Introduction
Non-Hodgkin’s lymphoma (NHL) has a far greater
inclination to disseminate to extranodal sites compared with
Hodgkin’s lymphoma (HL). The most common extranodal site is the
gastrointestinal tract, whereas NHL of the ureter is rare. Over the
past five decades, there have been limited case reports regarding
NHL of the ureter. Due to the limited number of cases, treatment of
the disease has not been unified. In the current study, a Chinese
male with NHL of the ureter was identified. The patient was treated
with surgery and chemotherapy. In combination with the relevant
literature, the diagnosis and treatment of malignant lymphomas of
the ureter are discussed in the present study.
Case report
Patient history
In October 2011, a 38-year-old Chinese male
complained of back pain and was admitted to Changzheng Hospital
(Shanghai, China) with left hydronephrosis, which was confirmed by
ultrasound. Ureteroscopy revealed a luminal stenosis of the left
ureter, thus, a ureteral double J stent was inserted. A
ureteroscopic biopsy was performed and histopathological
examination revealed a granuloma. Follow-up examination months
later showed no evidence of diminished hydronephrosis. Written
informed patient consent was obtained from the patient.
Patient examination
In February 2012, the patient was referred to Renji
Hospital (Shanghai, China) for further evaluation of the ureteral
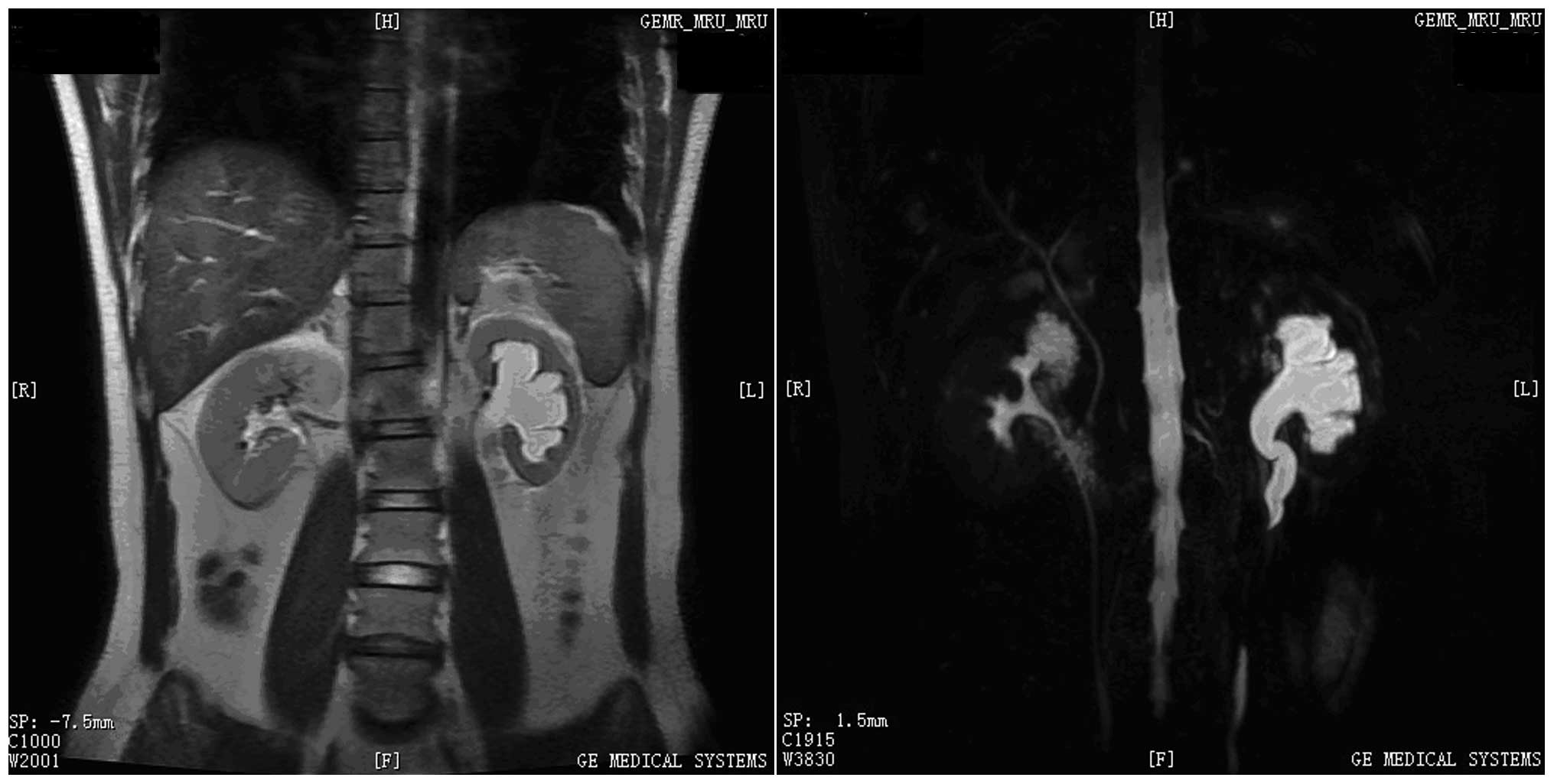
stenosis with uncertain etiology. Magnetic resonance imaging (MRI)
revealed an area of nodular soft-tissue density in the wall of the
left-middle ureter (Fig. 1). This
section of the ureteral wall exhibited low intensity on T1-weighted
images (WIs) and slight hyperintensity on T2-WIs. In addition, MRI
scans revealed retroperitoneal adenopathy and no abnormalities in
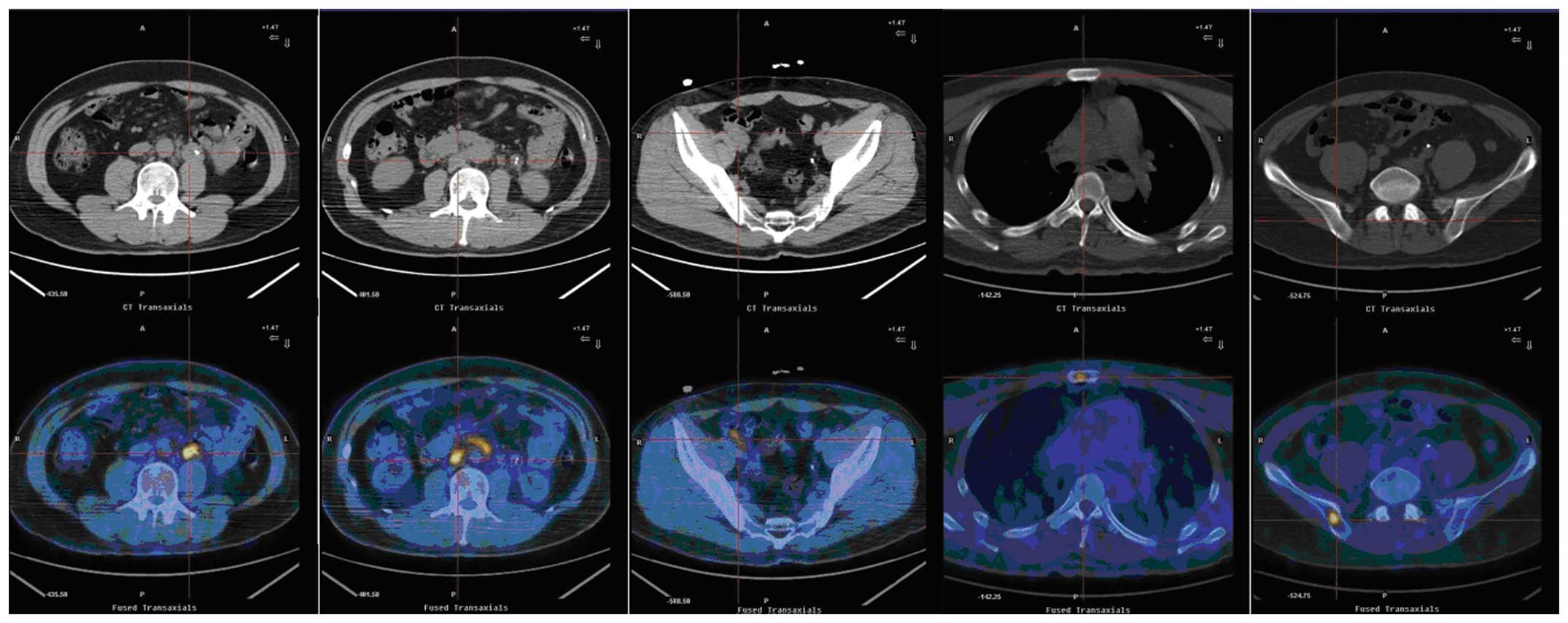
the right ureter. Positron emission tomography-computed tomography
(PET-CT) revealed an area of nodular soft-tissue density in the
wall of the left middle ureter at the L3 level (Fig. 2). This section had an area of
2.4×2.4 cm, with an average standardized uptake value (SUV) of 6.1.
The PET-CT scans also revealed paraaortic and iliac adenopathy with
an average SUV of 2.1–5.4. Increased fludeoxyglucose (FDG)
metabolism was also observed in the sternum and right ilium
(average SUV, 3.4–5.4). Radioisotope renography demonstrated that
the glomerular filtration rate (GFR) of the left kidney had
significantly decreased to 10.2 ml/min/1.73 m2, while
the GFR of the right kidney had reduced to 38.3 ml/min/1.73
m2. Subsequently, the patient underwent a left
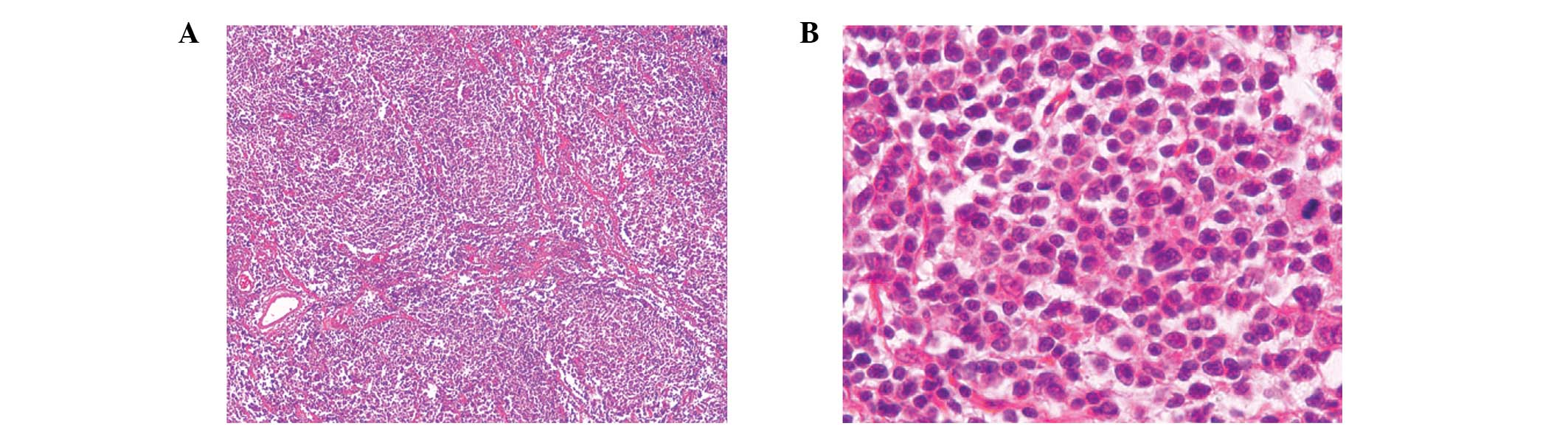
nephroureterectomy. Pathological examination revealed that the
left-middle ureter exhibited nodular thickening. Additional
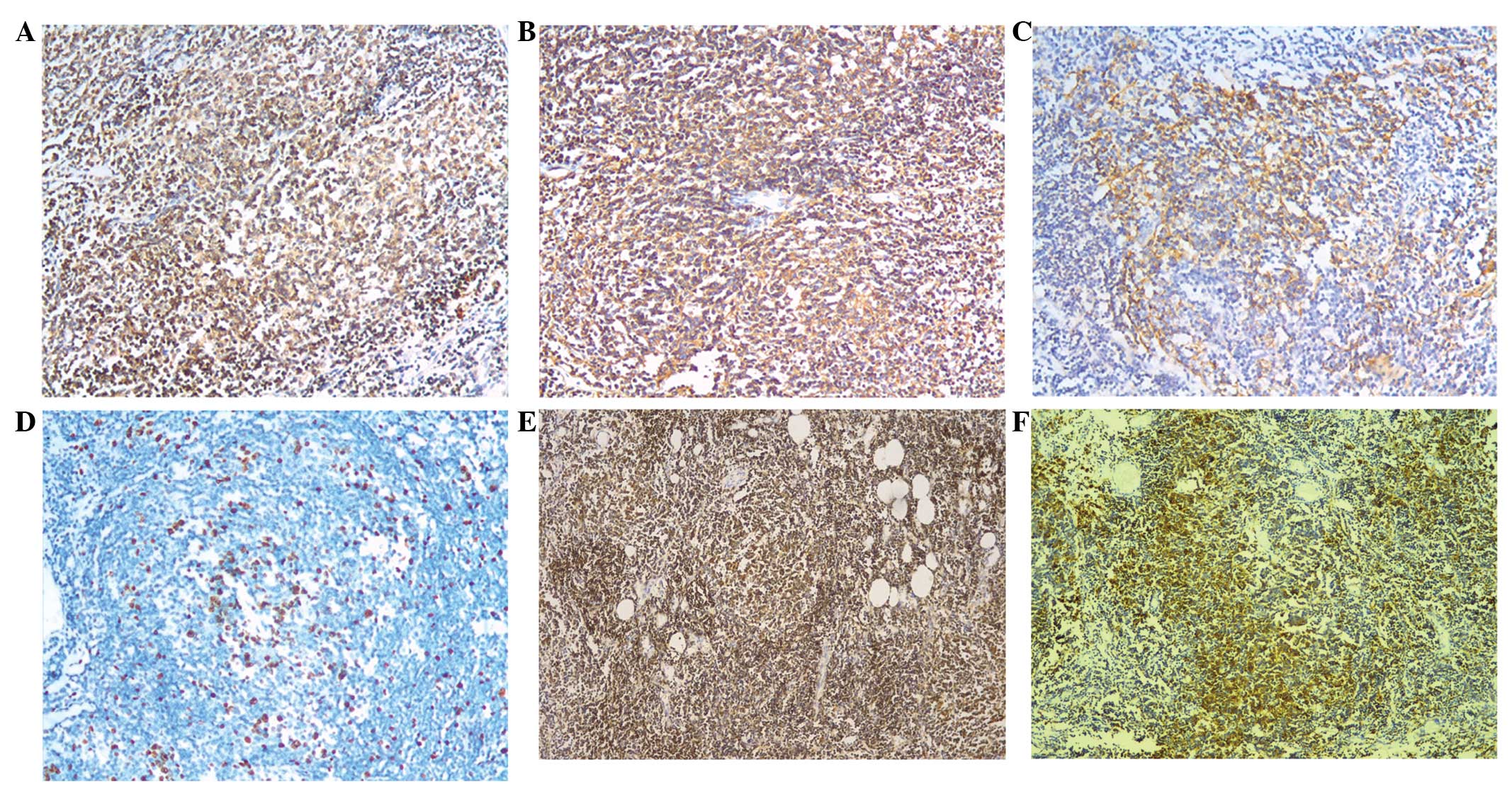
immunohistochemical analysis of the specimen (Figs. 3 and 4) revealed that the atypical lymphocytes
were positive for CD20, CD79α, CD21 and CD23. The Ki-67 index was
10% and Bcl-2 staining was positive in the follicular nodules.
Large, atypical cells were not observed and the tumor was diagnosed
as a grade 1 follicular NHL. Furthermore, histological examination
indicated chronic interstitial nephritis. The bone marrow biopsy
appeared normal and the bone marrow cytogenetic study revealed a
normal male karyotype.
Treatment and follow-up
The patient received systemic chemotherapy combining
375 mg/m2 rituximab, 750 mg/m2
cyclophosphamide, 20 mg/m2 liposomal doxorubicin, 1.4
mg/m2 vincristine and 80 mg methylprednisolone (R-CHOP),
which was administered every three weeks. Following three courses
of treatment, PET-CT examination revealed that the paraaortic
adenopathy had reduced by 50%, the iliac nodes had disappeared and
FDG metabolism in the sternum and right ilium was normal. Following
six courses of chemotherapy, the PET-CT scans showed negative for
residual tumor. Subsequently, the patient received a further two
courses of R-CHOP. Thus far, the follow-up period is seven months
and the current evaluation is that the patient is in complete
remission.
Discussion
Clinical manifestation of NHL is diverse and NHL has
highly variable outcomes. NHL has a far greater inclination to
disseminate to extranodal sites. The extranodal sites normally
affected by NHL include the gastrointestinal tract, testes,
ovaries, central nervous system, prostate, thyroid, bones and skin.
The most common extranodal site is the gastrointestinal tract,
whereas NHL of the ureter is rare. An extensive literature search
of the PubMed database only identified a few case reports (1–3,5–9) over
the past five decades that reported cases of NHL in the ureter. A
total of 20 patients with ureteral malignant lymphomas were
identified, the majority of which originated from Japan. Detailed
information regarding the 20 patients is shown in Table I. The median age of the patients
was 56 years, ranging between 12 and 74 years. The majority of the
patients exhibited no evident symptoms or only complained of flank
pain. Certain patients also presented with hematuria, renal colic
or postrenal azotemia. Imaging analysis revealed that the majority
of patients had hydronephrosis. The incidence was higher in males,
but there was no marked difference with regard to the side or site
of the ureteral malignant lymphoma. The most common pathological
type was NHL, including diffuse large B-cell lymphoma, follicular
B-cell lymphoma, small lymphocytic lymphoma and mucosa-associated
lymphoid tissue lymphoma. There were four cases of Hodgkin’s
lymphoma among the 20 patients.
 | Table ISummary of reported cases of malignant
lymphoma of the ureter. |
Table I
Summary of reported cases of malignant
lymphoma of the ureter.
| Age, years | Gender | Symptoms | Side | Site | Pathology | Treatment |
|---|
| 52 | F | Hematuria | L | U | HL | NU |
| 12 | M | Flank pain,
hydronephrosis | L | M | HL | NU, R |
| 35 | M | Asymptomatic,
hydronephrosis | L | L | NHL | NU, Chemo |
| 60 | M | Renal colic | R | L | NHL (mixed diffuse
and follicular) | Unknown |
| 59 | M | Cervical
lymphadenopathy | R | U | NHL | Unknown |
| 42 | M | Cervical
lymphadenopathy | R | U | NHL | Chemo |
| 69 | F | Asymptomatic,
hydronephrosis | L | M | NHL (follicular) | PU |
| 61 | M | Flank pain,
hydronephrosis | L | U | NHL (DLBCL) | Chemo |
| 62 | M | Postrenal
azotemia | Bil | U | NHL (small
lymphocytic) | Chemo |
| 52 | M | Flank pain,
hydronephrosis | L | L | HL | PU, R |
| 41 | F | Flank pain,
hydronephrosis | R | U | NHL (DLBCL) | Chemo, R |
| 54 | M | Asymptomatic,
hydronephrosis | R | M | HL | NU, Chemo |
| 72 | M | Asymptomatic,
hydronephrosis | R | U | NHL (MALT) | PU |
| 58 | M | Asymptomatic,
hydronephrosis | R | M | NHL (DLBCL) | NU, Chemo |
| 22 | F | Flank pain,
hydronephrosis | L | L | NHL | PU |
| 71 | M | Hematuria | R | M | NHL (follicular) | PU |
| 68 | M | Postrenal
azotemia | Bil | U | NHL (follicular) | NU, Chemo |
| 28 | M | Unknown | Unknown | Unknown | NHL (DLBCL) | Unknown |
| 74 | F | Flank pain,
urinoma | L | U | NHL (DLBCL) | PU, Chemo |
| 38 | M | Backache,
hydronephrosis | L | M | NHL (follicular) | NU, Chemo |
There is no particular imaging characteristic and in
the majority of cases, diagnosis was established according to the
histopathological study of tissue samples obtained from partial
ureterectomies or nephroureterectomies. Hashimoto et al
(6) reported that ureteral mucosal
biopsy by ureteroscopy was useful for obtaining enough tissue
sample to diagnosis. However, several other reports did not agree,
as the vessels and lymphatics of the ureters were longitudinally
oriented, thus, determined the direction of further tumor migration
(3). Adventitial arterial
involvement may allow the tumor to grow away from the wall. In the
present case, MRI scans revealed an area of nodular soft-tissue
density in the wall of the left-middle ureter. This section of the
ureteral wall demonstrated hyperintensity on T2-WIs, which favored
a neoplastic disease. PET-CT scans supported this hypothesis.
Although an ureteroscopic biopsy was obtained, histological
examination revealed a granuloma. Finally, the patient underwent a
nephroureterectomy due to the malfunction of the left kidney and
thereafter, the diagnosis was established.
Overall, 25–40% of NHL patients present with a
primary extranodal lymphoma. However, the definition of primary
extranodal lymphoma is controversial, particularly in patients
where nodal and extranodal sites are involved. Certain studies have
indicated that only patients with localized nidus have primary
extranodal lymphoma (10–13). Alternatively, studies that use a
more liberal criteria for extranodal lymphoma included patients
with a disseminated disease. These two definitions inevitably lead
to selection bias. Krol et al (14) hypothesized that any lymphoma that
is initially clinically dominant at an extranodal site should be
considered as a primary extranodal type, even if a disseminated
disease was identified. It is difficult to assess whether ureteral
lymphomas are primary. Lymphomas usually affect the ureter
indirectly with a mass effect caused by adjacent nodal disease.
Involvement of the proximal ureter by paraaortic adenopathy and the
distal ureter by iliac nodes is typical. However, this situation is
not absolute. Although distinguishing whether ureter lymphomas are
primary or caused by metastasis is difficult, for the patient in
the present study, it was important that the ureter was the initial
clinical location of the disease.
In conclusion, in cases where a partial ureteral
stenosis with ureteral wall thickening is observed by imaging
analysis, further histological examination of tissue samples should
be assigned as soon as possible, however, tissue biopsy via
ureteroscopy is not recommended.
References
|
1
|
Lebowitz JA, Rofsky NM, Weinreb JC and
Friedmann P: Ureteral lymphoma: MRI demonstration. Abdom Imaging.
20:173–175. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen HH, Panella JS, Rochester D, Ignatoff
JM and McVary KT: Non-Hodgkin lymphoma of ureteral wall: CT
findings. J Comp Assist Tomogr. 12:157–158. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buck DS, Peterson MS, Borochovitz D and
Bloom EJ: Non-Hodgkin lymphoma of the ureter: CT demonstration with
pathologic correlation. Urol Radiol. 14:183–187. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Curry NS, Chung CJ, Potts W and Bissada N:
Isolated lymphoma of genitourinary tract and adrenals. Urology.
41:494–498. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tozzini A, Bulleri A, Orsitto E, Morelli G
and Pieri L: Hodgkin’s lymphoma: an isolated case of involvement of
the ureter. Eur Radiol. 9:344–346. 1999.
|
|
6
|
Hashimoto H, Tsugawa M, Nasu Y, Tsushima T
and Kumon H: Primary non-Hodgkin lymphoma of the ureter. BJU Int.
83:148–149. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hara M, Satake M, Ogino H, Itoh M,
Miyagawa H, Hashimoto Y, Okabe M and Inagaki H: Primary ureteral
mucosa-associated lymphoid tissue (MALT) lymphoma-pathological and
radiological findings. Radiat Med. 20:41–44. 2002.PubMed/NCBI
|
|
8
|
Kawashima A, Shiotsuka Y, Nin M and Kokado
Y: Malignant lymphoma of the ureter: a case report. Hinyokika Kiyo.
51:269–272. 2005.(In Japanese).
|
|
9
|
Kubota Y, Kawai A, Tsuchiya T, Kozima K,
Yokoi S and Deguchi T: Bilateral primary malignant lymphoma of the
ureter. Int J Clin Oncol. 12:482–484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gospodarowicz MK, Sutcliffe SB, Brown TC,
et al: Patterns of disease in localized extranodal lymphomas. J
Clin Oncol. 5:875–880. 1987.PubMed/NCBI
|
|
11
|
Gospodarowicz MK and Sutcliffe SB: The
Extranodal Lymphomas. Semin Radiat Oncol. 5:281–300. 1995.
View Article : Google Scholar
|
|
12
|
Paryani S, Hoppe RT, Burke JS, et al:
Extralymphatic involvement in diffuse non-Hodgkin’s lymphoma. J
Clin Oncol. 1:682–688. 1983.
|
|
13
|
Rudders RA, Ross ME and DeLellis RA:
Primary extranodal lymphoma: response to treatment and factors
influencing prognosis. Cancer. 42:406–416. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krol AD, le Cessie S, Snijder S,
Kluin-Nelemans JC, Kluin PM and Noordjik EM: Primary extranodal
non-Hodgkin’s lymphoma (NHL): the impact of alternative definitions
tested in the Comprehensive Cancer Centre West population-based NHL
registry. Ann Oncol. 14:131–139. 2003.
|