Introduction
Postmenopausal osteoporosis is a type of systemic
bone disease, characterized by a reduction in bone density,
degradation of bone microstructure and an alteration in serum
markers of bone metabolism, including alkaline phosphatase (ALP),
estradiol (E2) and interleukin-6 (IL-6) in postmenopausal females.
This results in bone fragility and an increased risk of fracture.
Approximately a third of postmenopausal females suffer from
osteoporosis and this is usually due to the marked reduction in
estrogen levels (1). Hormone
replacement therapy (HRT) has been demonstrated to prevent bone
loss and commonly includes a combination of estrogen, progesterone
and progestin (2). However,
long-term HRT results in adverse side effects, including
hypocalcemia, worsening of renal impairment and osteonecrosis of
the jaw (3), and therefore novel
therapeutic strategies for the treatment of postmenopausal
osteoporosis are required. Bushen Huayu (BSHY) is used in
traditional Chinese medicine. It is based on the unique philosophy
of Chinese medicine, namely Bu Shen Hua Yu (complementing
the kidney system and resolving blood stasis). According to the
theory of traditional Chinese medicine, BSHY is able to improve the
function of the kidney system, strengthen bones, improve blood
circulation and relieve pain (4).
The aim of the present study was to investigate the therapeutic
effects of BSHY on bone density and morphology, as well as on serum
markers of bone metabolism in a postmenopausal osteoporosis animal
model, and to investigate the underlying mechanisms.
Materials and methods
Drugs and reagents
All Chinese medicinal materials used in the present
study were purchased from Hubei Shennong Bencao Prepared Herbal
Medicines Co., Ltd. (Xuchang, Henan, China) and were identified by
our laboratory. The herbarium specimens (no. SYRM-20090818-0090833)
were deposited in Hubei University of Medicine (Shiyan, Hubei,
China). The components were concentrated by decocting the solution
twice in a 10-fold volume of water at 100°C for 30 min. It was
found that 1 g BSHY corresponded with 1.2 g raw medicinal material.
The concentration of icariin, a quantity reference compound in the
extract, was 7.12 μg/g in a previous study (5). Nilestriol tablets were purchased from
Shanghai Hualian Pharmaceutical Co., Ltd. (Shanghai, China; no.
970710). Chloral hydrate (10%) was freshly prepared in the
laboratory. The E2 kit was purchased from Shenzhen Laerwen
Bioengineering Technology Co., Ltd. (Shenzhen, Guangdong, China)
and the IL-6 kit was purchased from Tianjin Jiuding Medical
Bioengineering Co., Ltd. (Tianjin, China).
Experimental animals
A total of 48 healthy, female Sprague-Dawley rats of
SPF grade, aged 3 months and weighing 215±45 g were provided by the
Hubei Laboratory Animal Center (Wuhan, Hubei, China; License no.
SCXK-E2005-0008). The rats were randomly divided into six groups:
the control group, the model group, the nilestriol group, the
low-dose BSHY (BSHY-L) group, the medium-dose BSHY (BSHY-M) group
and the high-dose BSHY (BSHY-H) group. All experiments were
conducted in accordance with the Guidelines for Ethical Conduct in
the Care and Use of Experimental Animals of the Hubei University of
Medicine and were approved by the ethics committee of the
university.
Oophorectomy model establishment
The animal models were established as previously
described with minor modifications (6). In the control group, sham surgery was
performed and only a small piece of fat tissue near the ovary was
removed. In all other groups, the ovaries were removed. Two weeks
after the surgery, 11.1, 22.2 and 44.4 g/kg BSHY was administered
by intragastric infusion to rats in the BSHY-L, BSHY-M and BSHY-H
treatment groups, respectively, whilst nilestriol (0.1 mg/kg) was
administered to rats in the nilestriol group. The animals in the
control and model groups underwent intragastric infusions of 10
ml/kg of 0.9% sodium chloride (equal volume to the other groups).
Treatment was performed for 12 weeks and was completed 24 h prior
to sample collection.
Sampling and detection
Following the drug treatment, blood and serum
samples were collected from all the rats. Serum ALP, E2 and IL-6
levels were determined. The rats were sacrificed by cervical
dislocation and the left and right femurs were collected. Dual
energy X-ray absorptiometry was used to measure the bone mineral
density in the right femur. Serial 4-μm sections were cut at the
proximal end of the left femur and the slices were stained using
hematoxylin and eosin. The slices were examined using microscopy
(Olympus BX43, Olympus Co., Tokyo, Japan) and the average number of
osteoblasts and osteoclasts was determined.
Statistical analysis
All data are expressed as the mean ± standard
deviation. The SPSS 13.0 software package was used for statistical
analysis (SPSS, Inc., Chicago, IL, USA). Statistical differences
were evaluated using one way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
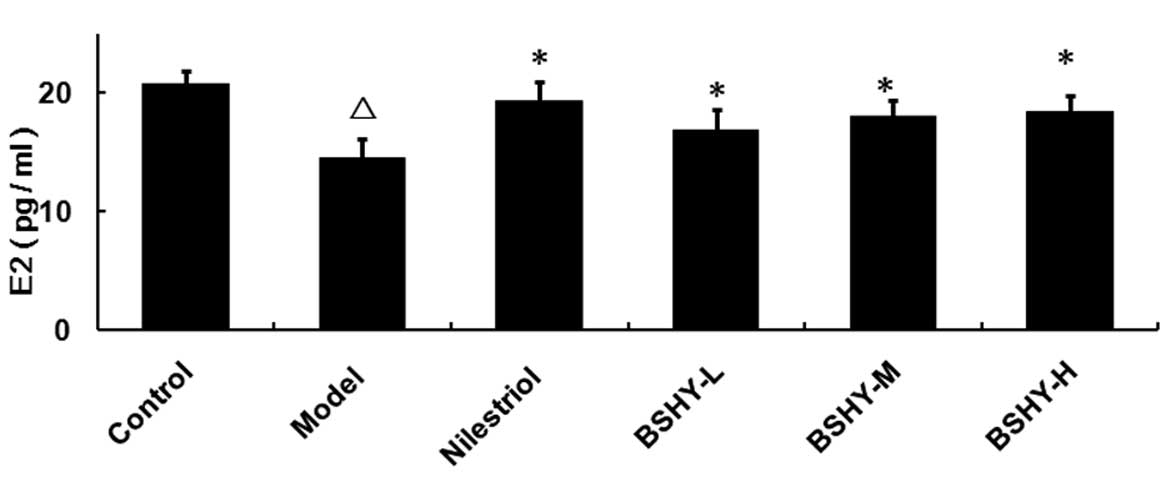
Effect of BSHY on the serum levels of E2
in oophorectomized rats
The serum E2 levels in the normal, model,
nilestriol, BSHY-L, BSHY-M and BSHY-H groups were 20.71±1.08,
14.54±1.61, 19.34±1.59, 16.89±1.71, 17.95±1.40 and 18.34±1.43
pg/ml, respectively. The serum levels of E2 in the model group were
significantly lower compared with the control group (P<0.05).
The levels of E2 in the nilestriol group were significantly higher
compared with the model group (P<0.05). Furthermore, compared
with the model group, the serum levels of E2 in all three BSHY
groups were significantly elevated (P<0.05), however, the levels
remained lower than the control group (Fig. 1).
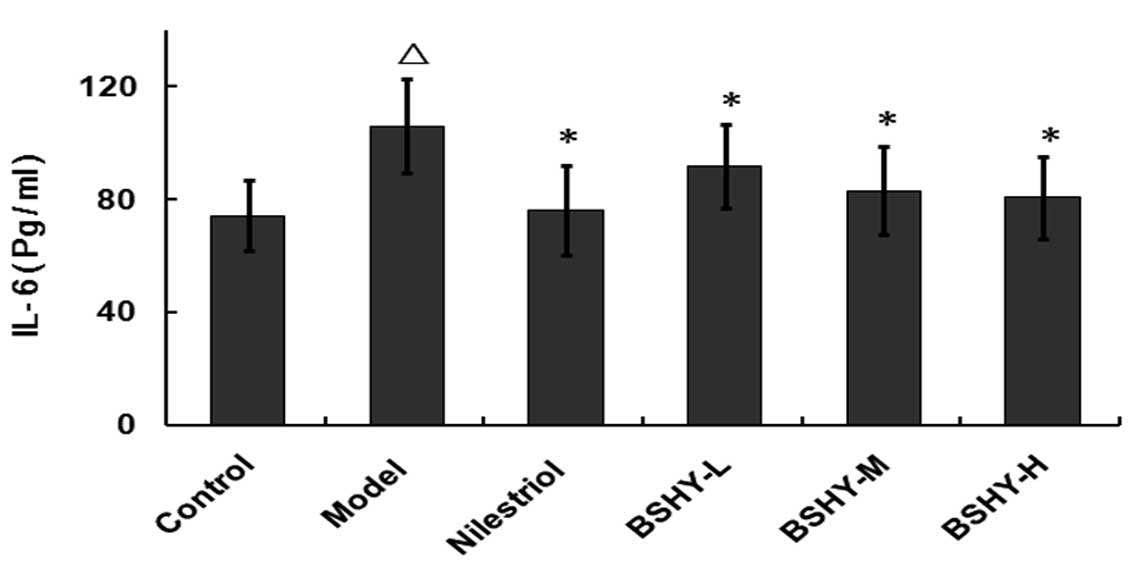
Effect of BSHY on serum IL-6 levels in
oophorectomized rats
The serum levels of IL-6 in the normal, model,
nilestriol, BSHY-L, BSHY-M and BSHY-H groups were 74.2±12.48,
105.93±16.50, 76.08±15.79, 91.85±14.81, 82.99±15.65 and 80.54±14.61
pg/ml, respectively. Compared with the control group, the serum
levels of IL-6 in the model group were significantly increased
(P<0.05), while the serum IL-6 levels in the BSHY-L, BSHY-M and
BSHY-H groups were significantly lower compared with the model
group (P<0.05; Fig. 2).
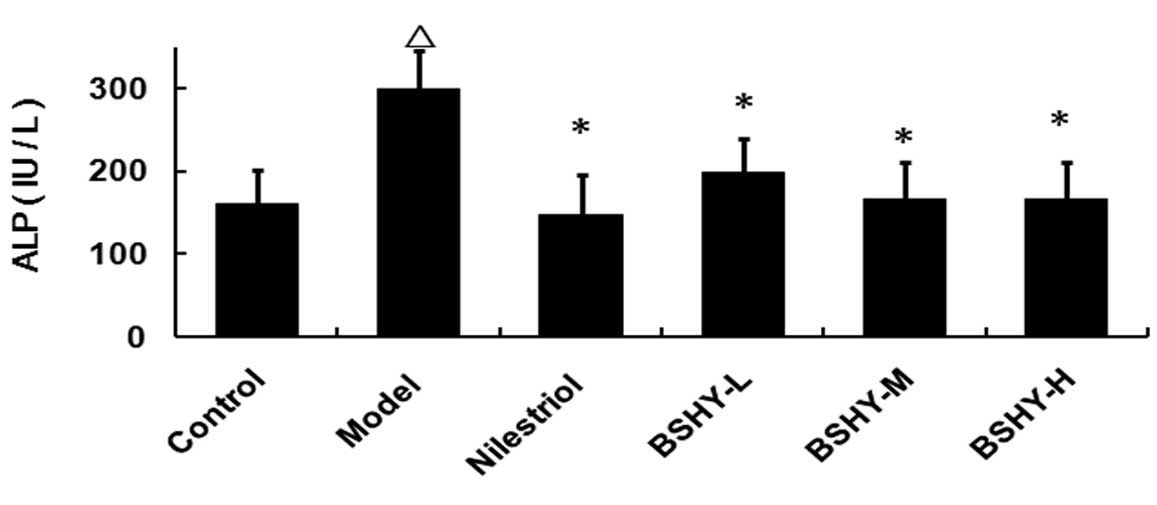
Effect of BSHY on the serum levels of ALP
in oophorectomized rats
The serum ALP levels in rats in the normal, model,
nilestriol, BSHY-L, BSHY-M and BSHY-H groups were 159.88±40.44,
299.13±45.79, 147.88±48.14, 197.75±41.74, 166.63±44.83 and
165.63±44.90 IU/l, respectively. Compared with the control group,
the serum ALP levels of rats in the model group were significantly
elevated (P<0.05), while the levels of ALP in the three BSHY
treatment groups were significantly lower compared with the model
group (P<0.05). However, no significant differences between the
three BSHY groups and the nilestriol group were identified
(P>0.05; Fig. 3).
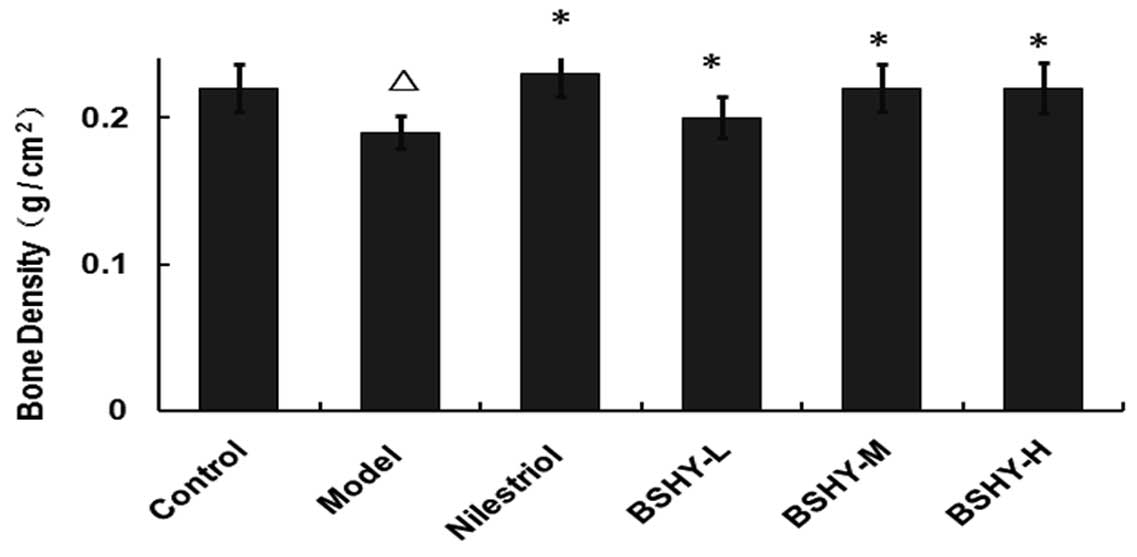
Effect of BSHY on bone density in the
bone metaphysis of the rat right femur
The bone density in the metaphysis of the right
femur in the normal, model, nilestriol, BSHY-L, BSHY-M and BSHY-H
groups were 0.22±0.016, 0.19±0.011, 0.23±0.016, 0.20±0.014,
0.22±0.016 and 0.22±0.017 g/cm2, respectively. The bone
density in the BSHY-L, BSHY-M and BSHY-H groups was elevated
compared with the model group (P<0.05) and no significant
differences between the BSHY treatment groups were observed
(P>0.05; Fig. 4).
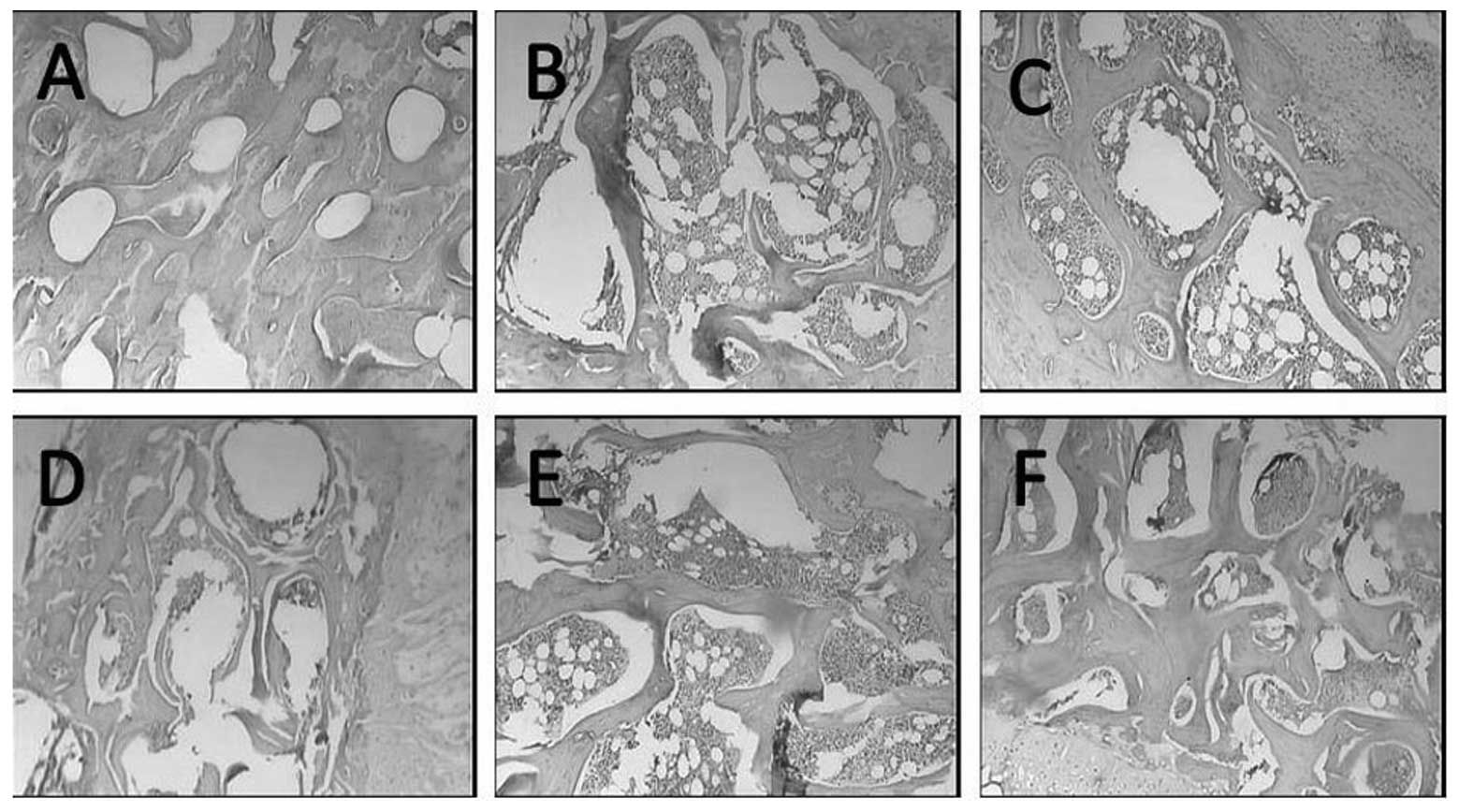
Alterations in bone tissue morphology in
the proximal end of the left femurs of oophorectomized rats
The bone trabeculae in the model group were
narrower, more lightly stained and more damaged arch-shaped
connections were observed compared with the control group. Bone
trabeculae fragments were also observed and the number was
significantly reduced compared with the control group. In addition,
the medullary cavity was enlarged in the model group and the
quantity of bone marrow was increased, compared with the control
group. However, compared with the model group, the bone trabeculae
in the three BSHY groups and the nilestriol group were all wider,
and the space and connections were markedly increased. Furthermore,
the medullary cavity was reduced in size (Fig. 5).
Discussion
Postmenopausal osteoporosis is a systematic
imbalance, in which the speed of bone resorption is greater than
that of bone formation. This disease is caused by estrogen
deficiency and results in microarchitectural changes, particularly
bone remodeling. Certain critical molecules co-ordinate the actions
of osteoblasts and osteoclasts during bone remodeling (7). As a result of estrogen deficiency
during bone remodeling osteoblasts release receptor activator of
NF-κB ligand (RANKL), a member of the tumor necrosis factor (TNF)
family. RANK binds to RANKL on osteoclasts, leading to the
differentiation, proliferation, multinucleation, activation and
survival of osteoclasts (8). In
addition, osteoblasts release markers, including TNF-α and IL-6
(9). IL-6 generation is attenuated
by sex hormones (10–12). IL-6 has been demonstrated to
directly increase the viability of osteoclasts, inhibit their
apoptosis and increase the length of their life cycle, resulting in
osteoporosis (13,14). In the present study, BSHY-treated
rats exhibited significantly elevated levels of E2, whilst levels
of IL-6 were significantly downregulated, indicating that BSHY may
attenuate the alterations induced in oophorectomized rats.
By contrast, ALP is a biomarker for osteoblasts and
the levels of ALP have been previously demonstrated to increase
when the level of estrogen increases in osteoblastic cells in
vitro (9). However, in the
present in vivo study, the serum levels of ALP in rats
treated with BSHY from the three groups decreased (P<0.05). This
may be due to the fact that half of all ALP is formed in the liver
and they may cross-react in the bone ALP assay. This result is
consistent with a previous study (15).
BSHY, a Chinese medicinal formulation, has been
proposed to supplement the kidney system and resolve blood stasis.
Among its components, Herba Epimedii (termed Yinyanghuo in
Chinese) and the active ingredient icariin, may have a potential
role in the prevention and treatment of osteoporosis by increasing
the mRNA expression levels of osteoprotegerin (a TNF-related
cytokine), bone morphogenetic protein (a promoter of osteogenesis)
and collagen I (synthesized by active osteoblasts) in
oophorectomized rats (16), as
well as inducing estrogen biosynthesis (17). Rhizoma Drynariae (termed
Gusuibu in Chinese) may enhance the treatment effects on
osteoporosis by reducing metabolic disorder (18). The anti-osteoporosis mechanisms of
other traditional Chinese medicines remain to be elucidated. They
may have a combined synergistic effect with Herba Epimedii and
Rhizoma Drynariae to enhance the actions and/or reduce the
side-effects.
Further studies are required to investigate whether
other bone resorption and bone formation parameters, including
RANKL, RANK, tartrate-resistant acid phosphatase, IL-1, calcium,
osteocalcin, TNF-α and deoxypyridinoline, are also involved in BSHY
treatment in oophorectomized rats.
In conclusion, in the present study, BSHY extract
was demonstrated to have a therapeutic effect on osteoporosis
caused by oophorectomy, and thus may be a potential therapeutic
treatment for postmenopausal osteoporosis. Furthermore, these
results suggest that the mechanism by which BSHY decreases the
serum levels of IL-6 may be by regulating E2.
Acknowledgements
This study was supported by the Class B Funding of
the Education Department of Hubei Province, China (Code no.
B20082412), The Public Health Project of Shiyan City (grant no.
2011057) and Hubei 2011 Research Project Funding (grant no. 4). The
authors would like to thank Mr. Hongliang Li, Mrs. Ming Liu and
Mrs. Hongli Guo for their technical support.
References
|
1
|
Cavalli L and Brandi ML: Age- and
gender-related macro- and micro-architecture changes in bone
structure and implications for treatment. Int J Clin Rheumatol.
6:359–369. 2011. View Article : Google Scholar
|
|
2
|
Lindsay R: Hormones and bone health in
postmenopausal women. Endocrine. 24:223–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanders S and Geraci SA: Osteoporosis in
postmenopausal women: considerations in prevention and treatment:
(women’s health series). South Med J. 106:698–706. 2013.
|
|
4
|
Yoldemir T, Erenus M and Durmusoglu F: The
impact of serum FSH and estradiol on postmenopausal osteoporosis
related to time since menopause. Gynecol Endocrinol. 28:884–888.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ouyang L, Wang X, Li H and Xiao Y:
Determination of Icariin in Bushenhuayu Electuary by HPLC. Zhong
Guo Yao Shi. 13:13–15. 2010.(In Chinese).
|
|
6
|
Joo MK, Park JJ, Lee BJ, Kim JH, Yeon JE,
Kim JS, Byun KS and Bak YT: The effect of a proton pump inhibitor
on bone metabolism in ovariectomized rats. Mol Med Rep.
7:1267–1272. 2013.PubMed/NCBI
|
|
7
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Craft CS, Broekelmann TJ, Zou W, Chappel
JC, Teitelbaum SL and Mecham RP: Oophorectomy-induced bone loss is
attenuated in MAGP1-deficient mice. J Cell Biochem. 113:93–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwak EJ, Lee YS and Choi EM: Effect of
magnolol on the function of osteoblastic MC3T3-E1 cells. Mediators
Inflamm. 2012:8296502012.PubMed/NCBI
|
|
10
|
Wang M, Ling G, Bei X, Junqing C, Peiqing
Z and Jie H: Clinical observation on 96 cases of primary
osteoporosis treated with kidney-tonifying and bone-strengthening
mixture. J Tradit Chin Med. 25:132–136. 2005.PubMed/NCBI
|
|
11
|
Hsieh TP, Sheu SY, Sun JS and Chen MH:
Icariin inhibits osteoclast differentiation and bone resorption by
suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis.
Phytomedicine. 18:176–185. 2011.PubMed/NCBI
|
|
12
|
McLean RR: Proinflammatory cytokines and
osteoporosis. Curr Osteoporos Rep. 7:134–139. 2009. View Article : Google Scholar
|
|
13
|
Mysliwiec J, Adamczyk M, Nikolajuk A and
Gorska M: Interleukin-6 and its considerable role in the
pathogenesis of thyrotoxicosis-related disturbances of bone
turnover in postmenopausal women. Endokrynol Pol. 62:299–302.
2011.PubMed/NCBI
|
|
14
|
Wang Y, Li LZ, Zhang YL, Zhu YQ, Wu J and
Sun WJ: LC, a novel estrone-rhein hybrid compound, concurrently
stimulates osteoprotegerin and inhibits receptor activator of NF-κB
ligand (RANKL) and interleukin-6 production by human osteoblastic
cells. Mol Cell Endocrinol. 337:43–51. 2011.PubMed/NCBI
|
|
15
|
Eastell R, Reid DM, Vukicevic S, Ensrud
KE, LaCroix AZ, Thompson JR, Thompson DD and Cummings SR: Effects
of 3 years of lasofoxifene treatment on bone turnover markers in
women with postmenopausal osteoporosis. Bone. 50:1135–1140.
2012.PubMed/NCBI
|
|
16
|
Song Y, Li SH, He ZJ, Zhang YW and Zhou
MW: Effect of icariin on osteoporosis in ovariectomized female
rats. Jun Yi Jin Xiu Xue Yuan Xue Bao. 33:400–403. 2012.(In
Chinese).
|
|
17
|
Yang L, Lu D, Guo J, Meng X, Zhang G and
Wang F: Icariin from Epimedium brevicornum Maxim promotes the
biosynthesis of estrogen by aromatase (CYP19). J Ethnopharmacol.
145:715–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Liu X, Zheng S, Jiang M, Xin C,
Lu X, Li F and Xiong Z: Metabonomic study on protective effect of
ethanol extracts of drynariae rhizoma on osteoporosis in rats urine
by using UPLC-MS/MS. Zhongguo Zhong Yao Za Zhi. 37:658–662.
2012.(In Chinese).
|