Introduction
Nocardia are gram-positive, aerobic,
slow-growing pathogens that are widespread in the environment and
are found in soil, vegetation, other organic matter, fresh water
and salt water (1). In humans,
they are mainly acquired through direct inhalation or inoculation.
Nocardia are rare opportunistic pathogens that particularly
affect patients who are on immunosuppressive therapy or
chemotherapy, post-transplant patients, and patients with human
immunodeficiency virus (HIV) infection. There are few studies and
case reports available on nocardiosis (2–9).
Thus, there are no optimal antimicrobial regimens available to
date. Furthermore, to the best of our knowledge, no large studies
or case series have been reported from China. Hence, the current
retrospective study was carried out to focus on the clinical
characteristics of Chinese patients with a confirmed diagnosis of
nocardiosis.
Materials and methods
Study design
The medical records of patients with a confirmed
diagnosis of nocardiosis, who were hospitalized at the First
Affiliated Hospital of Zhejiang University (Hangzhou, China)
between January 2000 and May 2013, were retrospectively reviewed.
The condition of all patients was confirmed as nocardiosis through
the presence of a positive culture of Nocardia. The possible
risk factors, clinical features, laboratory abnormalities,
treatments and outcomes of these patients were analyzed by the
review of all medical, epidemiological, clinical and laboratory
data by two infectious disease experts. Radiological results were
obtained from the review of radiographs by an independent
radiologist, who was blinded with regards to the clinical findings.
The ethics committee of the hospital reviewed and approved the
study protocol. Written informed consent was obtained from all
participants.
Specimens were inoculated in normal blood using
nonselective media. Nocardia species identification and
susceptibility tests were carried out only upon special request and
therefore the data on Nocardia assessments were available
for only a limited number of patients.
Statistical analysis
All statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous
variables were expressed as medians with interquartile ranges. The
percentages of patients in each category were calculated for
categorical variables.
Results
Demographic and epidemiological
characteristics
A total of 40 patients with clinical evidence for
nocardiosis and one or more positive Nocardia cultures from
various specimens were enrolled during the study period. Age,
gender, underlying diseases and corresponding treatment are
summarized in Table I.
 | Table IDemographic and epidemiological
characteristics of 40 patients with nocardiosis. |
Table I
Demographic and epidemiological
characteristics of 40 patients with nocardiosis.
| Characteristic | Number | % |
|---|
| Mean age (years) | 52.2 | 100.0 |
| Gender
distribution |
| Male | 26 | 65.0 |
| Female | 14 | 35.0 |
| Underlying
disease |
| Transplantation | 5 | 12.5 |
| Liver | 3 | 7.5 |
| Kidney | 2 | 5.0 |
| Chronic lung
disease | 6 | 15.0 |
| Solid
malignancy | 2 | 5.0 |
| Diabetes | 13 | 32.5 |
| Hypertension | 4 | 10.0 |
| Autoimmune
diseases | 8 | 20.0 |
| Chronic renal
disease | 4 | 10.0 |
| Hematological
disease | 3 | 7.5 |
| Hepatitis B
infection | 3 | 7.5 |
| HIV infection | 1 | 2.5 |
| Chronic drug use |
| Corticosteroids | 20 | 50.0 |
| Chemotherapy | 1 | 2.5 |
|
Immunosuppressants | 7 | 17.5 |
| Trauma | 5 | 12.5 |
| Other | 2 | 5.0 |
The median age of patients was 52 (range, 28–82)
years and 65.0% of the patients were male. A total of 72.5% (29/40)
of the patients had one or more underlying diseases; diabetes
(32.5%), autoimmune diseases (20.0%), chronic lung disease (15.0%),
solid organ transplant (12.5%) and chronic renal disease (10.0%)
were the most common underlying diseases. Five (12.5%) patients had
diseases due to trauma-related injuries such as road traffic
accidents and skin abrasion. Only one patient (2.5%) had HIV
infection. The remaining five patients had no definable underlying
diseases (12.5%). A total of 22 patients (55.0%) were found to be
immunocompromised due to corticosteroids (20 patients, including
seven patients on immunosuppressive therapy), chemotherapy (one
patient) or HIV infection (one patient).
Clinical features and laboratory
abnormalities
The clinical features and laboratory abnormalities
of the patients are shown in Table
II. The clinical features of nocardiosis are generally
non-specific. Fever, cough, the production of sputum and chest pain
were the most common symptoms observed among the study
participants. The majority of the patients presented a subacute
form of infection with one patient having a chronic form of
cutaneous abscess infection, and four patients presented an acute
form of infection. Overall, the pleuropulmonary region (n=34,
85.0%) was the most involved site among the study population, which
included the lung (n=29, 72.50%) and pleura (n=5, 12.5%). The other
sites involved included the skin and soft tissue (25.0%), brain
(12.5%), and pericardium (2.5%).
 | Table IIClinical characteristics and
laboratory abnormalities of 40 patients with nocardiosis. |
Table II
Clinical characteristics and
laboratory abnormalities of 40 patients with nocardiosis.
|
Characteristics | Number | % |
|---|
| Clinical
feature |
| Fever | 30 | 75.0 |
| Cough | 26 | 65.0 |
| Sputum
production | 20 | 50.0 |
| Chest pain | 9 | 22.5 |
| Hemoptysis | 3 | 7.5 |
| Cutaneous
abscess/ulcer | 5 | 12.5 |
| Shock | 1 | 2.5 |
| Headache | 4 | 10.0 |
| Laboratory
abnormalities |
| Leukocytosis | 21 | 52.5 |
| Neutrophilia | 30 | 75.0 |
| C-reactive protein
>8 mg/l | 19 | 73.1 |
Disseminated disease, defined as the isolation of
Nocardia from the bloodstream or its involvement in multiple
organs, was demonstrated in 30.0% of cases. Within the disseminated
disease group, ten patients (83.3%) had lung involvement, five
(41.7%) had central nervous system (CNS) involvement, five (41.7%)
had cutaneous abscess and one patient (8.3%) had suppurative
pericarditis. Bacteremia was present in five patients, one each
with renal transplantation, liver transplantation, autoimmune
hemolytic anemia, diabetes mellitus, and one patient without
underlying disease. The median time from the onset of symptoms to
the diagnosis of nocardiosis was 42 (range, 9–120) days. In
patients with and without underlying diseases, the median time from
hospital admission to diagnosis was 53.8 and 12.1 days,
respectively.
Leukocytosis (defined as a leukocyte count of
>10×109/l) was present in 21 (52.5%) patients.
Neutrophilia (>75% neutrophils) was present in 30 (75.0%)
patients. No patients had leukopenia (leukocyte count
<4×109/l) or neutropenia (neutrophil count
<0.5×109/l). C-reactive protein levels were available
in 26 (65.0%) patients and they markedly increased (defined as a
level >8 mg/l) in 19 (73.1%) patients with a range of 30.2 to
217 mg/l. Only three patients (7.5%) had a completely normal blood
count and chemistry panel on admission.
Diagnostic cultures were obtained from the following
clinical samples: sputum in 16 patients, bronchoalveolar lavage in
one, empyema drainage in 10, lung biopsy in three, abscess puncture
fluid in eight and blood in five patients.
Concomitant infections were commonly observed in the
study participants. A total of 40.0% of patients with pulmonary
nocardiosis were coinfected with other bacteria or fungi including
Stenotrophomonas maltophilia, Klebsiella pneumonia,
Streptococcus pneumoniae, Enterococcus faecalis, Aspergillus
and Candidiasis. One patient with pleurisy was coinfected
with Escherichia coli and another with Pseudomonas
aeruginosa. One patient with a brain abscess had a
Nocardia coinfection with Pseudomonas aeruginosa.
Major findings on chest computed
tomography (CT) and brain magnetic resonance imaging (MRI)
scans
Chest X-ray results of pulmonary nocardiosis are
usually non-specific and similar to those caused by other bacteria.
A total of 36 patients had serial chest CT scans and commonly
observed abnormalities were airspace opacities, nodules and masses,
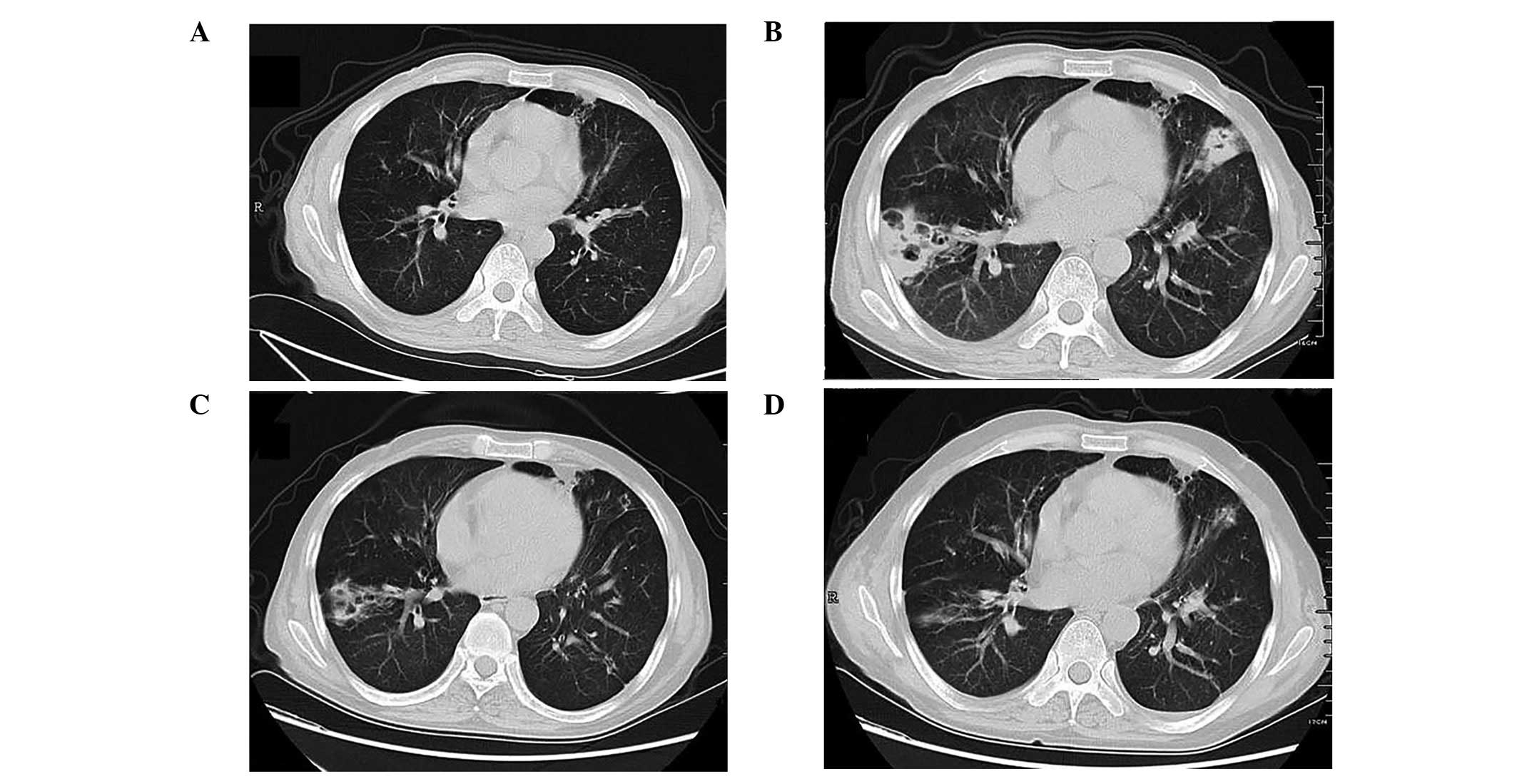
which accounted for 88.2% of cases. Cavitation was common in
patients with pulmonary nocardiosis (47.1%; Fig. 1); alveolar and interstitial
infiltration were less common. Pleural effusion or pleural nodules
were found alone or concurrently with lung lesions.
Results of 18F-fluorodeoxyglucose
(18F-FDG) positron emission tomography and CT scans were
available for two (5%) patients (one with pulmonary nocardiosis and
the other with a nocardial pleural nodule). An increased
18F-FDG uptake demonstrated in the lung and pleural
nodules in these patients was consistent with a previous study
(10). Chest pathology was
observed in seven patients (17.5%) and tissue pathology tests
revealed granulomatous inflammation or abscesses. The radiologic
patterns were not specific.
Five patients with brain nocardiosis had undergone
brain CT or MRI scans and were found to have a solitary
ring-enhancing lesion with central necrosis and surrounding edema
within the corresponding localization, which was associated with
abscesses (Fig. 2).
Treatment and outcome
Two patients were referred to a local hospital
immediately following diagnosis and thus were unavailable for
follow-up. Therefore, details of antimicrobial therapy were only
available for the remaining 38 patients.
A total of 27 (71.1 %) patients initially received
treatment with trimethoprim sulfamethoxazole (TMP-SMX). Of these,
four patients received TMP-SMX alone, while the others received it
in combination with: carbapenem (eight patients), amikacin (two
patients), ceftriaxone (six patients), quinolones (one patient),
linezolid (three patients), or carbapenem and amikacin (three
patients).
Three patients received treatment with quinolones
either as a monotherapy (one patient) or in combination with
minocycline or with TMP-SMX (one patient, respectively). The other
antibiotic regimens included a combination of amikacin and
carbapenem (one patient), ceftriaxone (four patients), carbapenem
(one patient), linezolid (one patient) and a combination of
amikacin, carbapenem and minocycline (one patient).
Following 2–4 weeks of initial treatment, the
patients were discharged as their clinical status had improved.
Their regimen was switched to the following oral treatments:
TMP-SMX; linezolid; TMP-SMX in combination with linezolid;
quinolones; TMP-SMX in combination with quinolones; and quinolones
in combination with minocycline.
Six patients (15.0%) succumbed during
hospitalization; of these, three had disseminated disease, two had
severe pneumonia and one succumbed due to hepatitis B virus-related
liver failure. Among the six deceased patients, a diagnosis of
nocardiosis was missed for three patients. The mortality rate was
higher among patients with disseminated disease (36.4%),
immunocompromised patients (22.7%), elderly patients >55 years
old (31.2%), patients who had CNS involvement (60.0%) and patients
with concomitant infections (31.2%). There was no significant
difference in mortality between males (19.2%) and females (14.3%).
Follow-up details for >3 months were available for 29 patients
(72.5%) and only 27 had follow-up details for one year. Other
patients were referred to other hospitals and thus were unavailable
for follow-up. Following hospital discharge, three patients
succumbed due to underlying diseases (one each with lung cancer,
renal failure and HIV infection), none of whom had CNS involvement.
Two patients did not receive neurosurgery and one patient who
received neurosurgery developed a concomitant infection with
Pseudomonas aeruginosa in the brain abscess.
Primary cutaneous nocardiosis was treated in all
patients. The duration of treatment ranged from one to three months
and three patients received antibiotic therapy in combination with
surgical treatment. One patient with disseminated disease involving
the lung, pericardium and subcutaneous regions recovered following
seven months of treatment, but recurrence of the disseminated
disease occurred one year following the discontinuation of
treatment. However, this patient recovered completely from the
second disseminated disease.
The most common initial diagnoses were pulmonary
fungal infection and tuberculosis. Due to the observation of
multiple nodules with cavities, mass and pleural effusion in the CT
scans at the time of initial presentation, a fungal infection was
highly suspected. A total of 17 patients (42.5%) initially received
antifungal therapy with voriconazole, caspofungin, amphotericin B
or fluconazole. The two patients with brain involvement received
praziquantel for antiparasitic treatment due to an initial
diagnosis of parasitic infection.
Discussion
The present retrospective study from a large
tertiary teaching hospital is the first report on serial cases of
nocardiosis in China. Similar to other reports, nocardiosis was
observed to be an emerging disease. While only one patient was
diagnosed with nocardiosis during 2000, approximately seven cases
were reported during 2012 at the site of the current study. This
increased trend is possibly due to the rise in the number of
immunosuppressed patients over the last several years (11). The most common underlying disease
in the patients with nocardiosis was diabetes (32.5%). Autoimmune
disease (20.0%), bronchiectasis and other structural lung
abnormalities, including chronic obstructive pulmonary disease
(15.0%), and organ transplantation (12.5%), were also observed as
risk factors in the study population. These observations were in
accordance with other published studies (4–8).
Notably, despite the hospital being a bone marrow transplantation
(BMT) center, none of the patients were BMT recipients. In
addition, the study site was in an area where HIV is not epidemic,
and only one patient in the study had HIV infection. Nocardiosis in
the study subjects had male predominance, which was similar to most
previously published studies (4,6,8,12).
The major risk factor in the study population was corticosteroid
treatment, with 20 (50.0%) of the patients having received systemic
corticosteroid therapy prior to the diagnosis of nocardiosis.
Chronic corticosteroid treatment at a high dose has been reported
to play a predisposing role in nocardiosis (2,3,6,13).
However, the present study results revealed that even short-term
corticosteroid treatment at a low dose (0.5–1 mg/kg, 3–6 weeks
duration) may be a risk factor for nocardiosis.
As reported in other studies (2,3,6–8),
pulmonary disease was the most common presentation (85.0%) and
30.0% of cases had a disseminated disease. The most frequent chest
CT results in the study group were airspace opacities, multifocal
nodules or masses; cavitation was common in pulmonary nocardiosis.
The brain images demonstrated lesions associated with
abscesses.
Clinical recognition of nocardial infection is
difficult due to its relatively low incidence rate and non-specific
manifestations. In the current study, the common initial diagnosis
was general bacterial infection, invasive fungal disease,
tuberculosis, parasitic infection or malignancy. In three patients,
an official diagnosis of nocardiosis was established only following
mortality. The median time between the onset of symptoms and
diagnosis was 42 days, and such a delay was also reported in
another published study (12).
This delay in diagnosis and treatment may be life threatening.
Primary pulmonary infection and skin/soft tissue infection are the
most common initial clinical symptoms and may result in
disseminated Nocardia infection if the initial therapy is
inadequate. In the present study, at least 50% of disseminated
nocardiosis cases were initially treated with antibiotics which is
sensitive to Nocardia, such as carbapenem, amikacin,
ceftriaxone or quinolones for suspected common bacterial infection.
Such antibiotic treatment was discontinued due to the initial
clinical improvement. However, the duration was not sufficient for
nocardiosis and resulted in a tendency to disseminate. To reduce
the delay in diagnosis and treatment, nocardiosis should be
considered in the differential diagnosis of immunosuppressed
patients and testing for Nocardia should be performed in
patients with risk factors for nocardiosis.
To the best of our knowledge, there have been no
randomized prospective trials published to date that have assessed
the most effective antimicrobial therapy for Nocardia
infection. In the present study, 71.1% of patients who received
antimicrobial agents were treated with TMP-SMX monotherapy or a
combination of TMP-SMX with other drugs. Alternative antibiotic
regimens included the combination of amikacin and carbapenem,
ceftriaxone, linezolid, quinolones, and minocycline.
TMP-SMX is currently accepted as the first-line
treatment for nocardiosis, although there are controversial reports
available concerning the sensitivity of TMP-SMX to Nocardia.
Certain studies have revealed high rates of resistance (16.1–43.0%)
of Nocardia to TMP-SMX (6,14,15).
However, a study by Brown-Elliott et al involving 522
clinical isolates in six major laboratories in the United States
reported that only 2% of the isolates were demonstrated to have
resistant minimum inhibitory concentrations of TMP-SMX and/or SMX
(16). Data from a study by Lai
et al revealed that 10% of isolates were resistant to
TMP-SMX (17). Furthermore, the
antimicrobial susceptibility of Nocardia to meropenem,
amikacin and ceftriaxone has also been reported to be high
(17). Clinical experience with
these drugs proved encouraging (18,19).
The mortality rate remains high with TMP-SMX monotherapy,
especially in patients with severe or disseminated disease. Thus, a
combination of TMP-SMX with amikacin and imipenem or ceftriaxone is
recommended as an empirical therapy in serious CNS-involved and
disseminated cases of Nocardia. Regimens with quinolones
have been described in several case reports, especially in patients
with CNS nocardiosis, due to their excellent cerebrospinal fluid
penetration and low toxicity (20,21).
Minocycline is another potentially useful drug (22,23)
whilst linezolid has been indicated to be an effective alternative
for the treatment of nocardiosis in certain studies (5,10,23–26).
Previous clinical experience obtained from the study site has
revealed that linezolid has a marked effectiveness in treating
Nocardia infection (24,26).
However, long-term use of linezolid should be avoided due to the
cost factor and adverse effects. Treatment for nocardiosis is
recommended to begin at the initial diagnosis, continue for several
weeks intravenously and then switch to oral therapy following
initial clinical improvement. For local empyema, including thoracic
empyema, brain and cutaneous abscesses, adjuvant surgical treatment
may be necessary.
The following were the key limitations of the
present study: i) the study was retrospective and ii) the majority
of the microbiological data concerning the identification of
species and their susceptibility were unavailable. Despite these
limitations, the current study results provide useful information
on the risk factors of nocardiosis. Nocardia infection is an
uncommon but emerging disease that may cause serious or
disseminated disease. Delays in the diagnosis and treatment of
nocardiosis may be life threatening. Patients with nocardiosis,
especially those who are at high risk, require careful clinical and
pathological evaluations. Early recognition of the disease and the
initiation of appropriate treatment are essential for a good
prognosis.
Acknowledgements
The authors thank Yang Qing for his assistance in
preparing the manuscript.
References
|
1
|
Wilson JW: Nocardiosis: updates and
clinical overview. Mayo Clin Proc. 87:403–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosman Y, Grossman E, Keller N, Thaler M,
Eviatar T, Hoffman C and Apter S: Nocardiosis: A 15-year experience
in a tertiary medical center in Israel. Eur J Intern Med.
24:552–557. 2013.PubMed/NCBI
|
|
3
|
Cattaneo C, Antoniazzi F, Caira M,
Castagnola C, Delia M, Tumbarello M, et al: Nocardia spp
infections among hematological patients: results of a retrospective
multicenter study. Int J Infect Dis. 17:e610–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garcia-Bellmunt L, Sibila O, Solanes I,
Sanchez-Reus F and Plaza V: Pulmonary nocardiosis in patients with
COPD: characteristics and prognostic factors. Arch Bronconeumol.
48:280–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambrosioni J, Lew D and Garbino J:
Nocardiosis: updated clinical review and experience at a tertiary
center. Infection. 38:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mootsikapun P, Intarapoka B and
Liawnoraset W: Nocardiosis in Srinagarind Hospital, Thailand:
review of 70 cases from 1996–2001. Int J Infect Dis. 9:154–158.
2005.
|
|
7
|
Saubolle MA and Sussland D: Nocardiosis:
review of clinical and laboratory experience. J Clin Microbiol.
41:4497–4501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castro JG and Espinoza L: Nocardia
species infections in a large county hospital in Miami: 6 years
experience. J Infec. 54:358–361. 2007.PubMed/NCBI
|
|
9
|
Tuo MH, Tsai YH, Tseng HK, Wang WS, Liu CP
and Lee CM: Clinical experiences of pulmonary and bloodstream
nocardiosis in two tertiary care hospitals in northern Taiwan,
2000–2004. J Microbiol Immunol Infect. 41:130–136. 2008.PubMed/NCBI
|
|
10
|
Zhao K, Dong MJ, Sheng ZK, Liu KF, Yang
SY, Liu ZF and Sheng JF: Elevated uptake of 18F-FDG in
PET/CT imaging of a nocardial pleural nodule. Clin Imaging.
36:383–385. 2012.
|
|
11
|
Hannaman MJ and Ertl MJ: Patients with
immunodeficiency. Med Clin North Am. 97:1139–1159. 2013. View Article : Google Scholar
|
|
12
|
Matulionyte R, Rohner P, Uckay I, Lew D
and Garbino J: Secular trends of nocardia infection over 15 years
in a tertiary care hospital. J Clin Pathol. 57:807–812.
2004.PubMed/NCBI
|
|
13
|
Martínez Tomás R, Menéndez Villanueva R,
Reyes Calzada S, Santos Durantez M, Vallés Tarazona JM, Modesto
Alapont M and Gobernado Serrano M: Pulmonary nocardiosis: risk
factors and outcomes. Respirology. 12:394–400. 2007.
|
|
14
|
Uhde KB, Pathak S, McCullum I Jr,
Jannat-Khah DP, Shadomy SV, Dykewicz CA, et al:
Antimicrobial-resistant Nocardia isolates, United States,
1995–2004. Clin Infect Dis. 51:1445–1448. 2010.PubMed/NCBI
|
|
15
|
Tremblay J, Thibert L, Alarie I,
Valiquette L and Pépin J: Nocardiosis in Quebec, Canada, 1988–2008.
Clin Microbiol Infect. 17:690–696. 2011.
|
|
16
|
Brown-Elliott BA, Biehle J, Conville PS,
Cohen S, Saubolle M, Sussland D, et al: Sulfonamide resistance in
isolates of Nocardia spp. from a US multicenter survey. J
Clin Microbiol. 50:670–672. 2012.
|
|
17
|
Lai CC, Liu WL, Ko WC, Chen YH, Tan HR,
Huang YT and Hsueh PR: Multicenter study in Taiwan of the in vitro
activities of nemonoxacin, tigecycline, doripenem, and other
antimicrobial agents against clinical isolates of various
Nocardia species. Antimicrob Agents Chemother. 55:2084–2091.
2011. View Article : Google Scholar
|
|
18
|
Garcia del Palacio JI and Martín Pérez I:
Response of pulmonary nocardiosis to ceftriaxone in a patient with
AIDS. Chest. 103:1925–1926. 1993.PubMed/NCBI
|
|
19
|
Lo W and Rolston KV: Use of imipenem in
the treatment of pulmonary nocardiosis. Chest. 103:951–952. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fihman V, Berçot B, Mateo J, Losser MR,
Raskine L, Riahi J, et al: First successful treatment of
Nocardia farcinica brain abscess with moxifloxacin. J
Infect. 52:e99–e102. 2006.
|
|
21
|
Tanioka K, Nagao M, Yamamoto M, Matsumura
Y, Tabu H, Matsushima A, et al: Disseminated Nocardia
farcinica infection in a patient with myasthenia gravis
successfully treated by linezolid: a case report and literature
review. J Infect Chemother. 18:390–394. 2012.
|
|
22
|
Ogawa T, Kasahara K, Yonekawa S, Nakagawa
C, Maeda K, Konishi M, et al: Nocardia beijingensis
pulmonary infection successfully treated with intravenous β-lactam
antibiotics and oral minocycline. J Infect Chemother. 17:706–709.
2011. View Article : Google Scholar
|
|
23
|
Lewis KE, Ebden P, Wooster SL, Rees J and
Harrison GA: Multi-system infection with Nocardia
farcinica-therapy with linezolid and minocycline. J Infect.
46:199–202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen Q, Zhou H, Li H and Zhou J: Linezolid
combined with trimethoprim-sulfamethoxazole therapy for the
treatment of disseminated nocardiosis. J Med Microbiol.
60:1043–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu X, Han F, Wu J, He Q, Peng W, Wang Y,
et al: Nocardia infection in kidney transplant recipients:
case report and analysis of 66 published cases. Transpl Infect Dis.
13:385–391. 2011. View Article : Google Scholar
|
|
26
|
Shen T, Wu L, Geng L, Wei Z and Zheng S:
Successful treatment of pulmonary Nocardia farcinica
infection with linezolid: case report and literature review. Braz J
Infect Dis. 15:486–489. 2011.
|