subcutaneous mass in the
posterior aspect of the lower leg.
Acknowledgements
This study was supported in part by the Foundation
for the Promotion of Medical Science.
References
|
1
|
Antonescu CR, Perry A and Woodruff JM:
Schwannoma (including variants). World Health Organization
Classification of Tumours of Soft Tissue and Bone. Fletcher CDM,
Bridge JA, Hogendoorn PCW and Mertens F: 4th edition. IARC Press;
Lyon: pp. 170–172. 2013
|
|
2
|
Riedl O and Frey M: Anatomy of the sural
nerve: cadaver study and literature review. Plast Reconstr Surg.
131:802–810. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Isobe K, Shimizu T, Akahane T and Kato H:
Imaging of ancient schwannoma. AJR Am J Roentgenol. 183:331–336.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim DH, Murovic JA, Tiel RL, Moes G and
Kline DG: A series of 397 peripheral neural sheath tumors: 30-year
experience at Louisiana State University Health Sciences Center. J
Neurosurg. 102:246–255. 2005.
|
|
5
|
Fellegara G and Bisceglia M: Intraneural
schwannoma. Int J Surg Pathol. 16:57–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogose A, Hotta T, Morita T, Otsuka H and
Hirata Y: Multiple schwannomas in the peripheral nerves. J Bone
Joint Surg Br. 80:657–661. 1998. View Article : Google Scholar
|
|
7
|
Jee WH, Oh SN, McCauley T, Ryu KN, Suh JS,
Lee JH, Park JM, Chun KA, Sung MS, Kim K, Lee YS, Kang YK, Ok IY
and Kim JM: Extraaxial neurofibromas versus neurilemmomas:
discrimination with MRI. AJR Am J Roentgenol. 183:629–633. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koga H, Matsumoto S, Manabe J, Tanizawa T
and Kawaguchi N: Definition of the target sign and its use for the
diagnosis of schwannomas. Clin Orthop Relat Res. 464:224–229.
2007.PubMed/NCBI
|
|
9
|
Ahmed AR, Watanabe H, Aoki J, Shinozaki T
and Takagishi K: Schwannoma of the extremities: the role of PET in
preoperative planning. Eur J Nucl Med. 28:1541–1551. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beaulieu S, Rubin B, Djang D, Conrad E,
Turcotte E and Eary JF: Positron emission tomography of
schwannomas: emphasizing its potential in preoperative planning.
AJR Am J Roentgenol. 182:971–974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benz MR, Czernin J, Dry SM, Tap WD,
Allen-Auerbach MS, Elashoff D, Phelps ME, Weber WA and Eilber FC:
Quantitative F18-fluorodeoxyglucose positron emission tomography
accurately characterizes peripheral nerve sheath tumors as
malignant or benign. Cancer. 116:451–458. 2010. View Article : Google Scholar
|
|
12
|
Antonescu CR, Brems H, Legius E and
Woodruff JM: Neurofibroma (including variants). World Health
Organization Classification of Tumours of Soft Tissue and Bone.
Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: 4th edition.
IARC Press; Lyon: pp. 174–176. 2013
|
|
13
|
Murphey MD, Smith WS, Smith SE, Kransdorf
MJ and Temple HT: From the archives of the AFIP. Imaging of
musculoskeletal neurogenic tumors: radiologic-pathologic
correlation. Radiographics. 19:1253–1280. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hachisuga T, Hashimoto H and Enjoji M:
Angioleiomyoma: a clinicopathologic reappraisal of 562 cases.
Cancer. 54:126–130. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupte C, Butt SH, Tirabosco R and
Saifuddin A: Angioleiomyoma: magnetic resonance imaging features in
ten cases. Skeletal Radiol. 37:1003–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|