Introduction
Parkinson’s disease (PD) is one of the most common
neurodegenerative disorders, and results from the progressive loss
of dopamine (DA)-containing neurons in the substantia nigra (SN)
and a decrease in DA concentration in the striatum (ST) (1).
1-Methyl-4-phenyl-1,2,3,6-tetra-hydropyridine (MPTP) is a potent
neurotoxin that causes selective nigral dopaminergic lesions,
resulting in clinical features similar to that of idiopathic PD in
human and nonhuman primates. A previous study demonstrated that
MPTP-induced dopaminergic cell degeneration was dependent on the
presence of dopamine transporter (DAT) (2). Therefore, the blockade of DAT
functions may attenuate the accumulation of neurotoxins into
dopaminergic neurons and the subsequent neurotoxicity. Several DA
uptake inhibitors have been examined in animals and a number of
these have demonstrated complete protection against DA depletion in
the mouse ST if administered prior to DA-depleting doses of MPTP.
The inhibition of DA reuptake may reverse motor deficits in
MPTP-treated primates (3,4).
α-Lobeline (lobeline), a lipophilic, nonpyridino,
alkaloidal constituent of Indian tobacco, is the predominant
alkaloid in a family of structurally-related compounds found in
Lobelia inflata(5). It has
been reported that lobeline may inhibit DA uptake into synaptic
vesicles and stimulate the reverse transportation of DA from
synaptic vesicles via interactions with the vesicular monoamine
transporter 2 (6). The aim of the
present study was to investigate whether lobeline exerted
neuroprotective effects in vivo using MPTP-induced mice
models of PD.
Materials and methods
Animal preparation and the MPTP-induced
model of PD
Male C57BL/6J mice (weight, 30±2 g; age, 4 months)
were supplied by the Shanghai Laboratory Animal Center (Shanghai,
China). The mice were maintained in cages (n=4 per cage) with
access to food and water ad libitum and under a 12/12-h
light/dark cycle. Prior to the experiments, all mice were trained
on the rotarod 3 times a day for 2 weeks. Mice were randomly
assigned to six groups (n=10 per group), comprising one group of
control mice and five groups of mice treated with MPTP.
MPTP-intoxicated mice were administered one subcutaneous (s.c)
injection of MPTP-HCl per day (30 mg/kg MPTP per day) for five
consecutive days. Lobeline-HCl (1 or 3 mg/kg lobeline,
respectively), GBR12935 (10 mg/kg) or vehicle (saline) were
administered via s.c injections for 11 consecutive days 30 min
prior to MPTP administration. These four groups of mice were known
as the lobeline (1 mg/kg)-treated, lobeline (3 mg/kg)-treated,
GBR12935 (10 mg/kg)-treated and MPTP-intoxicated (marked as
‘saline’ in figures) groups. In addition, a final group of mice,
the L-dopa-treated group, were administered 80 mg/kg L-dopa orally
for 11 days as positive controls. Mice in the control group
received s.c. injections of saline only. This study was approved by
the Zhengzhou University Life-Science Ethics Review Committee
(Zhengzhou University People’s Hospital, Zhengzhou, China).
Rotarod test
Rotarod tests were performed on the 7th, 9th and
11th days. The rotarod testing procedure was a modification of that
initially described by Rozas and Labandeira García (7). The overall rod performance (ORP)
score for each animal was calculated by the trapezoidal method as
the area under the curve in the plot of time-on-the-rod against
rotation speed. Time-on-the-rod at each speed was the mean of three
values obtained on the three days of testing.
Swim test
Swim tests were performed on the 12th day in water
tubs (length, 40 cm; width, 25 cm and height, 25 cm). The depth of
water (27±2ºC) was maintained at 15 cm. The animals were
acclimatized for 10 min one day prior to experimentation. The score
scales were as follows: 0, hind part sinks with head floating; 1,
occasional swimming using hind limbs while floating on one side; 2,
occasional floating, mostly swimming; 3, continuous swimming.
Immunohistochemistry
For tyrosine hydroxylase (TH) immunohistochemistry,
mice (n=3 per group) were sacrificed at the end of the swim test
and perfusion-fixed with 4% paraformaldehyde in 0.1 M
phosphate-buffered saline (PBS) (pH 7.4). Brains were removed,
postfixed in the same fixative solution for 12 h and dehydrated in
25% sucrose/PBS solution for 24 h. The entire midbrain and ST were
cryosectioned (20 μm for TH) and stored free-floating at 4ºC in a
solution of PBS with 0.2% sodium azide. Tissue sections were
incubated successively with a rabbit polyclonal anti-TH antibody
(1:400), a biotinylated-conjugated polyclonal goat anti-rabbit
antibody (1:400) and a horseradish-peroxidase-conjugated
avidin/biotin complex. All antibodies were purchased from Vector
Laboratories, Inc. (Burlingame, CA, USA). Peroxidase activity was
assessed using diaminobenzidine and sections were mounted on glass
slides.
The degree of neuronal loss was estimated using a
previously described method and an image analysis system (Quantimet
color 500, Leica Cambridge Ltd., Cambridge, UK) (8). Neurons were counted on five regularly
spaced sections covering the entire SN to estimate the total number
of neurons in the SN pars compacta (pc). The optical density (OD)
of TH immunoreactivity in the ST was measured using the image
analysis system (Quantimet color 500, Leica Cambridge Ltd.).
Statistical analysis
All values are expressed as the mean ± standard
error of the mean. Differences among means were analyzed using
one-way or two-way analysis of variance (ANOVA). When ANOVA showed
significant differences, pair-wise comparisons between means were
tested using Fisher or Newman-Keuls post-hoc tests. In all
analyses, the null hypothesis was rejected at a level of 0.05
(two-tailed, unless otherwise stated).
Results
Behavioral tests
Rotarod test
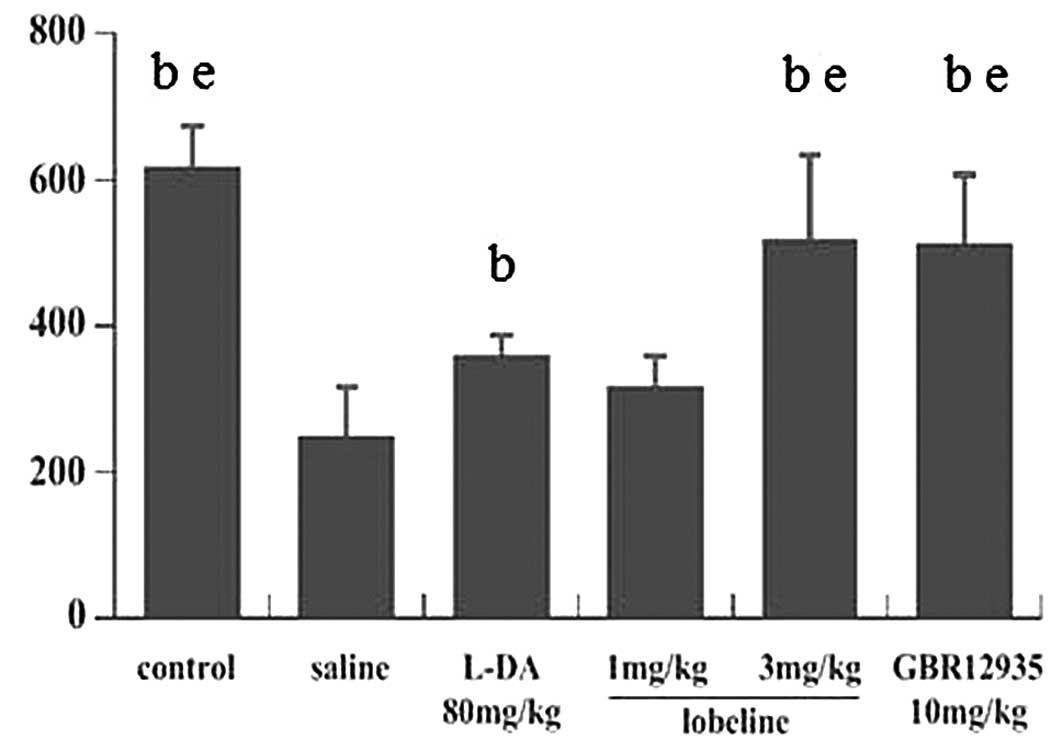
Mice underwent rotarod testing on the 7th, 9th and
11th days after the first day of MPTP intoxication, and the mean
ORP scores were calculated. In all six groups, the mean ORP scores
showed no significant changes throughout the experimental period.
The mean ORP score of the control group was 617.6±54.86. No
significant differences were identified between the lobeline (3
mg/kg)-treated, GBR12935-treated and control groups; however, the
mean ORP score of the MPTP-intoxicated group was significantly
lower than that of the control group. The mean ORP score of the
L-DA-treated group was lower than those of the lobeline (3
mg/kg)-treated and GBR12935-treated groups, although these three
groups all showed significantly increased ORP scores compared with
that of the MPTP-intoxicated group. However, the mean ORP score of
the lobeline (1 mg/kg)-treated group showed no significant
difference compared with that of the MPTP-intoxicated group
(Fig. 1).
Swim test
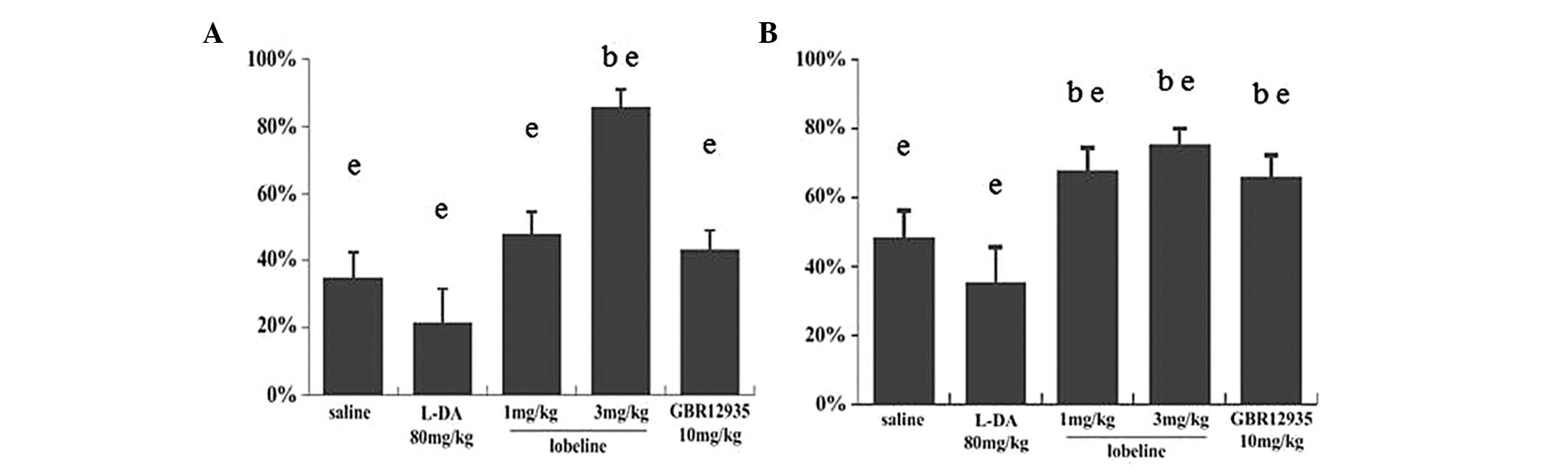
The swim test was performed once at the end of the
experiment, as forced swim tests may result in depression and
influence other behavioral tests. The results of the test indicated
that lobeline (3 mg/kg) reversed the motor deficit (Fig. 2). No significant differences in
swim score were identified between the lobeline (3 mg/kg)-treated,
GBR12935-treated and control groups. The swim score in the
L-DA-treated group was significantly higher than that of the
MPTP-intoxicated group and significantly lower than that of the
lobeline (3 mg/kg)-treated group. No significant difference was
found between the lobeline (1 mg/kg)-treated and MPTP-intoxicated
groups (Fig. 2). The results
supported the findings of the rotarod test.
Immunohistochemistry
TH immunostaining in the mesencephalon enabled
dopaminergic neurons in the SN (Fig.
3A, C, E, G, I and K) and ST (Fig.
3B, D, F, H, J and L) to be identified. MPTP intoxication
induced a loss of TH immunoreactivity in the SN. At the striatal
level, a sharp decrease in TH immunoreactivity was observed in the
ST of the MPTP-intoxicated (saline) mice compared with that of the
control mice. The decrease in the number of TH-positive neurons was
significant in the L-DA-treated group, while minimal in the
lobeline (3 mg/kg)-treated group. In addition, the OD of TH
immunostaining in the ST and the number of TH-positive neurons in
the SN showed less significant reductions in the lobeline (1
mg/kg)-treated and GBR12935-treated mice than that in the
MPTP-intoxicated mice (Fig.
4).
 | Figure 3Microphotographs of tyrosine
hydroxylase-immunostained sections from the (A,C,E,G,I,K) SN and
(B,D,F,H,J,L) ST in (A,B) control mice and MPTP-intoxicated mice
treated with (C,D) saline, (E,F) L-DA (80 mg/kg), (G,H) lobeline (1
mg/kg), (I,J) lobeline (3 mg/kg) and (K,L) GBR12935 (10 mg/kg).
Note that compared with other treatment options, mice treated with
lobeline (3 mg/kg) showed more structural integrity in the SN
(indicated by arrows). All treatment options showed more
condensation of immunoreactivity in the ST than that of the saline
group, with the exception of the L-DA (80 mg/kg) treatment group
(magnification, ×400). SN, substantia nigra; ST, striatum; MPTP,
1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine. |
Discussion
The dopaminergic neurotoxin MPTP was introduced in
the present study as a paradigm to produce a lesion of dopaminergic
neurons in mice. Using rotarod and swim tests, the overall
locomotor impairment of mice in different groups was evaluated.
MPTP-intoxicated mice showed lower scores than animals in the
control group (P<0.05). By contrast, mice that received
treatment with lobeline (3 mg/kg) exhibited significantly less
impaired mobile abilities than the MPTP-intoxicated mice
(P<0.05); however, no significant differences were identified
between the MPTP-intoxicated and lobeline (1 mg/kg)-treated groups
(P>0.05). In addition, the deficits in locomotor activity were
reversed by the administration of L-dopa and GBR12935.
Comparisons of TH immunoreactivity in the SN and ST
were performed among the five groups of animals. A marked decrease
in the number of TH-positive cells was observed in the
MPTP-intoxicated group (~65% compared with the control group). A
significant difference was also identified between the lobeline (3
mg/kg)-treated and control groups; however, the decrease was more
indiscernible (~15%). Of note, compared with the MPTP-intoxicated
group, the decrease in the number of TH-positive neurons appeared
more severe in the L-dopa (80 mg/kg)-treated group (~78%, with no
statistical significance), while the decreases in the lobeline (1
mg/kg)-treated (~52%) and GBR12935-treated (~56%) groups were less
marked.
The results of this study demonstrated that a higher
concentration of lobeline (3 mg/kg) may protect cells against
MPTP-induced toxicity in dopaminergic systems. L-dopa may have
reversed the toxin-induced locomotion deficits; however, it may
have accelerated cell death of dopaminergic neurons in the SNpc as
reported in a previous study (9).
It is of note that GBR12935 was also unable to prevent cells from
the disease progress. Unlike lobeline, which serves as a selective
inhibitor of DAT-mediated uptake without influence the
transporter-mediated release of neurotransmitter, GBR12935 also
acts as an inhibitor of DAT-mediated release at low concentrations
(5 nM) (10). This may result in
an increase in intracellular DA concentration in dopaminergic
cells, and increasing endocellular neurotoxins may result in
neurotoxicity and neuron death. However, GBR12935 remains an
effective agent for symptomatic treatment.
Lobeline is an atypical lipophilic, alkaloidal
nicotinic ligand derived from the plant Lobelia inflata.
Although it shares no obvious structural resemblance to
S(−)nicotine and the structure-function associations between
nicotine and lobeline do not suggest a common pharmacophore,
lobeline has shown mixed agonist- and antagonist-like effects, as
well as additional non-nicotinic effects (11). Previous epidemiological studies
have reported a significant inverse correlation between cigarette
smoking and the incidence of PD (12,13).
Furthermore, studies have demonstrated that nicotine may exert
neuroprotective effects against excitotoxic insults in neuronal
cultures (14,15). Additional studies have supported
the findings that nicotine exerts protective effects on
dopaminergic systems against MPTP toxicity in vivo(16,17).
One mechanism underlying this protective action may be its ability
to increase the expression of neurotrophic factors that are known
to promote survival of dopaminergic neurons (18,19).
Thus, the nicotine-like effects may also be accountable for the
protective effects of lobeline against MPTP-induced
neurotoxicity.
In conclusion, in addition to exerting nicotine-like
effects, lobeline, as an inhibitor of DAT, may protect dopaminergic
neurons against extracellular toxins, such as MPTP
(MPP+), by blocking DAT-mediated uptake, and may
increase the DA concentration in the synaptic cleft by inhibiting
reuptake of DA. Lobeline administration may thus result in the
treatment of symptoms and the deceleration of PD progress.
Therefore, the combination of several beneficial properties (high
blood-brain barrier penetration, DAT inhibition and neurotrophic
functions similar to nicotine) suggests that lobeline is a
potential preventive or therapeutic agent for the treatment of
PD.
References
|
1
|
Agid Y: Parkinson’s disease:
pathophysiology. Lancet. 337:1321–1324. 1991.
|
|
2
|
Ono S, Hirai K and Tokuda E: Effects of
pergolide mesilate on metallothionein mRNAs expression in a mouse
model for Parkinson disease. Biol Pharm Bull. 32:1813–1817. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fox SH, Visanji NP, Johnston TH,
Gomez-Ramirez J, Voon V and Brotchie JM: Dopamine receptor agonists
and levodopa and inducing psychosis-like behavior in the MPTP
primate model of Parkinson disease. Arch Neurol. 63:1343–1344.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tayarani-Binazir K, Jackson MJ, Rose S,
McCreary AC and Jenner P: The partial dopamine agonist pardoprunox
(SLV308) administered in combination with l-dopa improves efficacy
and decreases dyskinesia in MPTP treated common marmosets. Exp
Neurol. 226:320–327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barlow RB and Johnson O: Relations between
structure and nicotine-like activity: X-ray crystal structure
analysis of (−)-cytisine and (−)-lobeline hydrochloride and a
comparison with (−)-nicotine and other nicotine-like compounds. Br
J Pharmacol. 98:799–808. 1989.PubMed/NCBI
|
|
6
|
Dimatelis JJ, Russell VA, Stein DJ and
Daniels WM: The effects of lobeline and naltrexone on
methamphetamine-induced place preference and striatal dopamine and
serotonin levels in adolescent rats with a history of maternal
separation. Metab Brain Dis. 27:351–361. 2012. View Article : Google Scholar
|
|
7
|
Rozas G and Labandeira García JL:
Drug-free evaluation of rat models of parkinsonism and nigral
grafts using a new automated rotarod test. Brain Res. 749:188–199.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Damier P, Hirsch EC, Agid Y and Graybiel
AM: The substantia nigra of the human brain. I Nigrosomes and the
nigral matrix, a compartmental organization based on calbindin
D(28K) immunohistochemistry. Brain. 122:1421–1436. 1999. View Article : Google Scholar
|
|
9
|
Szego ÉM, Gerhardt E, Kermer P and Schulz
JB: A30P α-synuclein impairs dopaminergic fiber regeneration and
interacts with L-DOPA replacement in MPTP-treated mice. Neurobiol
Dis. 45:591–600. 2012.
|
|
10
|
Graham D and Langer SZ: Advances in
sodium-ion coupled biogenic amine transporters. Life Sci.
51:631–645. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McChargue DE, Collins FL Jr and Cohen LM:
Effect of non-nicotinic moist snuff replacement and lobeline on
withdrawal symptoms during 48-h smokeless tobacco deprivation.
Nicotine Tob Res. 4:195–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu R, Guo X, Park Y, Huang X, Sinha R,
Freedman ND, Hollenbeck AR, Blair A and Chen H: Caffeine intake,
smoking, and risk of Parkinson disease in men and women. Am J
Epidemiol. 175:1200–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Huang X, Guo X, Mailman RB, Park
Y, Kamel F, Umbach DM, Xu Q, Hollenbeck A, Schatzkin A and Blair A:
Smoking duration, intensity, and risk of Parkinson disease.
Neurology. 74:878–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sieber M: Neuroprotective properties of
nicotine. Curr Med Chem. 19:292–297. 2012. View Article : Google Scholar
|
|
15
|
Singh K, Singh S, Singhal NK, Sharma A,
Parmar D and Singh MP: Nicotine- and caffeine-mediated changes in
gene expression patterns of MPTP-lesioned mouse striatum:
Implications in neuroprotection mechanism. Chem Biol Interact.
185:81–93. 2010. View Article : Google Scholar
|
|
16
|
Li Y, Yu DQ, Peng Y, Feng YH, Zhang DM,
Zhao J, Zhang WQ and Sun YP: The protective effect of nicotine on
dopaminergic neuron of Parkinson’s disease mice. Zhongguo Ying Yong
Sheng Li Xue Za Zhi. 23:425–429. 2007.(In Chinese).
|
|
17
|
Parain K, Hapdey C, Rousselet E, Marchand
V, Dumery B and Hirsch EC: Cigarette smoke and nicotine protect
dopaminergic neurons against the
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine Parkinsonian toxin.
Brain Res. 984:224–232. 2003. View Article : Google Scholar
|
|
18
|
Harrod SB, Lacy RT, Zhu J, Hughes BA,
Perna MK and Brown RW: Gestational IV nicotine produces elevated
brain-derived neurotrophic factor in the mesocorticolimbic dopamine
system of adolescent rat offspring. Synapse. 65:1382–1392. 2011.
View Article : Google Scholar
|
|
19
|
Toulorge D, Guerreiro S, Hild A, Maskos U,
Hirsch EC and Michel PP: Neuroprotection of midbrain dopamine
neurons by nicotine is gated by cytoplasmic Ca2+. FASEB
J. 25:2563–2573. 2011. View Article : Google Scholar : PubMed/NCBI
|